Abstract
Purpose
The aim of this study was to investigate the effect of androgen deprivation therapy (ADT) on the health-related quality of life (HRQOL) of patients with prostate cancer (PC) and compare the changes in the HRQOL between ADT alone and ADT plus intensity-modulated radiation therapy (IMRT).
Materials and methods
Patients with PC were prospectively recruited between October 2018 and April 2020. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire and the PC-specific module (PR25) were administered before ADT (baseline) and at 3, 6, and 12 months after ADT. All patients received subcutaneous injections of 45 mg leuprolide acetate at 6-month intervals for 12 months.
Results
Fifty-five of the 71 patients (77.5%) completed the 12-month study. Twenty-two of the 55 patients received IMRT. There were no differences in the baseline characteristics with respect to IMRT. Compared with baseline, physical function and role function deteriorated after 3 months (p = 0.003, p = 0.019). However, the global quality of life (QOL) did not change over time. The symptom scales of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire indicated that there was a statistically significant deterioration in dyspnea and fatigue symptoms at 12 months (p = 0.004, p = 0.004). Responses to the QLQ-PR25 revealed that patients experienced an increase in hormonal treatment-related symptoms after 3, 6, and 12 months (p = 0.002, 0.001, and 0.004). Comparisons between the ADT group and ADT plus IMRT group showed that body function and role function did not differ between the two groups (p = 0.815, p = 0.759), and there was also no difference in global QOL (p = 0.624).
Conclusion
Our results indicate that treatment with leuprolide acetate at 6-month intervals was not accompanied by changes in global QOL, despite deterioration of body and role functions and hormonal treatment-related symptoms. The combination of ADT and IMRT did not lead to additional deterioration in the HRQOL.
Keywords: Androgen deprivation therapy, High-risk prostate cancer, Quality of life, Radiotherapy
1. Introduction
Although the prognosis and mortality rates associated with the treatment methods for prostate cancer (PC) are well documented, there is insufficient information regarding the effects of the different treatment strategies on the PC-specific quality of life (QOL) outcomes.1, 2, 3 The QOL of patients with PC is an important measure of the essential outcomes of treatment and provides appropriate information regarding their ability to perform activities of daily life after treatment.4 As patients with PC often have prolonged life expectancy, the QOL outcome of different treatment strategies is a major concern for male patients when selecting the appropriate treatment for themselves. Studies have reported that 16–19% of patients with local PC regret their choice of treatment.5,6 Some patients with local PC also experience clinical or biochemical failure after surgical or radiation therapy (RT).7 Therefore, a good understanding of the risks and benefits associated with the different treatment strategies may aid in reducing regret experienced by the patients regarding their treatment choice. Becuase surgical treatment is one of the treatment options, patients should be provided with sufficient information regarding the incidence of and various types of morbidities associated with the treatment and the possible effects on the health-related quality of life (HRQOL).8
Androgen deprivation therapy (ADT) serves as the backbone therapy for the treatment of metastatic PC and is also used for biochemical recurrence and as an adjuvant treatment after local treatment.9 Previous studies have reported that the effects of ADT differ among patients of difference races, and the assessment of QOL after ADT also shows racial differences.10,11 However, most of the studies on ADT evaluated the response to treatment and disease progression time as the primary endpoints. It is also important to determine the effectiveness of the various treatment strategies with respect to the HRQOL.
Therefore, the objective of the present study was twofold. The primary objective was to investigate the extent of changes in the QOL after hormonal therapy among patients with PC. The second objective was to compare the extent of changes in the QOL between patients with PC treated with ADT plus RT and those treated with ADT alone.
2. Materials and methods
2.1. Study design and population
This prospective longitudinal cohort study included newly diagnosed patients with PC and was conducted between October 2018 and April 2020 at Chonnam National University Hwasun Hospital. The inclusion criteria were as follows: (1) pathological diagnosis of a high risk or very high risk of PC, advanced-stage PC, or metastatic PC and (2) prescription of ADT alone or ADT with intensity-modulated radiation therapy (IMRT). All patients who already had received hormonal treatment for PC or had a double primary cancer before or after the diagnosis of PC were excluded. Other exclusion criteria were refusal to participate in the study or communication difficulties. All patients received subcutaneous injections of 45 mg leuprolide acetate at 6-month intervals for 12 months and 50 mg of an antiandrogen (bicalutamide) orally daily. Concurrent IMRT was performed from 3 months after the start of neoadjuvant ADT. All patients provided written informed consent and completed a self-administered questionnaire before administration of ADT and at 3, 6, and 12 months. The study protocol was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2018-079), and the study was conducted in accordance with the guidelines of the Declaration of Helsinki.
2.2. Measures of the HRQOL
In our study, HRQOL was the primary outcome. The HRQOL was assessed before treatment and at 3, 6, and 12 months after treatment using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and an accompanying PC-specific module (QLQ-PR25). The Korean version of the EORTC QLQ-C3012,13 and its prostate module (PR25)14,15 were used in this study. The EORTC QLQ-C30 is a 30-item scoring scale for global QOL, while the PR25 comprises 25 questions in 6 domains. The PR25 was designed to evaluate QOL associated with PC. All 55 questions were regrouped into 21 scales. On all scales, the item scores were summed and converted linearly to scale scores ranging from 1 to 100. A higher score on the functional scale indicates a higher level of function, but a higher score on the symptom scale indicates more severe symptoms. The domain scores for the QLQ-C30 and PR25 modules were calculated according to the scoring manual provided by the EORTC QOL group.12,14 We also collected patient data, including sociodemographic characteristics, such as marital status, education level, and smoking and drinking status. Clinical data, including the tumor, node, metastasis stage, Gleason score, Karnofsky performance status, and initial prostate-specific antigen (PSA) levels, were assessed.
2.3. Statistical analysis
We used descriptive analysis (Student t test and Chi-square test) for the evaluation of the baseline characteristics of the enrolled patients. The mean standard deviation on each scale of the EORTC QLQ-C30 and the PR25 module at each time point was calculated. The results at baseline and at 3, 6, and 12 months were compared, while case-wise deletion was used for any missing data. Repeated-measures analysis of variance with Bonferroni correction was used for comparing the domains of the HRQOL according to the time points. All analyses were conducted using MedCalc Statistical Software, version 19.3.1 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020), with p < 0.05 considered statistically significant. A clinically meaningful difference was defined as a difference of ≥10 points in the EORTC QLQ scores on a scale of 0–100.16
3. Results
3.1. Sample description and compliance
During the study period, 71 patients were recruited, and 55 (77.5%) patients completed the questionnaires at 3, 6, and 12 months. A total of 22 (40%) patients underwent IMRT from the 3-month time point. All patients were administered 45 mg of leuprolide acetate every 6 months for 12 months, and the serum testosterone levels effectively decreased. The patients and treatment characteristics are summarized in Table 1. The mean age of the patients was 76.3 years, and the mean PSA level was 35.7 ng/mL (standard deviation, 45.8). According to the clinical stage, 8 (14.5%), 42 (76.4%), and 5 (9.1%) patients had clinical stage T2, T3, and T4 disease, respectively. The number of patients with metastatic lymphadenopathy and bone metastasis was 6 (10.9%) and 7 (12.7%), respectively. At 12 weeks, 55 (100%) patients achieved serum testosterone levels within the castration range, and the levels were maintained in this range even after 12 months (Supplementary table1). Mortality was not reported during the study period.
Table 1.
Baseline characteristics of patients with prostate cancer (n = 55).
| Variable | Total (n = 55) | ADT (n = 33) | ADT + RT (n = 22) | p-value |
|---|---|---|---|---|
| Age (yr) | 76.3 ± 5.1 | 77.1 ± 4.4 | 75.0 ± 5.8 | 0.131 |
| Height (cm) | 164.8 ± 5.2 | 165.0 ± 4.5 | 164.5 ± 6.2 | 0.674 |
| Weight (kg) | 63.5 ± 8.5 | 63.4 ± 7.4 | 63.6 ± 10.0 | 0.912 |
| Comorbidity, any | 39 (70.9) | 24 (72.7) | 15 (68.2) | 0.718 |
| Living place | 0.273 | |||
| Urban | 25 (45.5) | 13 (39.4) | 12 (54.5) | |
| Rural | 30 (54.5) | 20 (60.6) | 10 (45.5) | |
| Education level | ||||
| Less than high school | 21 (38.2) | 13 (39.4) | 8 (36.4) | 0.563 |
| High school and above | 5 (9.1) | 4 (12.1) | 1 (4.5) | |
| Unknown | 29 (52.7) | 16 (48.5) | 13 (59.1) | |
| Economic activity | ||||
| Active | 15 (27.3) | 7 (21.2) | 8 (36.4) | 0.221 |
| None | 40 (72.7) | 26 (78.8) | 14 (63.6) | |
| Marital status | ||||
| Married | 35 (63.6) | 22 (66.7) | 13 (59.1) | 0.571 |
| Unmarried | 20 (36.4) | 11 (33.3) | 9 (40.9) | |
| Smoking | ||||
| Current | 8 (14.5) | 3 (9.1) | 5 (22.7) | 0.295 |
| Past | 19 (34.5) | 11 (33.3) | 8 (36.4) | |
| None | 28 (50.9) | 19 (57.6) | 9 (40.9) | |
| Drinking | ||||
| Current | 8 (14.5) | 4 (12.1) | 4 (18.2) | 0.394 |
| Past | 29 (52.7) | 18 (54.5) | 11 (50.0) | |
| None | 18 (32.7) | 11 (33.3) | 7 (31.8) | |
| Clinical T stage | ||||
| 2 | 8 (14.5) | 6 (18.2) | 2 (9.1) | 0.356 |
| 3 | 42 (76.4) | 23 (69.7) | 19 (86.4) | |
| 4 | 5 (9.1) | 4 (12.1) | 1 (4.5) | |
| Clinical N stage | ||||
| 0 | 49 (89.1) | 28 (84.8) | 21 (95.5) | 0.221 |
| 1 | 6 (10.9) | 5 (15.2) | 1 (4.5) | |
| Clinical M stage | ||||
| 0 | 48 (87.3) | 27 (81.8) | 21 (95.5) | 0.141 |
| 1b | 7 (12.7) | 6 (18.2) | 1 (4.5) | |
| Biopsy Gleason score | ||||
| 6 | 7 (12.7) | 4 (12.1) | 3 (13.6) | 0.939 |
| 7 | 19 (34.5) | 12 (36.4) | 7 (31.8) | |
| ≥ 8 | 29 (52.7) | 17 (51.5) | 12 (54.5) | |
| Tumor type | ||||
| Localized high risk | 45 (81.8) | 25 (75.8) | 20 (90.9) | 0.157 |
| Mets | 10 (18.2) | 8 (24.2) | 2 (9.1) | |
| PSA (ng/mL) | 35.7 ± 45.8 | 30.8 ± 45.2 | 43.2 ± 46.7 | 0.329 |
| Karnofsky performance status | ||||
| 100 | 50 (90.9) | 28 (84.8) | 22 (100) | 0.159 |
| 90 | 3 (5.5) | 3 (9.1) | 0 (0) | |
| 80 | 2 (3.6) | 2 (6.1) | 0 (0) | |
ADT = androgen deprivation therapy; RT = radiation therapy; PSA = prostate-specific antigen; Mets = Metastasis.
3.2. Longitudinal effects in patients treated with ADT
There was no statistically significant deterioration in the global health status/QOL based on the results of the EORTC QLQ-C30 (p = 1.0; Table 2). Among the functional scales, deterioration was noted in the physical function at 3, 6, and 12 months (p = 0.003, p = 0.001, and p < 0.001, respectively) and in the role function at 3 and 12 months (p = 0.019 and p = 0.0007, respectively; Table 2). The symptom scales of the EORTC QLQ-C30 indicated that fatigue and dyspnea were aggravated at 12 months (Table 2). The responses to the QLQ-PR25 revealed that the patients experienced an increase in hormonal treatment-related symptoms at 3, 6, and 12 months (p = 0.002, p = 0.001, and p = 0.004, respectively; Table 2).
Table 2.
Health-related quality of life of patients with prostate cancer after ADT for 1 year.
| Scale | Baseline |
3 months |
6 months |
12 months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p-value (vs. baseline) | Mean ± SD | p-value (vs. baseline) | p-value (vs. 3 months) | Mean ± SD | p-value (vs. baseline) | p-value (vs. 3 months) | p-value (vs. 6 months) | |
| QLQ-C30 functioning scale | ||||||||||
| Physical function (n = 53) | 85.8 ± 16.4 | 76.2 ± 22.0 | <0.001∗ | 73.5 ± 26.6 | <0.001∗ | 0.346 | 73.2 ± 24.1 | <0.001∗ | 0.210 | 0.554 |
| Role function (n = 53) | 87.7 ± 20.1 | 76.7 ± 24.9 | 0.003∗ | 79.2 ± 27.0 | 0.017 | 0.409 | 73.3 ± 29.0 | <0.001∗ | 0.292 | 0.071 |
| Emotional function (n = 21) | 86.7 ± 17.9 | 90.8 ± 17.1 | 0.131 | 90.8 ± 15.1 | 0.035 | >0.99 | 88.8 ± 15.8 | 0.230 | 0.586 | 0.459 |
| Cognitive function (n = 21) | 83.3 ± 14.3 | 89.6 ± 15.3 | 0.148 | 85.7 ± 15.7 | 0.545 | 0.234 | 82.5 ± 18.6 | 0.815 | 0.047 | 0.358 |
| Social function (n = 21) | 88.9 ± 12.6 | 86.5 ± 20.1 | 0.666 | 90.4 ± 15.7 | 0.724 | 0.286 | 88.8 ± 20.9 | >0.999 | 0.634 | 0.666 |
| Global quality of life (n = 21) | 63.1 ± 20.7 | 66.2 ± 24.5 | 0.559 | 63.4 ± 23.8 | 0.940 | 0.589 | 59.9 ± 23.1 | 0.570 | 0.260 | 0.560 |
| QLQ-C30 symptom scale | ||||||||||
| Fatigue (n = 53) | 19.6 ± 22.0 | 28.1 ± 21.1 | 0.023 | 23.8 ± 18.6 | 0.200 | 0.126 | 29.5 ± 19.0 | <0.001∗ | 0.642 | 0.038 |
| Nausea and vomiting (n = 53) | 1.9 ± 6.5 | 5.6 ± 17.2 | 0.083 | 2.2 ± 6.5 | 0.811 | 0.161 | 5.0 ± 10.5 | 0.058 | 0.766 | 0.038 |
| Pain (n = 52) | 9.3 ± 19.8 | 13.5 ± 19.4 | 0.219 | 9.6 ± 18.8 | 0.616 | 0.350 | 10.3 ± 23.5 | 0.707 | 0.322 | 0.896 |
| Dyspnea (n = 53) | 10.6 ± 19.3 | 16.3 ± 25.6 | 0.095 | 17.6 ± 23.9 | 0.020 | 0.727 | 23.2 ± 29.5 | 0.001∗ | 0.139 | 0.151 |
| Insomnia (n = 53) | 15.7 ± 23.0 | 22.0 ± 34.6 | 0.142 | 16.3 ± 25.6 | 0.880 | 0.220 | 16.3 ± 25.8 | 0.868 | 0.201 | >0.999 |
| Appetite loss (n = 53) | 13.1 ± 26.2 | 13.8 ± 27.8 | 0.878 | 13.8 ± 18.8 | 0.850 | >0.999 | 14.4 ± 21.0 | 0.709 | 0.850 | 0.811 |
| Constipation (n = 52) | 16.6 ± 27.8 | 19.8 ± 33.3 | 0.341 | 21.7 ± 27.6 | 0.172 | 0.582 | 22.4 ± 29.1 | 0.162 | 0.532 | 0.855 |
| Diarrhea (n = 52) | 1.9 ± 7.6 | 8.9 ± 23.1 | 0.033 | 8.3 ± 18.2 | 0.011 | 0.850 | 6.4 ± 18.9 | 0.090 | 0.376 | 0.497 |
| Financial difficulties (n = 21) | 12.7 ± 13.9 | 12.7 ± 26.8 | >0.999 | 9.5 ± 19.8 | 0.428 | 0.576 | 9.5 ± 23.4 | 0.493 | 0.576 | >0.999 |
| QLQ-PR25 scale | ||||||||||
| Urinary symptom (n = 21) | 23.0 ± 14.2 | 22.2 ± 17.1 | 0.836 | 26.8 ± 16.1 | 0.339 | 0.078 | 18.6 ± 14.5 | 0.104 | 0.376 | 0.026 |
| Bowel symptoms (n = 21) | 1.9 ± 4.6 | 2.4 ± 3.8 | 0.715 | 1.2 ± 2.9 | 0.329 | 0.267 | 3.5 ± 6.2 | 0.296 | 0.452 | 0.110 |
| Hormonal treatment-related symptom (n = 52) | 3.6 ± 5.2 | 10.4 ± 11.0 | <0.001∗ | 11.0 ± 10.5 | <0.001 | 0.640 | 9.6 ± 11.7 | <0.001 | 0.563 | 0.301 |
| Sexual activity (n = 51) | 93.1 ± 15.3 | 82.0 ± 30.9 | 0.018 | 90.8 ± 22.1 | 0.109 | 0.092 | 91.5 ± 19.4 | 0.440 | 0.030 | 0.340 |
| Sexual function (conditional) (n = 7) | 83.3 ± 23.7 | 79.7 ± 36.7 | 0.629 | 89.2 ± 18.3 | 0.618 | 0.602 | 80.9 ± 37.3 | 0.703 | 0.788 | 0.643 |
A Bonferroni correction was used to correct for multiple comparisons; because six parameters were studied, P = 0.0083 was considered to be significant. SD = standard deviation.
P < 0.0083, Bonferroni-adjusted P < 0.0083 for multiple paired t-tests.
3.3. Comparison of patients treated with ADT alone with those treated with ADT and IMRT
The number of patients who received ADT monotherapy and ADT and IMRT was 33 (19.6%) and 22 (30.1%), respectively. The mean age of the patients was 77.1 ± 4.4 and 75.0 ± 5.8, respectively, with no difference between the two groups (p = 0.131). There were no differences between the two groups in socioeconomic factors including educational level and residential area and health behavior factors including smoking and drinking. The average PSA levels were 30.8 ± 45.2 and 43.2 ± 46.7, respectively, pathological Gleason scores were mostly 8 or higher, and there was no difference between the two groups (51.5% vs 54.5%, p = 0.939). According to the pathological stage, the patients' stage was mainly T3 (69.7% vs 86.4%, p = 0.356), N0 (84.8% vs 95.5%, p = 0.221), and M0 (81.8% vs 95.5%, p = 0.141). The pathological stage did not differ between the two groups (Table 1).
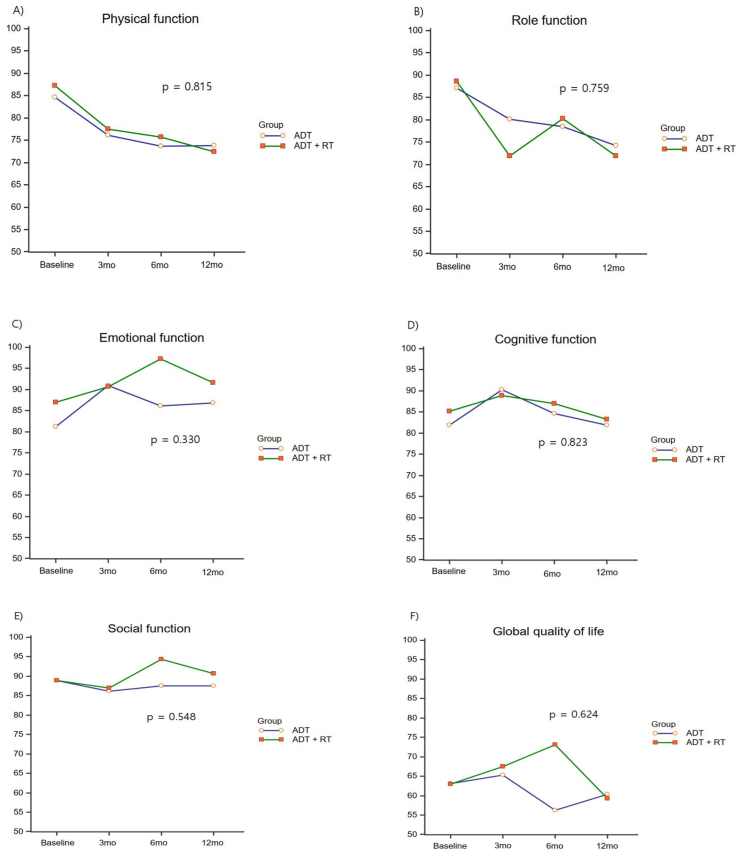
Physical function and role function showed the greatest decrease at 3 months after the start of treatment and continued to decrease afterward, but there were no differences between the two groups (p = 0.815, and p = 0.759, respectively). There were no differences between the two groups in emotional function, cognitive function, and social function (Fig. 1), and there were no significant changes before and after treatment (Table 2).
Fig. 1.
Changes in the QLQ-C30 function scale scores according to the type of ADT. ADT, androgen deprivation therapy.
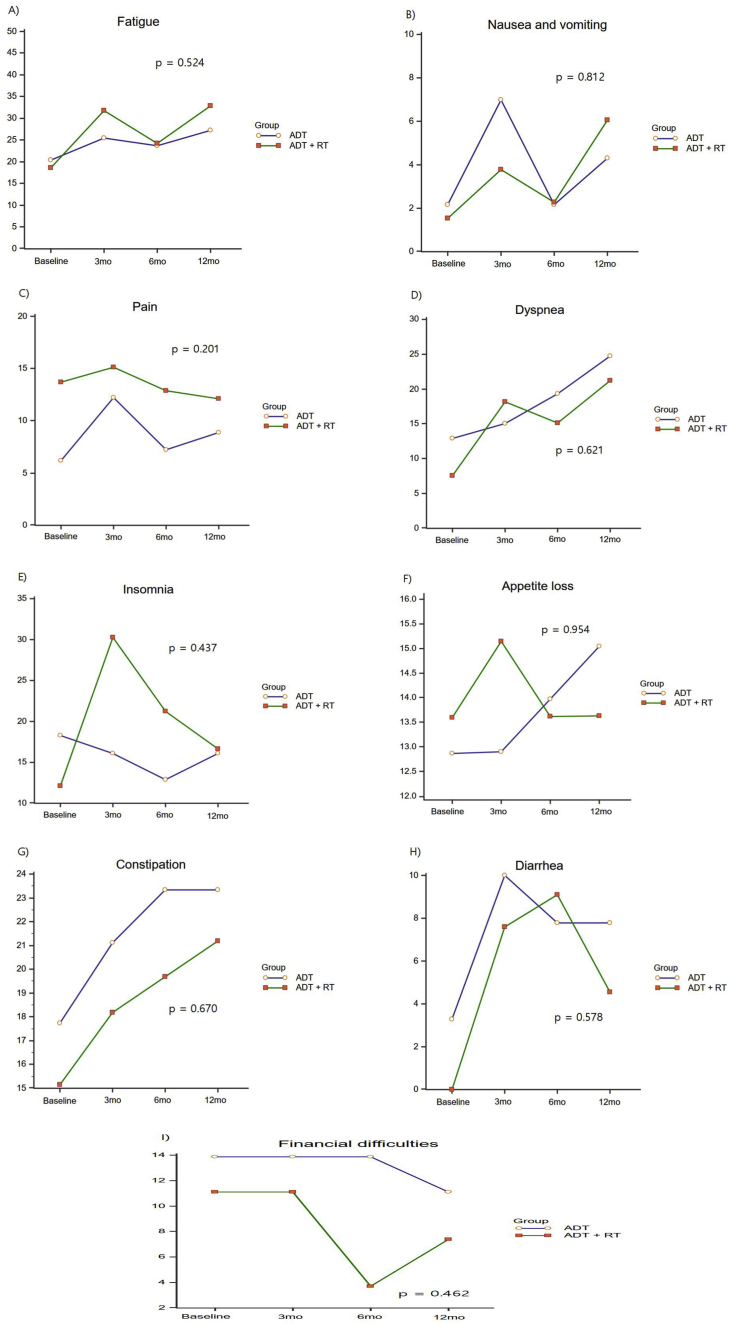
Global QOL did not show any difference between the two groups (p = 0.624; Fig. 1), and there was no significant change before and after treatment (p = 1; Table 2). Fatigue and dyspnea were elevated at 12 months compared with baseline in both groups, but there were no differences between the two groups (p = 0.524 and p = 0.621, respectively; Fig. 2). Nausea, vomiting, pain, decreased appetite, constipation, and diarrhea did not show any clinically significant changes, and there were no differences between the two groups (Fig. 2). Insomnia worsened at 3 months in the ADT plus IMRT group and improved at 6 and 12 months, but there was no difference between the two groups (p = 0.437; Fig. 2).
Fig. 2.
Changes in the QLQ-C30 symptom scale scores according to the type of ADT. ADT, androgen deprivation therapy.
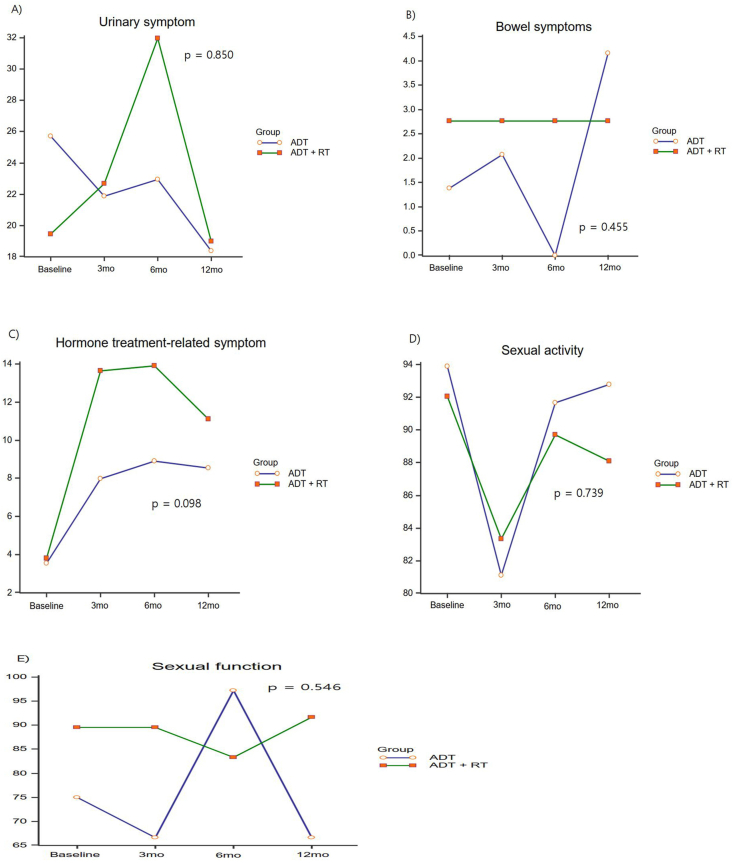
The urinary symptoms worsened at 6 months in the ADT + RT group but recovered to the baseline state at 12 months, and there was no difference between the two groups (p = 0.850; Fig. 3). Hormone therapy-related symptoms worsened significantly at 3 months, but there was no difference between the two groups (p = 0.098; Fig. 3). Sexual behavior significantly worsened at 3 months and improved after 3 months, but there were no differences between the two groups in both sexual activity and sexual function (p = 0.739 and p = 0.546, respectively; Fig. 3).
Fig. 3.
Changes in the QLQ-PR25 module scores according to the type of ADT. ADT, androgen deprivation therapy.
4. Discussion
Owing to the risk–benefit concerns associated with the treatment strategies for PC, the effect of the selected treatment strategy on the HRQOL should be evaluated, and adequate information should be provided to the patients. The main aim of this study was to determine the course of the QOL in patients with PC after administration of ADT with or without RT.
In our study, ADT or ADT with RT did not affect the global QOL, as indicated by the functional scale scores, of all patients; however, significant differences were observed in the physical and role function scores compared with those before treatment.
In a study that objectively evaluated the effect of ADT on the physical function and QOL, the physical component of the QOL after initiation of ADT was affected within 3 months.17 They enrolled 87 patients with ADT, 86 PC controls, and 86 healthy controls and assessed the physical function by conducting physical tests and measured the QOL using the Medical Outcomes Study Short-Form 36 questionnaire. Bola et al.18 conducted a study comparing RT plus ADT for 6 months and 36 months. At 6 months, all patients who received the RT plus ADT combination therapy showed a decrease in the physical and role functions. The long-term ADT group, maintained on androgen suppression for 2.5 years, showed further decrease in the functional levels, whereas the short-term group, which discontinued ADT after 6 months, showed no further decrease.18 Meanwhile, Shin et al.19 conducted a study on the change in the HRQOL according to surgical treatment in patients with PC and reported that surgery had no negative effect on the QLQ-C30 functional scale score over a time period of 1 year, except for the deterioration of the role function. Role function had a temporary decline at 3 months after surgery but returned to baseline at 12 months.
In the present study, the symptom scales of the EORTC QLQ-C30 indicated a statistically significant exacerbation of the dyspnea and fatigue symptoms at 12 months. Fatigue is one of the most common side effects in patients with cancer and has a major effect on the QOL of such patients; patients continue to experience fatigue even after treatment.20 Fatigue in patients receiving ADT may be associated with decreased testosterone levels and decreased skeletal muscle mass. To improve the QOL of patients receiving ADT, it is important to conduct routine screening to identify fatigue in patients.21 In our study, the dyspnea scale score increased by 13 points over 12 months compared with the pretreatment values. The mechanism underlying dyspnea in patients with cancer is not well understood due to the heterogeneous origins of shortness of breath. Administration of nonsteroidal antiandrogens, such as bicalutamide, in combination with a luteinizing hormone–releasing hormone analog leads to the development of dyspnea as a common side effect. A study comparing continuous ADT and intermittent ADT in patients with metastatic PC reported dyspnea in 6% and 12% of the patients, respectively.22 The authors estimated that the cause of dyspnea was the specific toxicity of nilutamide, which was administered as a therapeutic agent to the patients. However, a systemic review comparing antiandrogen administration and castration reported that dyspnea was not a prominent side effect of bicalutamide monotherapy or medical castration.23 Although it is difficult to determine the apparent cause of difficulty in breathing experienced by patients with PC who are receiving ADT, such difficulty in breathing is believed to be a side effect of ADT or a symptom after cancer progression and may also be associated with severe anemia.
We also evaluated the urinary symptoms, bowel symptoms, hormonal treatment-related symptoms, and sexual activity using the PR25, a module specialized for PC. We found that the two groups did not show any differences in this regard. Therefore, the data indicate that ADT had a greater effect on the symptom scores than the addition of IMRT. In a study comparing IMRT with 3D conformal radiotherapy in patients with advanced PC, the patients in the IMRT group showed baseline characters similar to those of the patients in our study, even the tumor stage and grade; however, only approximately 24% of these patients received ADT.24 The QLQ-C30 and PR25 scores were evaluated at 1 and 6 months after treatment and showed no clinically significant changes compared with the scores recorded before treatment. In contrast, in the aforementioned study by Bolla et al.,18 the hormonal treatment-related symptom score increased in all patients who received combination therapy with ADT and RT for 6 months. The long-term group, which continued receiving ADT for 36 months, presented with an even more elevated symptom score, whereas for the short-term group, which discontinued ADT, the scores almost recovered to the previous scores. Shin et al.19 reported that the PC-specific HRQOL of Korean patients, as assessed using the EORTC QLQ-PR25, showed a significant deterioration of most symptom scores, with the exception of bowel symptoms, at 3 months postoperatively compared with the baseline condition after radical prostatectomy. The urinary symptoms worsened at 3 months, and incontinence significantly worsened at 3 months, although patients recovered to nearly the baseline status at 12 months. However, hormonal treatment-related symptoms, sexual activity, and sexual function were significantly worse at 3 months and remained poorer than the baseline condition at 12 months. Some studies in which the EORTC QLQ-PR25 was used have reported improvement in the urinary symptoms after administration of ADT in patients with advanced disease.18,25,26 In our study, these symptoms tended to improve, but the results were not significant.
Shim et al.’s27 study assessed ADT treatment with three types of LHRH agonists, including leuprolide, in patients with PC and found that the level of chemical castration remained low after 3 months. In our study using leuprolide, the testosterone level fell to the castration level after 3 months and was maintained at this level continuously. In particular, the physical function continued to be decreased after 3 months in both groups compared with before ADT, which is known to be related to the testosterone level.28
To the best of our knowledge, previous studies have not directly compared the HRQOL between ADT and ADT and IMRT. Our findings may aid patients in making more appropriate decisions by giving them practical experience in selecting a treatment modality for advanced PC. The limitations of our study include the lack of randomization for treatment and the relatively small sample size. This study reports results for only 12 months of treatment. Thus, long-term follow-up studies are required. Most changes in the HRQOL predominantly occurred during the first 3 months after administration of ADT or RT, but additional studies on long-term changes are required.29 The strengths of this study include the prospective longitudinal cohort design and use of validated and standardized health-related questionnaires during the follow-up period. A limitation of this study is the lack of patient randomization, but there was no significant difference between groups at baseline. Another limitation of our study is the heterogeneity of the stage of PC in patients, which may have affected the improvement in the QOL on administration of ADT. According to previous studies, the HRQOL tended to improve in patients with symptoms of advanced PC after ADT.30
5. Conclusion
In conclusion, our prospective study indicates that leuprolide acetate treatment at 6-month intervals was not accompanied by significant changes in the global health status/QOL, despite a deterioration in the physical function, role function, and hormonal treatment-related symptoms. The combination of ADT and IMRT did not lead to any further deterioration in the HRQOL compared with ADT monotherapy.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This study was supported by a grant (HCRI 19017) from the Chonnam National University Hwasun Hospital Institute for Biomedical Science.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2020.11.001.
Appendix A. Supplementary data
The following is/are the Supplementary data to this article:
References
- 1.Hamdy F.C., Donovan J.L., Lane J.A., Mason M., Metcalfe C., Holding P. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 2.Lardas M., Liew M., van den Bergh R.C., De Santis M., Bellmunt J., Van den Broeck T. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur Urol. 2017;72:869–885. doi: 10.1016/j.eururo.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Whiting P.F., Moore T.H., Jameson C.M., Davies P., Rowlands M.A., Burke M. Symptomatic and quality-of-life outcomes after treatment for clinically localised prostate cancer: a systematic review. BJU Int. 2016;118:193–204. doi: 10.1111/bju.13499. [DOI] [PubMed] [Google Scholar]
- 4.Schaake W., de Groot M., Krijnen W.P., Langendijk J.A., van den Bergh A.C. Quality of life among prostate cancer patients: a prospective longitudinal population-based study. Radiother Oncol. 2013;108:299–305. doi: 10.1016/j.radonc.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Schroeck F.R., Krupski T.L., Sun L., Albala D.M., Price M.M., Polascik T.J. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.H., Ha Y.S., Jeong S.J., Kim S., Kim W.J., Jang T.L. Factors related to patient-perceived satisfaction after robot-assisted radical prostatectomy based on the expanded prostate cancer index composite survey. Prostate Cancer Prostatic Dis. 2013;16:341–345. doi: 10.1038/pcan.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal P.K., Sadetsky N., Konety B.R., Resnick M.I., Carroll P.R. Cancer of the Prostate Strategic Urological Research E. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 8.Kirschner-Hermanns R., Jakse G. Quality of life following radical prostatectomy. Crit Rev Oncol Hematol. 2002;43:141–151. doi: 10.1016/s1040-8428(02)00026-4. [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich A., Aus G., Bolla M., Joniau S., Matveev V.B., Schmid H.P. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Cobran E.K., Young H.N., Chen R.C., Chen X., Reeves J., Godley P.A. Race and Time to Receipt of Androgen Deprivation Therapy Among Men With Metastatic Prostate Cancer. J Natl Med Assoc. 2019;111:246–255. doi: 10.1016/j.jnma.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Shavers V.L., Brown M.L. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Yun Y.H., Park Y.S., Lee E.S., Bang S.M., Heo D.S., Park S.Y. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 14.van Andel G., Bottomley A., Fossa S.D., Efficace F., Coens C., Guerif S. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Canc. 2008;44:2418–2424. doi: 10.1016/j.ejca.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Park J., Shin D.W., Yun S.J., Park S.W., Jeon S.S., Kwak C. Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire for patients with prostate cancer - EORTC QLQ-PR25. Oncology. 2013;85:299–305. doi: 10.1159/000355689. [DOI] [PubMed] [Google Scholar]
- 16.Maringwa J., Quinten C., King M., Ringash J., Osoba D., Coens C. Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol. 2011;22:2107–2112. doi: 10.1093/annonc/mdq726. [DOI] [PubMed] [Google Scholar]
- 17.Alibhai S.M., Breunis H., Timilshina N., Johnston C., Tomlinson G., Tannock I. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 18.Bolla M., de Reijke T.M., Van Tienhoven G., Van den Bergh A.C., Oddens J., Poortmans P.M. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 19.Shin D.W., Lee S.H., Kim T.H., Yun S.J., Nam J.K., Jeon S.H. Health-Related Quality of Life Changes in Prostate Cancer Patients after Radical Prostatectomy: A Longitudinal Cohort Study. Cancer Res Treat. 2019;51:556–567. doi: 10.4143/crt.2018.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger A.M., Mooney K., Alvarez-Perez A., Breitbart W.S., Carpenter K.M., Cella D. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geerkens M.J.M., Pouwels N.S.A., Beerlage H.P. The effectiveness of lifestyle interventions to reduce side effects of androgen deprivation therapy for men with prostate cancer: a systematic review. Qual Life Res. 2020;29:843–865. doi: 10.1007/s11136-019-02361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langenhuijsen J.F., Badhauser D., Schaaf B., Kiemeney L.A., Witjes J.A., Mulders P.F. Continuous vs. intermittent androgen deprivation therapy for metastatic prostate cancer. Urol Oncol. 2013;31:549–556. doi: 10.1016/j.urolonc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Kunath F., Grobe H.R., Rucker G., Motschall E., Antes G., Dahm P. Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database Syst Rev. 2014;6 doi: 10.1002/14651858.CD009266.pub2. CD009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lips I., Dehnad H., Kruger A.B., van Moorselaar J., van der Heide U., Battermann J. Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys. 2007;69:656–661. doi: 10.1016/j.ijrobp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Nabid A., Carrier N., Martin A.G., Bahary J.P., Lemaire C., Vass S. Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur Urol. 2018;74:432–441. doi: 10.1016/j.eururo.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 26.You D., Jeong I.G., Kim S.W., Chung B.H., Cho J.S., Lee H.M. Impacts of leuprolide acetate on quality of life in patients with prostate cancer: a prospective multicenter study. Scand J Urol Nephrol. 2010;44:399–405. doi: 10.3109/00365599.2010.508048. [DOI] [PubMed] [Google Scholar]
- 27.Shim M., Bang W.J., Oh C.Y., Lee Y.S., Cho J.S. Effectiveness of three different luteinizing hormone-releasing hormone agonists in the chemical castration of patients with prostate cancer: Goserelin versus triptorelin versus leuprolide. Invest Clin Urol. 2019;60:244–250. doi: 10.4111/icu.2019.60.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy T.A., Blackman M.R., Harman S.M., Tobin J.D., Schrager M., Metter E.J. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metabol. 2002;283:E284–E294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 29.Spry N.A., Kristjanson L., Hooton B., Hayden L., Neerhut G., Gurney H. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Canc. 2006;42:1083–1092. doi: 10.1016/j.ejca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Albertsen P.C., Aaronson N.K., Muller M.J., Keller S.D., Ware J.E., Jr. Health-related quality of life among patients with metastatic prostate cancer. Urology. 1997;49:207–216. doi: 10.1016/S0090-4295(96)00485-2. discussion 16-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.