Abstract
Resveratrol, a natural antioxidant, anti-inflammatory plant extract, was found to have a protective effect in poultry subjected to heat stress. In this study, we strove to characterize resveratrol on intestinal of duck exposed to acute heat stress and investigate the underlying mechanism. A total of 120 Shan-ma ducks (60 days old) were randomly divided into 2 groups. The control group was fed a basal diet, and the resveratrol group was fed a basal diet supplemented with 400 mg/kg resveratrol. Animals in 2 groups were kept at a temperature of 24°C ± 2°C for 15 d. Then, animals of both groups were placed in an artificial climate room at 39°C. Twelve ducks of each group were sacrificed for sampling at 0, 30, and 60 min, respectively. Results indicated that resveratrol increased the ratio of villus height to crypt depth, increased the number of goblet cells, and reduced the histopathological damage of jejunum caused by acute heat stress. Furthermore, the gene expression of heat shock proteins (HSP60, HSP70, and HSP90) and tight junction proteins (CLDN1 and OCLN) was significantly increased in the resveratrol group compared to that in the control groups. Simultaneously, resveratrol significantly activated the SIRT1-NRF1/NRF2 signaling pathways, improved ATP level of jejunum, and increased SOD and CAT antioxidant enzymes activities. In addition, we found that the NF-κB/NLRP3 inflammasome signaling pathways were repressed under acute heat stress. Meanwhile, supplement resveratrol further inhibited the NLRP3 inflammasome pathway, decreased protein level of NLRP3 and caspase1 p20, reduced the secretion of IL-1β. Taken together, our results indicate that resveratrol against the oxidative damage and inflammation injury in duck jejunum induced by heat stress via active SIRT1 signaling pathways.
Key words: resveratrol, acute heat stress, jejunum, SIRT1
INTRODUCTION
Accompanied by the global warming issue, heat stress could play a crucial role in affecting poultry performance. Hyperthermia reduces poultry food intake and daily weight gain, induces tissue injury, and reduces reproduction performance (Ma et al., 2014; Roushdy et al., 2020). Further study found that heat stress impairs mitochondria functions and induces cell oxidative injury (Huang et al., 2015). The intestine is the main organ where the digestion and absorption of nutrients occur, as well as an important innate immune barrier to prevent the invasion of pathogens (Ulluwishewa et al., 2011). Heat stress damages the intestinal of poultry, resulting in both decreased intestinal villus height to crypt depth ratio and the number of proliferating cells, and increased plasma endotoxin concentration (Nanto-Hara et al., 2020). At the same time, heat stress directly alters jejunal tight junction protein cause an impaired intestinal barrier, and leading to inflammation (Koch et al., 2019). Therefore, maintaining the intestinal barrier integrity and improving the antioxidant capacity within this organ has been suggested as a potential target for relieving heat stress injury.
Resveratrol is a natural polyphenol with anti-inflammatory, antioxidant, antiaging, and anticancer properties that have attracted increasing attention in recent decades (Koch et al., 2019; Meng et al., 2021). The study indicated administration of resveratrol (400 mg/kg) as a dietary supplement exerts a protective effect against heat-induced injury of the chicken jejunum by inhibiting the expression of transcription factor-nuclear factor κB (NF-κB) (Liu et al., 2016). Dietary resveratrol supplementation can inhibit heat stress-induced high-activated innate immunity and inflammatory response in the spleen of yellow-feather broilers via inhibiting the activation of NF-κB, MAPK, and PI3K/AKT signaling pathways (He et al., 2019). Recently, a study in Pekin ducks confirmed that dietary resveratrol supplement also improves carcass traits and meat quality (Yu et al., 2021). Significantly, resveratrol is considered as an activator of silent information regulator 1 (SIRT1) (Lagouge et al., 2006). However, the effect of resveratrol on poultry whether it is related to the SIRT1 signaling pathway is still unclear. SIRT1 signaling pathway plays an important role in antioxidant and anti-inflammation. SIRT1 deacetylated the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator-1 α (PGC-1α), where it induces expression of nuclear respiratory factor 1 (NRF1) and binds to the mitochondrial transcription factor A (TFAM) promoter to enhance mitochondrial biogenesis and function (Hwang et al., 2013). Activation of the SIRT1/PGC-1α pathway also enhances nuclear factor-like 2 (NRF2) nuclear translocation and increases the transcription of antioxidant target genes (Han et al., 2019). Moreover, a study confirmed that resveratrol could attenuate the inflammatory response by reducing ROS production and inhibiting NLRP3 activation (Zou et al., 2018).
In this study, we investigated the effects of resveratrol administration on intestinal injury and intestinal barrier integrity in ducks under acute heat stress (39°C for 30–60 min) (Zeng et al., 2014), focusing on the activation of SIRT1, NF-κB, and NLRP3 signaling pathways, and antioxidant and anti-inflammatory capacity. This study facilitates a better understanding of the effects of acute heat stress on intestinal damage and provides the theoretical basis of using resveratrol to prevent heat stress injury in ducks.
MATERIALS AND METHODS
Animals and Experimental Design
A total of 120 female Shan Ma ducks, aged 60 d, were bred in Zhong-kai Education Scientific Research Base. The ducks were randomly divided into 2 groups, each group of birds was further subdivided into 6 replicate groups (10 ducks per replicate). The Control (C) group was fed with a standard diet (Table 1) and water ad libitum, and the Resveratrol (Res) group was fed the basal diet supplemented with 400 mg/kg resveratrol (Aladdin, China). The resveratrol dose used was selected based on a previous study (Liu et al., 2016). Animals were kept at 24°C ± 2°C in an environmentally controlled room. After 15 d, the animals of both groups were placed in an artificial climate room at 39°C (without food and water). Twelve ducks of each group were weighed (Table S1) and sacrificed using carbon dioxide for sampling at 0, 30, and 60 min, respectively. Serum samples were collected. And jejunum samples were carefully dissected, collected, and stored at −80°C for use in subsequent experiments.
Table 1.
Composition and nutrient levels of basal diet.
| Ingredients | 0–4 w | 5–12 w |
|---|---|---|
| Corn | 38.5 | 29 |
| Wheat | 12 | 12.5 |
| Barley | 5 | 8 |
| Wheat bran | 3 | 12.8 |
| Sorghum | 7 | 9 |
| Soybean meal | 21 | 9 |
| Corn DDGS | 9 | 15.5 |
| Limestone | 1.39 | 1.50 |
| CaHPO4 | 1.30 | 0.9 |
| Salt | 0.3 | 0.3 |
| L-Lys.HCl | 0.33 | 0.32 |
| DL-Met | 0.15 | 0.13 |
| Thr | 0.03 | 0.05 |
| Premix1 | 1 | 1 |
| Total | 100 | 100 |
| Calculate nutrient level | ||
| ME, | 2.80 | 2.65 |
| CP | 18.5 | 16.0 |
| Ca | 0.90 | 0.85 |
| NPP | 0.66 | 0.38 |
| Lys | 1.10 | 0.85 |
| Met | 0.45 | 0.41 |
| Trp | 0.22 | 0.21 |
| Thr | 0.70 | 0.61 |
| Arg | 1.10 | 0.61 |
The premixes provided per kilogram of diet:VA 4,000 IU,VD3 1 500 IU,VE 15 mg,VK3 2 mg,VB1 1.5 mg,VB2 8 mg,VB6 2.5 mg,VB12 0.02 mg,Choline chloride 1,000 mg,Calcium Pantothenate 10 mg,Folic acid 1 mg,Nicotinic acid 50 mg,Biotin 0.2 mg,Fe 60 mg,Cu 8 mg,Mn 47 mg,Zn 60 mg,I 0.2 mg,Se 0.15 mg, β-glucanase 0.15 g (15,000 IU), and β-xylanase, 0.15 g (15,000 IU).
Experimental procedures were approved by the Zhong-kai University of Agriculture and Engineering (ZHKU, China) Animal Care and Ethics Committee (NO.2020081011) in compliance with the Ministry of Agriculture and Food requirements for the Care and Use of Animals for Scientific Purposes.
Morphology and Histology Analysis
Jejunum samples were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm-thick slides. After deparaffinization and dehydration, the sections were stained with hematoxylin and eosin. The structure of the jejunum was observed using an ML51 MShol microscope (MSho, Guangzhou, China), and analyzed using an MShol Digital Imaging System (MSho). Villi height and crypt depth of the jejunum was measured from at least 10 well-oriented villi. Goblet cells were visually identified and counted per unit area in a 400 × field of view.
Measurement of the Antioxidant Parameters
The total antioxidant capacity (T-AOC) level, the activity of SOD, CAT, and GSH-Px, the ability to inhibit hydroxyl radicals (AIHR), and MDA levels were measured in jejunal tissue homogenate samples and serum samples. AIHR and GSH-Px indexes were measured using commercial detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). T-AOC, SOD, CAT, and MDA indexes were measured using a commercial detection kit (Beyotime, Shanghai, China).
RNA Extraction and qRT-PCR Analysis
Total RNA was extracted and purified from the jejunum using RNAiso Plus (TaKaRa, Tokyo, Japan). Reverse transcription was performed using PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer's instructions. All primers were designed using Primer 5.0 software (Table 2) and synthesized by Sangon Biotech (Shanghai, China) Co., Ltd. Real-time quantitative RT-PCR was performed in a total reaction volume of 20 μL, which included 10 μL of the 2 × SYBR Premix EX Taq master mix (Genstar, Beijing, China), 0.8 μL of each of the forward and reverse primers (10 μM), 1 μL of the cDNA template, and 8.2 μL of sterilized water. The qRT-PCR analysis was performed using the Applied Biosystems Quant Studio 7 Flex Real-Time PCR System (Thermo Fisher, Waltham, MA). Gene expression values were determined using the 2−ΔΔCT method and normalized to the expression levels of β-actin.
Table 2.
Gene primers sequence.
| GenBank No. | Genes | Primer sequence (5’-3’) | Annealing temperature (°C) |
|---|---|---|---|
| XM_038165410.1 | β-actin | F-5’-ATGTCGCCCTGGATTTCG-3’ | 60 |
| R-5’-CACAGGACTCCATACCCAAGAA-3’ | |||
| XM_005021880.5 | HSP10 | F-5’-AGTTCCTTCCCCTGTTTGAT-3’ | 60 |
| R-5’-GCTTGTAGCACTTTCCCTTGA -3’ | |||
| XM_027461761.2 | Hsp60 | F-5’-AGCCAAAGGGCAGAAATG-3’ | 60 |
| R-5’-TACAGCAACAACCTGAAGACC-3’ | |||
| NM_001310775.1 | HSP70 | F-5’-CCC CCA GAT CGA GGT TAC TTT -3’ | 55 |
| R-5’-CTC CCA CCC GAT CTC TGT TG -3’ | |||
| XM_027458158.2 | HSP90 | F-5’-TAT TAC ACC TCC GCA TCT GG -3’ | 55 |
| R-5’-CGG AAG CTC CAA ACC CTC T -3’ | |||
| XM_013108556.4 | CLDN1 | F-5’-TCATGGTATGGCAACAGAGTGG-3’ | 60 |
| R-5’-CGGGTGGGTGGATAGGAAGT-3’ | |||
| XM_038184905.1 | TJP1 | F-5’-ACGCTGGTGAAATCAAGGAAGAA-3’ | 60 |
| R-5’-AGGGACATTCAACAGCGTGGC-3’ | |||
| XM_038168987.1 | OCLN | F-5’-CAGGATGTGGCAGAGGAATACAA-3’ | 60 |
| R-5’-CCTTGTCGTAGTCGCTCACCAT-3’ | |||
| XM_038180256.1 | mucin-2 | F-5’-CAAGTCCTGGGCAGAGAAAG-3’ | 60 |
| R-5’-CTGCAGAACAGAAGCAATCG-3’ | |||
| XM_038181120.1 | SIRT1 | F-5’-GGATGATATGACGCTGTGGC-3’ | 55 |
| R-5’-GAAGTCTACAGCAAGGCGTG-3’ | |||
| XM_005031663.5 | PGC-1α | F-5’-CCAGTACAGCAATGAACCCG-3’ | 55 |
| R-5’-TCTTCTGCCTCCTGAGTTGG-3’ | |||
| XM_038187113.1 | NRF1 | F-5’-AGTATTTGGAGCTGCACCCT-3’ | 55 |
| R-5’-CCTGGGTCATCTTGTCCACT-3’ | |||
| XM_038181334.1 | TFAM | F-5’-GAGTTGCCAGCATCACAGA-3’ | 55 |
| R-5’-TCCCAGGACTTCATTTCGTT-3’ | |||
| XM_027460923.2 | NRF2 | F-5’-GCTGGAGTTAGACGAGGAG-3’ | 60 |
| R-5’-AGGGCTTGTGATTGTGCT-3’ | |||
| XM_005015345.5 | HO-1 | F-5’-ATGCCTACACTCGCTATCTG-3’ | 60 |
| R-5’-GCAAGGTCCATCTCAAGG-3’ | |||
| XM_038166869.1 | IL-1β | F-5’-TCATCTTCTACCGCCTGGAC-3’ | 60 |
| R-5’-GTAGGTGGCGATGTTGACCT -3’ | |||
| XM_005027491.5 | TNF-α | F-5’-ACCCCGTTACAGTTCAGACG-3’ | 60 |
| R-5’-CTGGTTACAGGAAGGGCAAC-3’ |
Protein Extraction and Nuclear Protein Extraction
Total protein from the jejunum was extracted using RIPA cell lysis buffer (Beyotime, Beijing, China). Nuclear proteins were extracted using a Minute Cytosolic and Nuclear Extraction Kit for Frozen/Fresh Tissues (Invent Biotechnologies, Beijing, China). The protein concentration was estimated using BCA Protein Assay Kit (Beyotime). Samples were mixed with SDS-PAGE Sample Loading Buffer 6X (Beyotime), and incubated for 10 min at 100°C.
Western Blot Analysis
Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with phosphate-buffered saline with Tween 20 (PBST) containing 5% fat-free milk, PVDF membranes were coincubated with the antibodies anti-NRF1(1: 1,000, Proteintech, Wuhan, China); anti-NRF2(1: 1,000, Proteintech); anti-PCNA(1: 1,000, Proteintech); anti-β-actin (1: 1,000, Sigma-Aldrich, St. Louis, MO), anti-IκBα (1: 1,000, Proteintech, China), anti-P50 (1: 1,000, Proteintech), anti-NLRP3 (1: 1,000, Wanleibio, Shenyang, China), anti-ASC (1: 1,000, Wanleibio), anti-pro-Caspase1(1: 1,000, Wanleibio); anti-Caspase1-p20 (1: 1,000, Wanleibio) at 4°C overnight. Subsequently, PVDF membranes were incubated with the corresponding secondary antibody at 37°C for 1 h. Proteins were detected using the ECL kit (Beyotime, Beijing), and visualized using a Tanon-5200Multi (Tanon, Shanghai, China) device. Densitometry analysis was performed using the Image J software (National Institutes of Health, Bethesda, MD).
Determination of Cytokines
The levels of IL-1β and TNF-α in jejunal tissue homogenate samples were determined using commercial detection kits (CUSABIO, Wuhan, China).
Statistical Analysis
GraphPad Prism 7.1 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. Multiple comparison analysis was performed using a 2-way ANOVA followed by Tukey's post hoc correction for multiple comparisons. All experimental data were analyzed using the means ± standard deviation (S.E.M.). Specific symbols (*) indicate statistically significant differences for *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
Resveratrol Maintains the Morphology of Jejunum
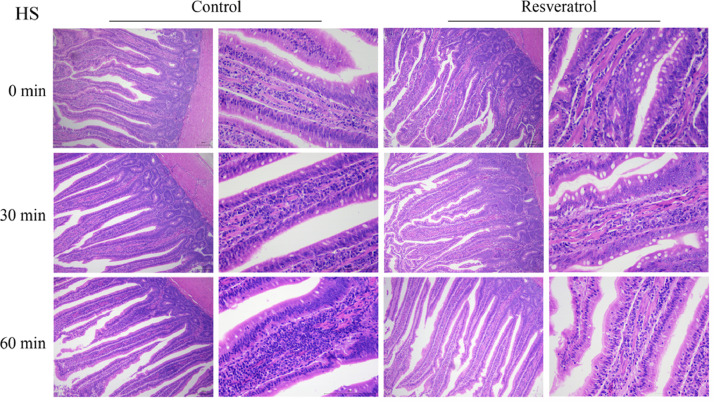
To evaluate the influence of resveratrol on heat stress-exposed jejunum histopathology, the jejunum of the ducks was dissected for morphological analyses. We found that jejunum villus vessels were congested and bleeding under heat stress conditions, and there was diffuse lymphocyte infiltration. Dietary supplementation of resveratrol effectively alleviated jejunal mucosal damage caused by acute heat stress (Figure 1). In addition, the severity of the lesion was determined by computing the histopathological scores. The crypt depth of jejunum in the heat stress group was increased (P < 0.05), and the ratio of villus height to crypt depth was decreased (P < 0.05) compared to those in the control group. The number of goblet cells in the jejunum was significantly increased with dietary supplementation of 400 mg/kg resveratrol, as well as the ratio of villus height to crypt depth (P < 0.05). Though there was no significant difference in villus length (Table 3).
Figure 1.
The representative figure of jejunal morphologies (H & E Staining, × 100, × 400) jejunum.
Table 3.
Structure indexes of jejunum morphologies.
| Index | Control | HS 30 min | HS 60 min | Resveratrol (400 mg/kg) |
||
|---|---|---|---|---|---|---|
| Control | HS 30 min | HS 60 min | ||||
| Villus height (μm) | 859.43 ± 22.25 | 896.80 ± 28.68 | 898.59 ± 19.21 | 877.38 ± 16.83 | 883.88 ± 24.34 | 889.77 ± 34.31 |
| Crypt depth (μm) | 173.57 ± 6.02Ab | 199.30 ± 8.67b | 200.19 ± 6.01Ba | 127.53 ± 5.54Bc | 202.59 ± 6.78b | 228.16 ± 10.62Aa |
| Villus height/Crypt depth | 4.99 ± 0.07B | 4.51 ± 0.09 | 4.49 ± 0.05A | 6.96 ± 0.15A | 4.35 ± 0.04 | 3.90 ± 0.06B |
| Goblet cell | 42.95 ± 2.04B | 39.30 ± 1.32B | 40.70 ± 1.42B | 53.95 ± 1.91A | 51.15 ± 1.70A | 57.65 ± 1.94A |
Note: Different lowercase letters (a, b) indicate significant difference in different heat stress time (P < 0.05), and different capital letters (A, B) indicate that there is significant difference between the control group and resveratrol group during the same heat stress time (P < 0.05), n = 3.
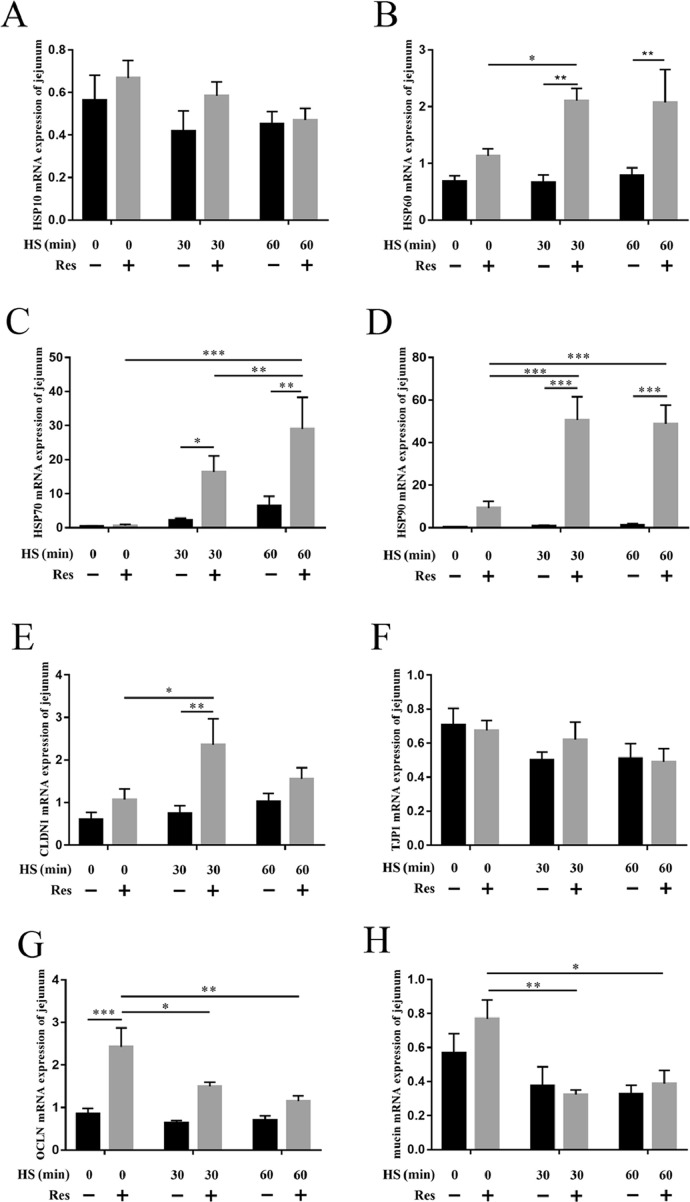
Resveratrol Enhanced Heat Shock Protein and Intestinal Barrier-Related Gene mRNA Expression
The mRNA levels of heat shock protein (HSP) 70 and 90 were increased after heat stress exposure, while HSP 10 and 60 mRNA expression levels did not present significant differences compared with the control group. In contrast, the mRNA levels of HSP60, 70, and 90 in the jejunum were significantly increased in the resveratrol group when subjected to heat stress compared to those in the control group. Resveratrol also promoted HSP10 expression, although not significant (Figures 2A–2D). Results indicated resveratrol enhanced levels of HSPs expression upon heat stress exposure. In addition, the mRNA expression levels of tight junction proteins were examined, which are essential for the integrity of the intestinal barrier. Tight junction protein 1 (TJP1) and mucin mRNA expression levels were downregulated during heat stress. Supplement resveratrol promoted the expression of claudin 1 (CLDN1), especially at 30 min heat stress (P < 0.01), compared with the control group. Furthermore, the expression levels of occludin (OCLN) were also increased by resveratrol. Although it was downregulated during heat stress, it was still higher than the control group (Figures 2E–2H).
Figure 2.
The mRNA expression of HSPs and tight junction protein in the jejunum. mRNA expression of (A) HSP10, (B) HSP60, (C) HSP70, (D) HSP90, (E) CLDN1, (F) TJP1, (G) OCLN, and (H) mucin. Data are presented as means ± SEM, n = 5. Difference analysis was performed using two-way ANOVA followed by Tukey's multiple comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001. Abbreviations: HSP, heat shock protein.
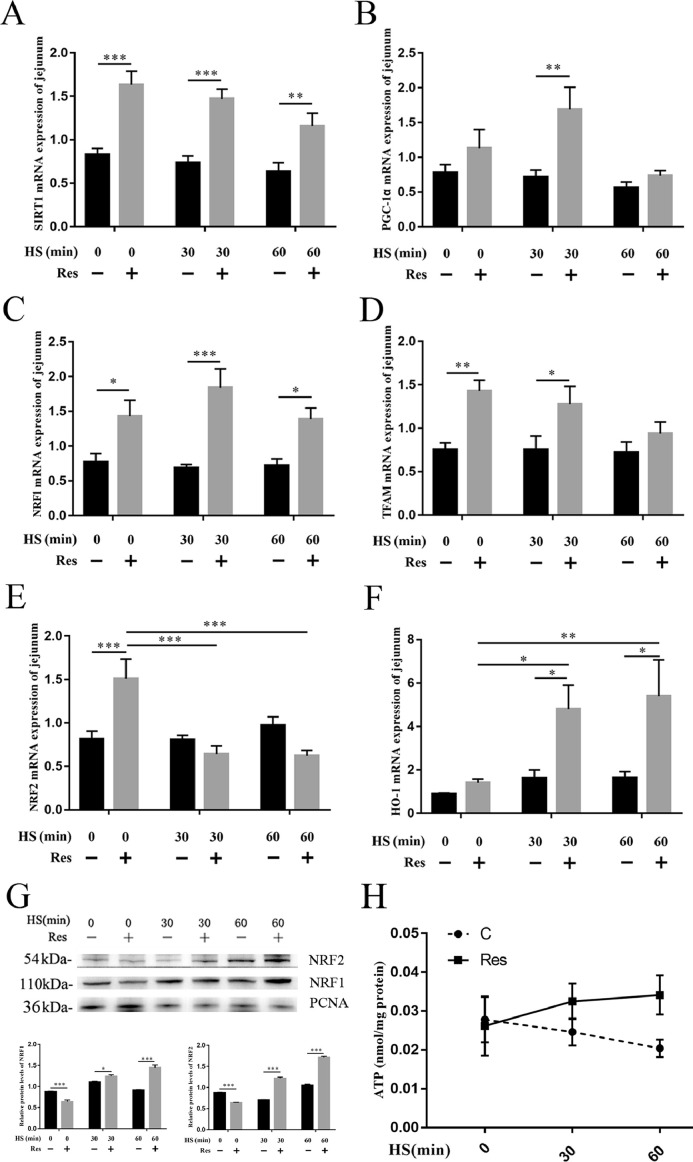
Resveratrol Activates the SIRT1/PGC-1α Signaling Pathway
Resveratrol is one of the strongest activators of SIRT1. To determine whether resveratrol is effective in ducks, the activation of the SIRT1/PGC-1α signaling pathway was detected, and the level of NRF1, NRF2 in the nucleus was investigated. Compared with that of the control group, the mRNA expression level of SIRT1, PGC-1α, NRF1, and TFAM in the jejunum of the resveratrol group was significantly increased (Figures 3A–3D). And, the protein NRF1 in the nucleus was increased in the resveratrol group on exposure to heat stress. The ATP level in the jejunum of the control group was reduced upon exposure to heat stress. While the ATP level of the resveratrol group was significantly increased upon heat stress exposure (Figure 3G). In addition, the mRNA expression of NRF2 was increased in the resveratrol group at normal temperature, but it was significantly downregulated after heat stress. Interestingly, the protein NRF2 in the nucleus was significantly increased in the resveratrol group under heat stress, and the downstream molecule heme oxygenase-1 (HO-1) mRNA expression in the resveratrol group was significantly higher than that in the control group after heat stress (Figures 3E–3F).
Figure 3.
Effect of resveratrol on SIRT1 signaling pathway of jejunum. The mRNA expression levels of (A) SIRT1, (B) PGC-1α, (C) NRF1, (D) TFAM, (E) NRF2, and (F) HO-1; (G) the protein expression levels of NRF1 and NRF2; (H) the levels of ATP in the jejunum. Data are presented as means ± SEM, n = 5. Difference analysis was performed using two-way ANOVA followed by Tukey's multiple comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001.
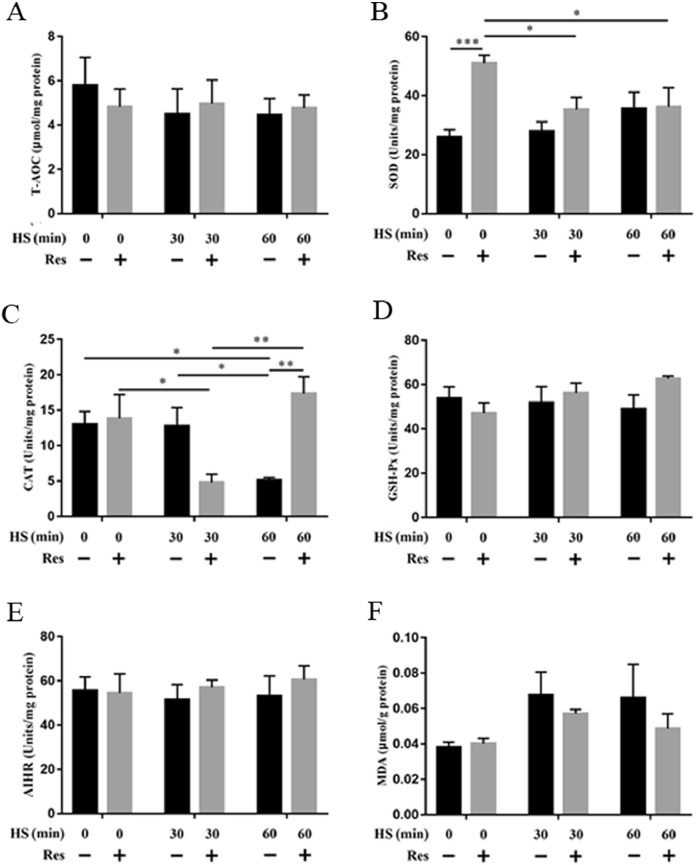
Resveratrol Elevates the Antioxidant Capacity of Ducks
The resveratrol group presented significantly higher serum antioxidant activity than the control group (Figures S1A–F). The T-AOC level of the resveratrol group was significantly increased than the control group after 30 min of heat stress. Serum SOD activity was also significantly increased in the resveratrol group after heat stress, whereas it was downregulated in the control group during heat stress. Furthermore, serum CAT activity was significantly decreased during heat stress, especially at 30 min heat stress, while rapidly normalized in the resveratrol group. Finally, the serum levels of MDA were significantly decreased in the resveratrol group. These results show that resveratrol enhances duck serum antioxidant capacity and decreases the levels of MDA. Meanwhile, the activity of several antioxidant enzymes in the jejunum was determined (Figures 4A–4F). SOD activity was significantly increased in the resveratrol group in comparison with the control group, although they were both downregulated during heat stress. CAT activity in the jejunum was significantly decreased during heat stress in the control group, while rapidly normalized in the resveratrol group. Furthermore, same to the result of serum antioxidant activity, there were no significant changes in the AIHR levels and GSH-Px activity between the control and resveratrol groups. The content of MDA in the resveratrol group was decreased during heat stress compared to that in the control group.
Figure 4.
Effect of resveratrol on antioxidative enzymes activities of jejunum. (A) The T-AOC level in jejunum tissues. (B) The SOD activity in jejunum tissues. (C) The CAT activity in jejunum tissues. (D) The GSH-Px activity in jejunum tissues. (E) The AIHR activity in jejunum tissues. (F) The MDA content in jejunum tissues. Data are presented as means ± SEM, n = 5. Difference analysis was performed using two-way ANOVA followed by Tukey's multiple comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001. Abbreviations: AIHR, ability to inhibit hydroxyl radicals; T-AOC, total antioxidant capacity.
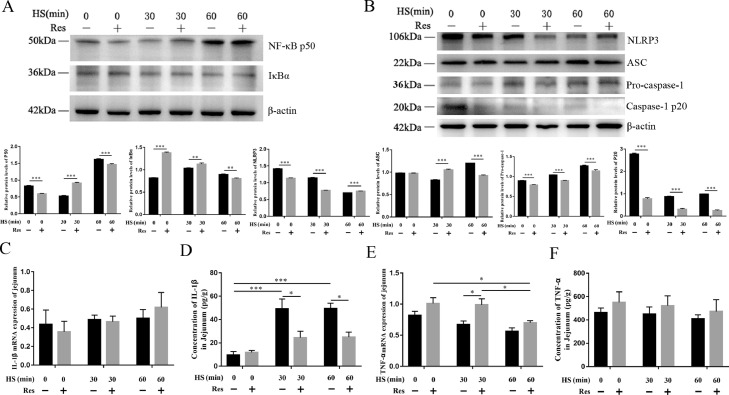
Resveratrol Alleviates Jejunal Inflammation by Inhibiting the NLRP3 Inflammasome Pathway
To investigate the effect of resveratrol on inflammation of jejunum during acute heat stress, the levels of proteins associated with the NF-κB/ NLRP3 inflammasome pathways were assessed by immunoblotting. NF-κB P50 protein level was increased during heat stress both in the control and resveratrol group, whereas the protein levels of IκBα were higher in the resveratrol group than those in the control group. In the control group, heat stress did not result in any significant change in the protein levels of IκBα. Meanwhile, in the resveratrol group, IκBα protein levels were downregulated, and the expression levels after 60 min of heat stress were close to the control group (Figure 5A). Interestingly, the protein levels of NLRP3 and caspase1 p20 were significantly reduced during heat stress in both the control and resveratrol groups. Furthermore, the protein levels of NLRP3 and caspase1 p20 were significantly downregulated in the resveratrol group compared to those in the control group (Figure 5B). In addition, the mRNA and protein levels of IL-1β and TNF-α in the jejunum were determined (Figures 5C–5F). The mRNA expression levels of IL-1β were not significantly different between the control and the resveratrol group, while the protein concentrations of IL-1β were significantly lower in the resveratrol group compared with that in the control group. In contrast, the basal mRNA and protein levels of TNF-α in the resveratrol group were higher than those in the control group, while no significant change was observed in protein level after heat stress exposure.
Figure 5.
Effect of resveratrol on NF-κB and NLRP3 signaling pathway and inflammation of jejunum. (A) Protein expression of NF-κB P50 and IκBα. (B) Protein expression of NLRP3, ASC, pro-caspase-1, and Caspase-1 p20. (C, D) mRNA expression and concentration of IL-1β. (E, F) mRNA expression and concentration of TNF-α. Data are presented as means ± SEM, n = 3. Difference analysis was performed using two-way ANOVA followed by Tukey's multiple comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001.
DISCUSSION
Heat stress is one of the most important factors that affect the production performance of poultry (Tang et al., 2021). Till now, no effective drugs are available to treat heat stress in poultry. Resveratrol is a natural compound that has strong antioxidant and anti-inflammatory effects, thus standing as a potential dietary supplement against heat stress-induced damage. Although the beneficial effects of resveratrol have been widely proven, resveratrol has a low bioavailability, which limits its applicability (Walle, 2011). This study aims to characterize the effects of resveratrol on duck exposed to acute heat stress, focusing on the activation of SIRT1, NF-κB, and NLRP3 signaling pathways, and antioxidant and anti-inflammatory capacity in the jejunum.
The villi length and crypt depth ratio (V/C) reflects the digestion and absorption capacity of the intestine (Wickramasuriya et al., 2019). In this study, the V/C of jejunum, and the number of goblet cells in jejunal mucosa were increased by resveratrol, which alleviated intestinal villus congestion and lymphocyte infiltration, effectively protected the intestinal of duck exposed during acute heat stress. Moreover, results indicated that resveratrol effectively increased the mRNA expression of HSP60, 70, and 90 in the jejunum. Heat shock proteins function as molecular chaperones and play an important role in stress tolerance (Driedonks et al., 2015). HSP60 binds to unfolded protein molecules, promotes protein folding, and consequently prevents protein aggregation (Hasan et al., 2020). HSP70 is related to protein homeostasis and the production of anti-inflammatory cytokines, while HSP90 is involved in promoting the maturation of several receptors and kinases (Zininga et al., 2018). A previous study indicated that heat stress significantly reduced the mRNA expression of tight junction proteins, and the permeability of tight junctions between cells was increased with heat stress (Cheng et al., 2019). The tight junctions between cells are composed of different molecules, including CLDN1, TJP1, OCLN (Suzuki, 2020). In our study, we found that the mRNA expression of TJP1 and mucin in the jejunum was inhibited during acute heat stress. The mRNA expression of CLDN1 and OCLN was effectively increasing with the supplement of resveratrol. The effect of resveratrol contributed to maintaining the gut barrier, possibly by initiating heme-oxygenase-1 (HO-1) dependent signaling, which is a downstream molecule of the SIRT1 signaling pathway (Wang et al., 2016).
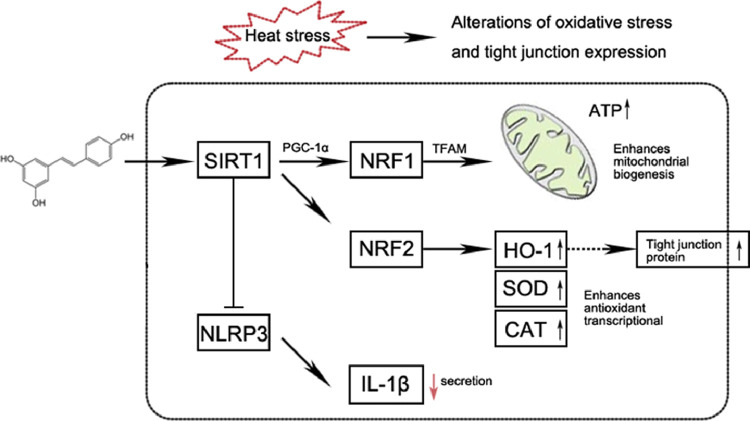
Resveratrol is a natural chemical activator of SIRT1, and it can directly or indirectly affect the redox reaction function of cells (Hwang et al., 2013). The activation of SIRT1 promotes NRF2 nuclear translocation, mediates the transcription of antioxidant enzymes, including HO-1, SOD, CAT, and enhances the antioxidant capacity (Kim et al., 2018). MDA, as the final production of lipid peroxidation, is typically taken as an important indicator for oxidative stress (Khaliq et al., 2018). And, the T-AOC capacity was considered as a measure of antioxidant defense system functions, which are recently used to monitor the development and extent of damage due to oxidative (Song et al., 2020). Thus, the levels of the MDA and T-AOC and activates of these antioxidants were evaluated. In our study, although the serum T-AOC was increased with acute heat stress, SOD and CAT activity were decreased, and MDA levels were increased, suggesting that the balance between the production and removal of hydrogen peroxide in the body is disturbed during acute heat stress. SOD and CAT antioxidant enzymes activities were increased with resveratrol supplement, that enhanced antioxidant capacity of jejunum and serum. In addition, a study in chicken confirmed that resveratrol supplements can rescue Cd-induced excessive mitophagy and increase SIRT1, PGC-1α, NRF1, and TFAM mRNA transcription (Zhang et al., 2020). PGC-1α was engaged in the transcriptional control of NRF1 and TFAM (Santos et al., 2011). And TFAM, which is a principal mitochondrial gene-regulator, is essential for mitochondrial DNA maintenance along with mitochondrial dynamics, contributing to ROS scavenging and cell survival (Kang et al., 2007). As an effector of nucleo-mitochondrial interactions, the NRF1 was also stated to be eminently pertinent to the biogenesis of mitochondria (Hickson-Bick et al., 2008). More importantly, it is thought that mitochondrial DNA contributes to mitochondrial content and ATP production by encoding essential components of oxidative phosphorylation (Kunkel et al., 2016). A previous study was indicated that heat stress damages the mitochondria of muscular cells and induced excessive ROS in chickens (Huang et al., 2015). In our study, resveratrol increases the mRNA expression of PGC-1α, NRF1, TFAM, NRF2, and HO-1, and promoted the nucleus translocation of NRF-1, NRF-2, enhances mitochondrial biogenesis and the antioxidant transcriptional response to acute heat stress (Figure 6).
Figure 6.
Model of the effect of resveratrol on duck intestine under acute heat stress.
In the inflammatory response, we found protein expression of P50 was increased under acute heat stress, but the expression of IκBα was not altered. And, the expression level of IκBα was higher in the resveratrol group compared with that in the control group. This indicates that the NF-κB signaling pathway may not have been activated upon exposure to acute heat stress. In line with our results, a previous study has indicated that heat shock inhibits NF-κB activation in a dose and time-dependent manner (Schell et al., 2005). Interestingly, although the mRNA expression level of IL-1β did not change significantly, the secretion of IL-1β was significantly downregulated in the resveratrol group. A study in the NLRP3 signaling pathway indicated although the caspase-1 p20 was reduced during acute heat stress, the protein level of caspase-1 p20 was significantly downregulated in the resveratrol group compared to the control group. Recently, a study demonstrated that heat stress can reduce NLRP3 activation and decrease the expression of IL-1β and IL-18 in vivo, And the decreased activation of NLRP3 may be related to the increase of HSP70 (Martine et al., 2019). In other studies, resveratrol was also found to protect ischemia-induced cell damage by inhibiting NLRP3 activation in vitro (Feng et al., 2020), and RNA interference of SIRT1 expression or administration of the SIRT1 inhibitor sirtinol prevented the inhibition of resveratrol on NLRP3 (He et al., 2017; Zou et al., 2018).
In conclusion, acute heat stress resulted in a negative impact on the morphological alterations of duck jejunum, destroyed intestinal tight junctions, and caused intestinal oxidative stress. Dietary supplementation of 400 mg/kg resveratrol effectively increased the V/C ratio of jejunum and significantly increased the number of goblet cells in jejunum mucosa. Resveratrol activated the SIRT1 signaling pathway, improved the activity of antioxidant enzymes, increased HSPs and tight junction protein mRNA expression upon exposure to acute heat stresses. Finally, we found acute heat stress repressed NF-κB/NLRP3 inflammasome signaling pathway. And, supplement resveratrol further inhibited the NLRP3 inflammasome pathway decreased the secretion of IL-1β.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the National Key Technologies R & D Program of China (2018YFE0128200), Natural Science Foundation of Guangdong Province (2018A030313630, 2020A1515110451), Guangzhou Science and Technology Plan (201804010412), Modern Agriculture Waterfowl Industry Technology System Innovation Team of Guangdong (2020KJ137)
Author contributions: CY, WL, and YH contributed to the hypothesis generation, experimental design, data interpretation, and manuscript preparation. PL, SC, and ZD conducted the experiments. XF, DX, and YT contributed to the data interpretation
Data availability statement: All data generated or analyzed in this study are included in this published article.
DISCLOSURES
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101459.
Appendix. Supplementary materials
Figure S1. Effect of resveratrol on antioxidative enzymes activities of serum. (A) The T-AOC level of serum. (B)The SOD activity of serum. (C) The CAT activity of serum. (D) The GSH-Px activity of serum. (E) The AIHR activity of serum. (F) The MDA content of serum. Data are presented as means ± SEM, n = 5. Difference analysis was performed using Two-way ANOVA followed by Tukey's multiple comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001.
REFERENCES
- Cheng Y.F., Chen Y.P., Chen R., Su Y., Zhang R.Q., He Q.F., Wang K., Wen C., Zhou Y.M. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019;98:4767–4776. doi: 10.3382/ps/pez192. [DOI] [PubMed] [Google Scholar]
- Driedonks N., Xu J., Peters J.L., Park S., Rieu I. Multi-level interactions between heat shock factors, heat shock proteins, and the Redox system regulate acclimation to heat. Front. Plant. Sci. 2015;6:999. doi: 10.3389/fpls.2015.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H., Mou S.Q., Li W.J., Zhang N., Zhou Z.Y., Ding W., Bian Z.Y., Liao H.H. Resveratrol inhibits ischemia-induced myocardial senescence signals and NLRP3 inflammasome activation. Oxid. Med. Cell Longev. 2020;2020 doi: 10.1155/2020/2647807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Li S., Lv Y., Yang D., Li J., Yang Q., Wu P., Lv Z., Zhang Z. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019;1:5555–5565. doi: 10.1039/c9fo01152h. [DOI] [PubMed] [Google Scholar]
- Hasan S.S., Kang D., Park J., Choi H.W., Shim K. Acute heat stress induces the differential expression of heat shock proteins in different sections of the small intestine of chickens based on exposure duration. Animals (Basel) 2020;10:1234. doi: 10.3390/ani10071234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson-Bick D.L., Jones C., Buja L.M. Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J. Mol. Cell Cardiol. 2008;44:411–418. doi: 10.1016/j.yjmcc.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Hwang J.W., Yao H., Caito S., Sundar I.K., Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D., Kim S.H., Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Khaliq H., Jing W., Ke X., Ke-Li Y., Peng-peng S., Cui L., Wei-wei Q., Zhixin L., Hua-Zhen L., Hui S., Ju-Ming Z., Ke-Mei P. Boron affects the development of the kidney through modulation of apoptosis, antioxidant capacity, and Nrf2 pathway in the African ostrich chicks. Biol. Trace Elem. Res. 2018;186:226–237. doi: 10.1007/s12011-018-1280-7. [DOI] [PubMed] [Google Scholar]
- Kim E.N., Lim J.H., Kim M.Y., Ban T.H., Jang I.A., Yoon H.E., Park C.W., Chang Y.S., Choi B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging (Albany NY) 2018;10:83–99. doi: 10.18632/aging.101361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Thom U., Albrecht E., Weikard R., Nolte W., Kuhla B., Kuehn C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. 2019;116:10333–10338. doi: 10.1073/pnas.1820130116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel G.H., Chaturvedi P., Tyagi S.C. Mitochondrial pathways to cardiac recovery: TFAM. Heart Fail. Rev. 2016;21:499–517. doi: 10.1007/s10741-016-9561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Liu L., Fu C., Yan M., Xie H., Li S., Yu Q., He S., He J. Resveratrol modulates intestinal morphology and HSP70/90, NF-kappaB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016;7:1329–1338. doi: 10.1039/c5fo01338k. [DOI] [PubMed] [Google Scholar]
- Ma X., Lin Y., Zhang H., Chen W., Wang S., Ruan D., Jiang Z. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reprod. Sci. 2014;145:182–190. doi: 10.1016/j.anireprosci.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Martine P., Chevriaux A., Derangère V., Apetoh L., Garrido C., Ghiringhelli F., Rébé C. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death. Dis. 2019;10:256. doi: 10.1038/s41419-019-1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T., Xiao D., Muhammed A., Deng J., Chen L., He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules. 2021;26:229. doi: 10.3390/molecules26010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanto-Hara F., Kikusato M., Ohwada S., Toyomizu M. Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 2020;57:284–290. doi: 10.2141/jpsa.0190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roushdy E.M., Zaglool A.W., Hassan F. Thermal stress consequences on growth performance, immunological response, antioxidant status, and profitability of finishing broilers: transcriptomic profile change of stress-related genes. Trop. Anim. Health Prod. 2020;52:3685–3696. doi: 10.1007/s11250-020-02405-4. [DOI] [PubMed] [Google Scholar]
- Santos J.M., Tewari S., Goldberg A.F.X., Kowluru R.A. Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radic. Biol. Med. 2011;51:1849–1860. doi: 10.1016/j.freeradbiomed.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M.T., Spitzer A.L., Johnson J.A., Lee D., Harris H.W. Heat shock inhibits NF-kB activation in a dose- and time-dependent manner. J. Surg. Res. 2005;129:90–93. doi: 10.1016/j.jss.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Song X., Zhang J., Li J., Jia L. Acetylated polysaccharides from pleurotus geesteranus alleviate lung injury via regulating NF-κB signal pathway. Int. J. Mol. Sci. 2020;21:2810. doi: 10.3390/ijms21082810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim. Sci. J. 2020;91:e13357. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.P., Li W.H., Liu Y.L., Lun J.C., He Y.M. Heat stress aggravates intestinal inflammation through TLR4-NF-kappaB signaling pathway in Ma chickens infected with Escherichia coli O157:H7. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann. NY Acad. Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wang N., Han Q., Wang G., Ma W.P., Wang J., Wu W.X., Guo Y., Liu L., Jiang X.Y., Xie X.L., Jiang H.Q. Resveratrol protects oxidative stress-induced intestinal epithelial barrier dysfunction by upregulating heme oxygenase-1 expression. Dig. Dis. Sci. 2016;61:2522–2534. doi: 10.1007/s10620-016-4184-4. [DOI] [PubMed] [Google Scholar]
- Wickramasuriya S.S., Kim E., Cho H.M., Shin T.K., Kim B., Lee M., Seo S., Heo J.M., Choi H. Differential effects of dietary methionine isomers on broilers challenged with acute heat stress. J. Poult. Sci. 2019;56:195–203. doi: 10.2141/jpsa.0180072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Fang C., Ma Y., He S., Ajuwon K.M., He J. Dietary resveratrol supplement improves carcass traits and meat quality of Pekin ducks. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T., Li J.J., Wang D.Q., Li G.Q., Wang G.L., Lu L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperones. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang C., Ge J., Lv M.W., Talukder M., Guo K., Li Y.H., Li J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020;11:1856–1868. doi: 10.1039/c9fo02287b. [DOI] [PubMed] [Google Scholar]
- Zininga T., Ramatsui L., Shonhai A. Heat shock proteins as immunomodulants. Molecules. 2018;23:2846. doi: 10.3390/molecules23112846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P., Liu X., Li G., Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018;17:3212–3217. doi: 10.3892/mmr.2017.8241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of resveratrol on antioxidative enzymes activities of serum. (A) The T-AOC level of serum. (B)The SOD activity of serum. (C) The CAT activity of serum. (D) The GSH-Px activity of serum. (E) The AIHR activity of serum. (F) The MDA content of serum. Data are presented as means ± SEM, n = 5. Difference analysis was performed using Two-way ANOVA followed by Tukey's multiple comparisons. *P < 0.05, **P < 0.01 and ***P < 0.001.