Abstract
Background
We analyzed the relationship between biochemical recurrence (BCR) and the status of positive surgical margin (PSM) in patients with pT3a prostate cancer (PCa).
Materials and methods
Patients (n = 150) who underwent radical prostatectomy for pT3a PCa without nodal/distant metastasis were retrospectively reviewed between 2010 and 2013. The data regarding the status of PSM including the number, length, and margin Gleason score were collected. The predictors of BCR were analyzed using Cox regression hazard models. BCR-free survival was compared between the patients with negative surgical margin (NSM) and with PSM using Kaplan–Meier curves and log-rank tests.
Results
PSM was noted in 74 patients (49.3%). Seventy-six patients (50.7%) had NSM and 38 patients (25.3%) had single PSM. Twenty patients (13.3%) had two PSMs and 16 patients (10.7%) had ≥3 PSMs. In total patients, the multivariate analysis demonstrated that a pathological Gleason score of ≥8 was significantly associated with BCR [hazard ratio (HR), 2.173; 95% confidence interval (CI), 1.244–3.797; P = 0.038]. In patients with PSM, the number of PSM more than two was significantly associated with BCR (HR, 2.723; 95% CI, 1.256–5.902; P = 0.011). PSM length of ≥3 mm was also a significant predictive factor (HR, 1.024; 95% CI, 0.994–1.055, P = 0.042). Patients with the highest margin Gleason score of ≥4 had poorer BCR-free survival than those with that of 3/no surgical margin.
Conclusions
Number (more than one), length (≥3 mm), and higher margin Gleason score (≥4) of PSM were significantly associated with an increased likelihood of BCR in patients with pT3a PCa.
Keywords: Biochemical recurrence, Extraprostatic extension, Positive surgical margin, Radical prostatectomy
1. Introduction
Prostate cancer (PCa) is the second most common malignancy in men in western countries and has a high mortality rate [1,2]. Patients with biochemical recurrence (BCR) have a considerably worse prognosis compared with those without BCR, and they often develop distant metastasis and suffer a cancer-related death [3,4]. In previously published studies, various predictive factors of BCR, such as preoperative prostate-specific antigen (PSA), pathologic Gleason score (pGS), pathologic T stage (pT), the invasion of seminal vesicles, and high percent tumor volume (PTV) have been reported [5, 6, 7].
It is also known that both extraprostatic extension (EPE) and positive surgical margin (PSM) can offer prognostic information. EPE usually increases BCR risk 1.5-fold over confined disease [8]. Furthermore, the reported incidence of a PSM in PCa is 8.8%–42% (median, approximately 20%) [9]. Alkhateeb et al. reported that the BCR-free survival rate of patients with PSM was 79.9% and that PSM was a significant predictive factor of BCR [10]. Other studies have suggested that one may be a more important predictive factor than the other; however, in most cases, it is challenging to distinguish between them [11]. Some studies investigated the impact of PSM status including length or Gleason score of PSM [12, 13], but they did not comprehensively analyze the PSM status, especially in pT3 disease.
In this study, we evaluated the impact of a PSM status on BCR in patients with pT3a PCa to demonstrate the predictive impact of PSM status along with the extent of EPE. We investigated the presence of PSM as well as the number, length, and margin Gleason score (mGS) of PSM.
2. Materials and methods
In total, 645 patients who underwent open radical prostatectomy (RP) or robot-assisted RP from February 2010 to December 2013 were retrospectively reviewed. Patients with nodal or distant metastasis and those who had received neoadjuvant therapy were excluded. Among the 645 patients, 150 had pT3a disease and satisfied the inclusion criteria. BCR was defined according to the National Comprehensive Cancer Network guidelines for PCa, which include PSA persistence and recurrence [14]. Patient data, including age, preoperative PSA, pGS, prostate volume, lymphovascular invasion (LVI), and PTV were collected. In addition, information regarding the PSM status, including the number, length, and mGS was obtained. Regular follow-ups were conducted at 6-month intervals. All prostatectomy specimens were coated with ink and sectioned into 3–4 mm slices that were analyzed by a single pathologist. PSM was defined as a tumor that extends to the surface of the prostate, for which surgeons cut across the tissue plane [1]. Samples with one positive slice were considered to have solitary PSM and those with two or more positive sections obtained from different locations of the prostate were considered to have multiple PSMs. The extent of EPE was measured as per the width and depth that indicated the tumor diameter beyond the normal confines of the prostate as per the International Society of Urological Pathology consensus recommendations [15].
We analyzed the clinicopathological factors using the Chi-square test and an independent sample t-test. BCR-free survivals between the patients with negative surgical margin (NSM) and patients with PSM were analyzed using the log-rank tests and Kaplan–Meier method. Univariate and multivariate Cox proportional hazard models were used to demonstrate the predictive factors of BCR and hazard ratio (HR) of each significant factor. Patients were stratified based on pGS (≤7 and ≥8), number of PSM (0, 1, 2, and ≥3), and length of PSM (<3 mm and ≥3 mm). All statistical tests were analyzed using the SPSS software package (version 22.0; IBM, Armonk, NY).
The study protocol was approved by the Institutional Review Board of the Asan Medical Center. The study design was in accordance with the principles of the Declaration of Helsinki.
3. Results
The preoperative and postoperative characteristics of 150 patients with pT3a PCa in our cohort are summarized in Table 1. The mean follow-up duration was 41.46 ± 6.49 months. Of all patients, 74 patients (49.3%) had PSM and BCR occurred in 53 patients (35.3%). A total Gleason score of ≥8 was identified in 44 patients (29.3%). Among all patients with PSM, 38 (25.3%) had solitary PSM, 20 patients (13.3%) had two PSMs, and 16 patients had more than two PSMs. The mean length of PSM was 4.69 ± 8.89 mm. The number of patients with mGS of 3 and ≥4 were 28 (18.7%) and 46 (30.7%), respectively.
Table 1.
Preoperative and postoperative patient characteristics.
| Variables | Total patients (n = 150) |
|---|---|
| Age (y) | 66.23 ± 6.96 |
| Preoperative PSA (ng/ml) | 13.45 ± 13.51 |
| Prostate volume (ml) | 32.63 ± 13.64 |
| EPE width (mm) | 9.41 ± 12.78 |
| EPE depth (mm) | 0.54 ± 1.164 |
| Diabetic mellitus, n (%) | 29 (19.3%) |
| Hypertension, n (%) | 70 (46.7%) |
| Body mass index (kg/m2) | 24.49 ± 2.49 |
| Pathological Gleason score, n (%) | |
| 7≤ | 106 (70.7%) |
| ≥8 | 44 (29.3%) |
| Percent tumor volume (%) (gm) | 22.4 ± 17.80 |
| Lymphovascular invasion, n (%) | 34 (22.7%) |
| No. of PSM, n (%) | |
| 0 | 76 (50.7) |
| 1 | 38 (25.3) |
| 2 | 20 (13.3) |
| ≥3 | 16 (10.7) |
| Margin Gleason grade, n(%) | |
| 3 | 28 (18.7%) |
| 4 or 5 | 46 (30.7%) |
| Length of PSM (mm) | 4.69 ± 8.89 |
| Biochemical recurrence, n (%) | 53 (35.3%) |
| Operation method, n (%) | |
| Open | 69 (46.0%) |
| Robot | 81 (54.0%) |
| Follow-up duration (mo) | 41.46 ± 6.49 |
PSM, positive surgical margin; PSA, prostate-specific antigen.
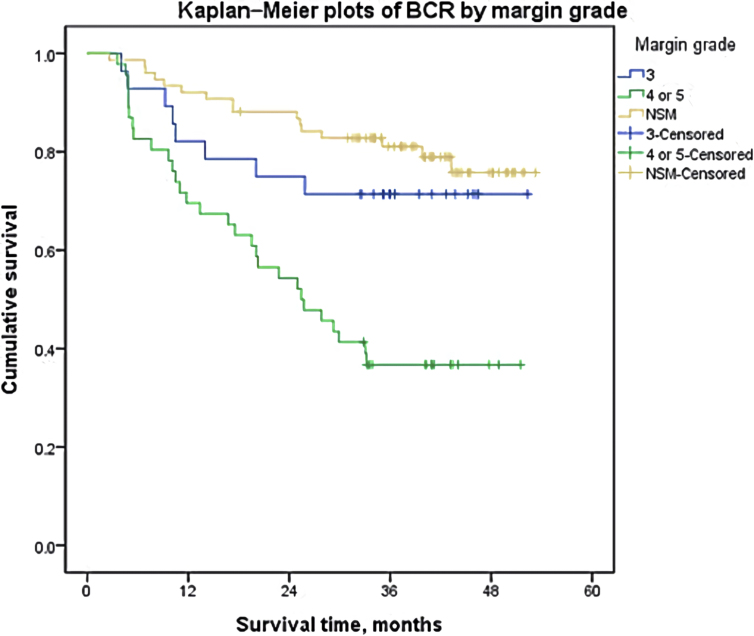
The Kaplan–Meier curves and log-rank tests were conducted to demonstrate the BCR-free survival for multiple predictive factors. Fig. 1 shows BCR-free survival according to the number of PSMs. BCR-free survival varies significantly regarding the number of PSM (NSM vs. 1 PSM, P = 0.035; NSM vs. 3 PSMs, P < 0.001; 1 PSM vs. ≥3 PSMs, P = 0.002; and 2 PSMs vs. 3 PSMs, P = 0.024), except for patients with one PSM and two PSMs (P = 0.536), The patients with one or two PSMs showed significantly poorer BCR-free survival than patients with NSM and patients with more than two PSMs showed worst prognosis regarding BCR-free survival. The mean BCR-free survival period were 45.80 ± 1.73, 37.25 ± 2.99, 35.27 ± 4.11, and 19.99 ± 4.17 months, respectively. Patients who had a PSM length of ≥3 mm had significantly shorter BCR-free survival (Fig. 2; median, 25.742 vs. not reached, P < 0.001). Fig. 3 shows the BCR-free survival according to mGS. Patients with mGS of 3 did not differ from those with NSM in terms of BCR-free survival (P = 0.343). However, those with mGS of 4 or 5 had poorer BCR-free survival than those with a score of 3 (P = 0.010).
Fig. 1.
BCR-free survival according to the number of PSM. BCR, biochemical recurrence; PSM, positive surgical margin.
Fig. 2.
BCR-free survival according to PSM length.
Fig. 3.
BCR-free survival according to the margin grade.
The results of the univariate and multivariate Cox proportional hazard regression analyses for all patients are shown in Table 2. In the univariate analysis, preoperative PSA (P = 0.011), pGS (P = 0.012), EPE width (P < 0.001), LVI (P < 0.001), and the presence of PSM (P < 0.001) were significant predictors of BCR-free survival. In the multivariate analysis, a higher pGS (≥8) was significantly associated with BCR-free survival [HR, 2.173; 95% confidence interval (CI), 1.244–3.797; P = 0.038]. In addition, LVI [HR, 2.033; 95% CI, 1.145–3.609; P = 0.015] and the presence of PSM [HR, 2.350; 95% CI, 1.279–4.315; P = 0.006] were also significant predictors of BCR-free survival. The results of the univariate and multivariate Cox proportional hazard regression analyses of BCR-free survival in pT3a patients with PSM are shown in Table 3. In the univariate analysis, EPE width (P = 0.010), more than two PSMs (P = 0.003), length of PSM (P = 0.017), and mGS (≥4, P = 0.013) were significantly associated with BCR-free survival. In the multivariate analysis, more than two PSMs (HR, 2.723; 95% CI, 1.256–5.902; P = 0.011), mGS ≥ 4 (HR, 2.356; 95% CI, 1.060–5.238; P = 0.035), and length of PSM ≥3 mm was a significant predictive factor of BCR (HR, 1.024; 95% CI, 0.994–1.055; P = 0.042).
Table 2.
Univariate and multivariate Cox proportional hazard regression analyses for BCR-free survival in pT3a patients.
| Univariate |
Multivariate |
||
|---|---|---|---|
| P | HR (95% CI) | P | |
| Age | 0.634 | ||
| Preoperative PSA (ng/ml) | 0.011 | 1.011 (0.995–1.028) | 0.177 |
| Prostate volume | 0.819 | ||
| Diabetes mellitus | 0.252 | ||
| Hypertension | 0.744 | ||
| Body mass index | 0.811 | ||
| Pathologic Gleason score | |||
| ≤7 | Ref. | Ref. | Ref. |
| ≥8 | 0.012 | 2.173 (1.244–3.797) | 0.038 |
| Lymphovascular invasion | <0.001 | 2.033 (1.145–3.609) | 0.015 |
| EPE width | <0.001 | 1.012 (0.992–1.032) | 0.189 |
| EPE depth | 0.878 | ||
| Resection margin | |||
| Negative | Ref. | Ref. | Ref. |
| Positive | <0.001 | 2.350 (1.279–4.315) | 0.006 |
| Percent tumor volume | 0.731 | ||
| Operation method (open vs. robot) | 0.534 | ||
BCR, biochemical recurrence; CI, confidence interval; HR, hazard ratio; PSA, prostate specific antigen, EPE, extraprostatic extension.
Table 3.
Univariate and multivariate Cox proportional hazard regression analyses for BCR-free survival in pT3a patients with PSM.
| Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|
| P | HR (95% CI) | P | |
| Age | 0.629 | ||
| Baseline PSA | 0.211 | ||
| Prostate volume | 0.420 | ||
| Diabetes mellitus | 0.479 | ||
| Hypertension | 0.726 | ||
| Body mass index | 0.800 | ||
| pGS | |||
| ≤7 | Ref. | ||
| ≥8 | 0.119 | ||
| Lymphovascular invasion | 0.121 | ||
| EPE width | 0.010 | 1.003 (0.978–1.028) | 0.817 |
| EPE depth | 0.690 | ||
| PSM status | |||
| No. of PSM | |||
| 1 | Ref. | Ref. | Ref. |
| 2 | 0.750 | 1.324 (0.595–2.949) | 0.492 |
| ≥3 | 0.003 | 2.723 (1.256–5.902) | 0.011 |
| mGS | |||
| 3 | Ref. | Ref. | Ref. |
| ≥4 | 0.013 | 2.356 (1.060–5.238) | 0.035 |
| Length of PSM | |||
| <3 mm | Ref. | Ref. | Ref. |
| ≥3 mm | 0.015 | 1.024 (0.994–1.055) | 0.008 |
| Percent tumor volume | 0.815 | ||
| Operation method (open vs. robot) | 0.823 | ||
BCR, biochemical recurrence; CI, confidence interval; HR, hazard ratio; PSM, positive surgical margin; PSA, prostate-specific antigen; pGS, pathologic Gleason score; EPE, extraprostatic extension; mGS, margin Gleason score.
4. Discussion
There have been various reports of studies that have attempted to identify predictive factors for BCR because BCR is significantly related to distant metastasis and cancer-related death [16, 17].
The present results demonstrate a precise understanding regarding the impact of PSM status on BCR in patients with pT3a PCa. PCa with seminal vesicle invasion usually demonstrates BCR in 40%–60% of the patients [11]. This suggests that pT3b disease has severe adverse effects on BCR and can mask the predictive impact of PSM status on BCR. Therefore, we evaluated only pT3a patients in this study.
Zhang L. et al demonstrated the result of a meta-analysis showing that PSM is an independent prognostic factor of BCR [18]. Alkhateeb S. et al claimed that PSM is considered to be an important factor in disease recurrence, and the reported incidences of PSM after RP vary depending on several factors, including surgical techniques, patient characteristics, and the use of different methods for pathological examinations [10]. Conversely, Stephenson A.J. et al reported that PSM status, including location, number, and extent, was not a significant predictive factor of BCR after RP [19]. In addition, Srigley et al. reported that PSM may result from artifacts or an intraprostatic incision during the surgery [20].
Although many studies have shown different significance and incidence of PSM after RP, they usually have indicated an increased risk of BCR in the presence of PSM; this is validated by a greater need for secondary treatment [10, 19, 21, 22, 23]. Some large multi-institutional studies demonstrated that patients with PSM had BCR at a rate two times higher than those with NSM, even after adjusting for age, pGS, PSA, and pathologic stage [19, 24, 25, 26].
According to previous studies, the 5-year failure-free survival was 48%–76% [27,28] in patients with pT3a PCa without PSM and 33%–55% [22,28, 29, 30] in those with both pT3a disease and PSM. The 10-year failure-free survival was 46%–90% and 20%–53%, respectively, for these patients. These results demonstrate that the presence of PSM significantly increases disease recurrence. Our results were similar to those of studies in which patients with both pT3a PCa and PSM had a higher rate of BCR than those with pT3a PCa without PSM [22,28] (50.0% vs. 21.1%; HR, 2.350; 95% CI, 1.279–4.315; P = 0.006).
Along with the impact of the presence of PSM on BCR, we also showed that the multiplicity, longer length, and the higher mGS of PSM were the important predictive factors of BCR. The multiplicity of PSM has previously been reported as a predictive factor for BCR [6]. In our study, patients with two PSMs did not differ from patients with one PSM regarding BCR-free survival (P = 0.492), but patients with more than two PSMs had a significantly higher risk of BCR (HR, 2.723; 95% CI, 1.256–5.902; P = 0.011). The absence of significant difference in BCR-free survival between patients with a solitary PSM and those with two PSMs may be explained by the relatively small number of patients included in our study.
In addition, we categorized patients with PSM into two groups according to the PSM length (<3 mm and ≥3 mm) to verify the impact of PSM length on BCR. The cut-off value of 3 mm was used in this study based on several previous reports [7, 13]. Shikanov et al. have reported that a PSM of ≥3 mm or multifocal PSMs can contribute to BCR after RP [31]. In this study, Cox regression analysis demonstrated that patients with a PSM of ≥3 mm had poorer BCR-free survival (P = 0.042).
Recently, the importance of the mGS of PSM as a predictive factor for BCR has been also described by some physicians [12, 32]. Kates et al. have demonstrated that lower mGS at the site of PSM were significantly associated with reduced risk of BCR [12]. We demonstrated that patients with mGS of ≥4 had significantly poorer BCR-free survival than those with mGS of 3. In contrast, patients with mGS of 3 exhibited no significant difference regarding BCR-free survival compared with the patients with NSM. These results suggest that pathologists should report mGS of PSM to help predict the risk of BCR after RP and provide supportive information for the adjuvant therapy.
In our study, many confounding factors that attributed to BCR were adjusted. We analyzed patients with pT3a PCa only who had no distant or nodal metastasis; none of the patients received neoadjuvant therapy. Few studies have evaluated the PSM status in only pT3a patients; therefore, the present results provide additional clinical information regarding the impact of PSM on BCR in pT3a PCa. Furthermore, the artificial stretch injury that may be misinterpreted as PSM was minimized because all the surgeries were performed by two expert surgeons who had previously performed >500 RPs. Therefore, the impact of PSM status in pT3a PCa would have been revealed more clearly in this study than in other studies.
The PSM status, including the number, length, and mGS were significant predictors of BCR. The EPE width had the tendency to predict BCR; however, the impact of the PSM status was more significant. Our study results suggest the reporting of PSM status on the pathological results because they could be predictive factors of BCR. Adjuvant treatment should be considered if number of PSM more than one, PSM length ≥3 mm, or mGS ≥4 in pT3a PCa patients.
In the Cox regression analysis, the pGS was a significant prognostic factor of BCR in the total cohort, but did not show statistical significance in patients with PSM. This might be due to two reasons. First, the impact of the PSM status may have outweighed the impact of pGS on BCR. Second, the relatively small number of patients may have made the results statistically insignificant. Regardless of these results, it is clear that the increased number of PSM is an important predictive factor of BCR. Along with the PSM status, LVI was significantly related to BCR-free survival. In the previous studies, the presence of LVI demonstrated a significant predictive effect regarding BCR in patients with localized PCa [33, 34, 35]. Our study results on LVI showed similar results to those of other studies with an HR of 2.033 (95% CI, 1.145–3.609; P = 0.015).
According to one meta-analysis about the accuracy of magnetic resonance imaging (MRI) for local staging of PCa, the sensitivity and specificity regarding the detection of EPE were 0.57 (95% CI, 0.49–0.64) and 0.91 (95% CI, 0.88–0.93), respectively [36]. These results suggest that more than half of EPE could be predicted by MRI. Therefore, as the PSM status has been proved as a significant adverse pathological feature in our study and several other studies, surgeons should be aware of not making PSM during the surgery in patients with suspicious EPE on MRI by more wide and precise excision of the prostate.
This study has several limitations. Firstly, this study was retrospective in nature and was based on medical records; thus, a potential inherent bias may exist. Second, the follow-up duration was relatively short. Third, the sample size was relatively small. Thus, we recommend that further studies including a larger sample size with a longer follow-up duration should be conducted to verify our results.
Despite the limitations of this study, it has revealed the impact of PSM status more precisely than previous studies, and the sample size is reasonable, considering that this was a single-center study.
5. Conclusions
The PSM number of more than two, PSM length of 3 mm or longer, and the mGS 4 or 5 were significantly associated with an increased risk of BCR in pT3a PCa patients. When EPE is suspicious before surgery, it must be done carefully to minimize the occurrence of PSM. When both EPE and PSM are encountered after the surgery, the precise PSM status should be carefully examined and patients should be considered for early secondary treatment.
Authors' contributions
W Lee: manuscript writing and data analysis; B Lim: data analysis; YS Kyung: manuscript editing; C-S Kim: projection development and manuscript editing.
Funding sources
No funding to declare.
Research involving human participants and/or animals
As this was a retrospective study, this study did not contain any contact with human participants or animals performed by any of the authors.
Informed consent
As this was a retrospective study, no informed consent was required for this study by the internal review board.
Conflicts of interest
All authors have no conflict of interest to declare.
Acknowledgments
The authors would like to thank Enago (http://www.enago.co.kr) for the English language review.
Contributor Information
Wonchul Lee, Email: teetee512@naver.com.
Bumjin Lim, Email: lbj1986@hanmail.net.
Yoon Soo Kyung, Email: kyungys@amc.seoul.kr.
Choung-Soo Kim, Email: cskim@amc.seoul.kr.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA A Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Koontz B.F. Impact of primary Gleason grade on risk stratification for Gleason score 7 prostate cancers. Int J Radiat Oncol Biol Phys. 2012;82(1):200–203. doi: 10.1016/j.ijrobp.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Thompson I.M. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190(2):441–449. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Isbarn H. Long-term data on the survival of patients with prostate cancer treated with radical prostatectomy in the prostate-specific antigen era. BJU Int. 2010;106(1):37–43. doi: 10.1111/j.1464-410X.2009.09134.x. [DOI] [PubMed] [Google Scholar]
- 5.Vis A.N., Schroder F.H., van der Kwast T.H. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol. 2006;50(2):258–265. doi: 10.1016/j.eururo.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Somford D.M. Prognostic relevance of number and bilaterality of positive surgical margins after radical prostatectomy. World J Urol. 2012;30(1):105–110. doi: 10.1007/s00345-010-0641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sooriakumaran P. The impact of length and location of positive margins in predicting biochemical recurrence after robot-assisted radical prostatectomy with a minimum follow-up of 5 years. BJU Int. 2015;115(1):106–113. doi: 10.1111/bju.12483. [DOI] [PubMed] [Google Scholar]
- 8.Tourinho-Barbosa R. Biochemical recurrence after radical prostatectomy: what does it mean? Int Braz J Urol. 2018;44(1):14–21. doi: 10.1590/S1677-5538.IBJU.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iczkowski K.A., Lucia M.S. Vol. 2011. Prostate Cancer; 2011. Frequency of positive surgical margin at prostatectomy and its effect on patient outcome; p. 673021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhateeb S. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol. 2010;183(1):145–150. doi: 10.1016/j.juro.2009.08.132. [DOI] [PubMed] [Google Scholar]
- 11.Swanson G.P., Basler J.W. Prognostic factors for failure after prostatectomy. J Canc. 2010;2:1–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Kates M. Importance of reporting the gleason score at the positive surgical margin site: analysis of 4,082 consecutive radical prostatectomy cases. J Urol. 2016;195(2):337–342. doi: 10.1016/j.juro.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Servoll E. The length of a positive surgical margin is of prognostic significance in patients with clinically localized prostate cancer treated with radical prostatectomy. Urol Int. 2014;93(3):289–295. doi: 10.1159/000362342. [DOI] [PubMed] [Google Scholar]
- 14.Mohler J.L. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14(1):19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 15.Tan P.H. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 5: surgical margins. Mod Pathol. 2011;24(1):48–57. doi: 10.1038/modpathol.2010.155. [DOI] [PubMed] [Google Scholar]
- 16.Pagano M.J. Predictors of biochemical recurrence in pT3b prostate cancer after radical prostatectomy without adjuvant radiotherapy. Prostate. 2016;76(2):226–234. doi: 10.1002/pros.23114. [DOI] [PubMed] [Google Scholar]
- 17.Blute M.L. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001;165(1):119–125. doi: 10.1097/00005392-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol. 2018;16(1):124. doi: 10.1186/s12957-018-1433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephenson A.J. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182(4):1357–1363. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 20.Srigley J.R. Key issues in handling and reporting radical prostatectomy specimens. Arch Pathol Lab Med. 2006;130(3):303–317. doi: 10.5858/2006-130-303-KIIHAR. [DOI] [PubMed] [Google Scholar]
- 21.Chang S.S., Cookson M.S. Impact of positive surgical margins after radical prostatectomy. Urology. 2006;68(2):249–252. doi: 10.1016/j.urology.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 22.Galetti T.P. Positive surgical margins after radical prostatectomy. Urologia. 2014;81(1):16–24. doi: 10.5301/uro.5000060. Italian. [DOI] [PubMed] [Google Scholar]
- 23.Silberstein J.L., Eastham J.A. Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J Urol. 2014;30(4):423–428. doi: 10.4103/0970-1591.134240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porres D. Organ-limited prostate cancer with positive resection margins. Importance of adjuvant radiation therapy. Urologe. 2012;51(9):1246–1252. doi: 10.1007/s00120-012-2871-0. German. [DOI] [PubMed] [Google Scholar]
- 25.Ro Y.K. Biochemical recurrence in Gleason score 7 prostate cancer in Korean men: significance of the primary Gleason grade. Korean J Urol. 2012;53(12):826–829. doi: 10.4111/kju.2012.53.12.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegel T. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 27.Hull G.W. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167(2 Pt 1):528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 28.Pfitzenmaier J. Positive surgical margins after radical prostatectomy: do they have an impact on biochemical or clinical progression? BJU Int. 2008;102(10):1413–1418. doi: 10.1111/j.1464-410X.2008.07791.x. [DOI] [PubMed] [Google Scholar]
- 29.Kupelian P. Correlation of clinical and pathologic factors with rising prostate-specific antigen profiles after radical prostatectomy alone for clinically localized prostate cancer. Urology. 1996;48(2):249–260. doi: 10.1016/S0090-4295(96)00167-7. [DOI] [PubMed] [Google Scholar]
- 30.Roehl K.A. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 31.Shikanov S. Length of positive surgical margin after radical prostatectomy as a predictor of biochemical recurrence. J Urol. 2009;182(1):139–144. doi: 10.1016/j.juro.2009.02.139. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R. Extraprostatic extension (EPE) of prostatic carcinoma: is its proximity to the surgical margin or Gleason score important? BJU Int. 2015;116(3):343–350. doi: 10.1111/bju.12911. [DOI] [PubMed] [Google Scholar]
- 33.Baydar D.E. Prognostic significance of lymphovascular invasion in clinically localized prostate cancer after radical prostatectomy. Sci World J. 2008;8:303–312. doi: 10.1100/tsw.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman C.M. Lymphovascular invasion as a predictor of disease progression in prostate cancer. Am J Surg Pathol. 2000;24(6):859–863. doi: 10.1097/00000478-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S. Lymphovascular invasion is an independent predictor of prostate-specific antigen failure after radical prostatectomy in patients with pT3aN0 prostate cancer. Int J Urol. 2008;15(10):895–899. doi: 10.1111/j.1442-2042.2008.02140.x. [DOI] [PubMed] [Google Scholar]
- 36.de Rooij M. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol. 2016;70(2):233–245. doi: 10.1016/j.eururo.2015.07.029. [DOI] [PubMed] [Google Scholar]