Abstract
Purpose
This prospective registry study evaluated the feasibility of stereotactic magnetic resonance imaging (MRI)–guided radiation therapy for the local treatment of isolated prostate cancer recurrence within the gland or prostate bed after primary radiation therapy.
Methods and Materials
Patients with isolated recurrence without any regional or distant extension after treatment by external radiation therapy of the prostate gland/bed or by prostate brachytherapy were included. A 173-second Fast Imaging with Steady state Precession (TrueFISP) sequence was used for MRI simulation, and the gross tumor volume was delineated using multimodal images. The initial treatment plan varied from 27.5 Gy in 5 fractions to 38.7 Gy in 9 fractions and was adapted at each session, if necessary. The primary endpoint was acute toxicities (according to the Common Terminology Criteria for Adverse Events v5.0 criteria). Secondary endpoints were the effects of the adaptive treatment on target volume coverage, late toxicities, and oncologic events.
Results
Twenty patients were included. After a minimum follow-up of 6 months, grade 2 dysuria (from grade 1 at baseline; n = 1), grade 2 polyuria (n = 1), grade 1 urinary incontinence (n = 1), grade 1 urinary pain (n = 2), and grade 1 diarrhea (n = 1) were reported.
All initial treatment plans met the tumor coverage objectives, with a mean 95% planning target volume value of 95.7%. No plan exceeded the bladder and rectum dose constraints, but 8 exceeded the urethra dose constraints because of urethra proximity to the planning target volume. The initial plan was adapted in 7 patients (35%). The tumor coverage improved by 3.7% compared with the predicted plan (P = .0001) without increase in the dose to organs at risk. The biochemical control rate for the whole cohort was 75% (15/20 patients) including the 4 patients who received androgen-deprivation therapy.
Conclusions
MRI-guided reirradiation for isolated recurrence within the prostate or in the prostate bed appears to be safe with excellent dosimetric results.
Introduction
Prostate cancer is the most common cancer in men in Europe.1 Despite the many advances in the available treatment techniques (prostatectomy, brachytherapy, and external radiation therapy), recurrence rates vary from 15% to 40% after primary external beam radiation therapy (EBRT), depending on the prognostic group.2,3 However, thanks to new prostate imaging modalities, such as prostate magnetic resonance imaging (MRI) and positron emission tomography (PET) with choline or prostate-specific membrane antigen (PSMA), 10% to 40% of recurrences are detected locally and are considered as potentially curable.4, 5, 6 For many years, treatment of local recurrences was mainly based on androgen deprivation therapy (ADT).7 Unfortunately, this treatment is only palliative, and can dramatically decrease the quality of life.8
In recent years, local treatment with curative intent has only been considered as an option for localized recurrences. Radical prostatectomy,9 cryotherapy, and high-intensity focused ultrasound10,11 have been evaluated for local recurrences, but their efficacy and morbidity are not optimal. Salvage treatment with EBRT or brachytherapy is now increasingly proposed.12 MR-guided radiation therapy (MRgRT) is a new radiation therapy modality.13 Specifically, MRIdian Linac is a radiation therapy system developed by Viewray in which a 0.35 Tesla MRI system is coupled to a multileaf collimator-equipped linear accelerator. The system allows increasing the prescribed dose while sparing the organs at risk (OARs) by (1) improving the tumor/OAR delineation accuracy thanks to better soft-tissue MR contrast compared with computer tomography (CT); (2) adapting treatments to the daily anatomy, target volumes, and OAR positioning, using an integrated treatment planning system (TPS); and (3) tracking targets by continuous MRI acquisition during irradiation. This technique might be interesting for the treatment of local recurrences of prostate cancer after EBRT/brachytherapy because at each fraction, it allows the accurate delivery of high dose by continuously monitoring the neighboring sensitive OARs. The objective of this monocenter prospective registry was to evaluate the feasibility (safety and dosimetric quality) of adaptive MRgRT for isolated local recurrence in the prostate gland/bed after primary radiation therapy.
Methods and Materials
Inclusion criteria
Inclusion was prospectively proposed to all patients with isolated recurrence within the prostate or in the prostate bed after primary radiation therapy (EBRT or brachytherapy). PET with choline or PSMA was systematically performed to confirm the absence of visible metastases. No histology confirmation was required if strong evidence suggested local prostatic cancer recurrence: prostate-specific antigen (PSA) increase confirmed by 2 consecutive measurements, lesion with high evidence of tumor recurrence on prostate diffusion-weighted images–MRI, and choline or PSMA avidity in prostate. Other inclusion criteria were Eastern Cooperative Oncology Group performance status = 0 or 1, no previous intestinal or genitourinary radiation-induced toxicity of grade 3 or higher, >12-month interval between the primary EBRT or brachytherapy and adaptive MRgRT, no MRI contraindication (presence of non-MRI compatible implanted cardiac devices, claustrophobia, psychiatric disorders, metal objects), and absence of bilateral hip prostheses (that could alter the treatment plan quality). This study was registered in the French Health Data Hub (registration number: #1802) and was approved by our local research committee (ICM-ART 2020/01). All patients signed an informed consent form before treatment.
Simulation
All patients underwent CT simulation directly followed by MRI simulation using the MRIdian apparatus to ensure reproducibility of the anatomic configuration. MR and CT images were rigidly registered for target volume delineation, whereas only the MR images were used for other organs. Furthermore, for dose calculation, CT to MR image registration was performed using an elastic registration algorithm. No contrast agent was needed because soft tissue contrast in MR images was considered sufficient. During the CT simulation, MRI dummy surface coils with similar electron attenuation properties to real MRI coils were placed on the custom immobilization device. MRI images were acquired with a Fast Imaging with Steady state Precession (TrueFISP) sequence (T1/T2, free breathing, 173 seconds; resolution of 1.5 × 1.5 mm; field of view of 45 × 30 × 36 cm).
Treatment planning
The gross tumor volume (GTV) was delineated using the data from the MRI simulation images without injection, MRI diagnostic images (mainly with diffusion-weighted images), and/or PET with choline (or PSMA) images, when useful. An isotropic margin of 3 mm was used for the planning target volume (PTV) extension. In all cases, GTV corresponded to a volume smaller than the entire prostate or the prostate bed area. Dose prescription and OAR dose constraints were determined as described in the Groupe d'Etude des tumeurs urogénitales Association Française d'Urologie (GETUG AFU) 31 protocol.14 The initial treatment plan varied from 27.5 Gy in 5 fractions to 38.7 Gy in 9 fractions and was adapted at each session, if necessary. The considered OARs were rectum (V27 <2 cm3; V12 <20%), bladder (V27 <5 cm3; V12 <15%), urethra (V36 <1 cm3, V24 urethra + 3 mm <30%), and femoral heads. Treatment planning was done using the Viewray TPS, with normalization on D50 (100% of the prescribed dose covers 50% of the target volume), while ensuring 95% PTV coverage within the 95% isodose.
Daily adaptive treatment workflow
Patients were positioned to target the dose to the prostate gland or bed volume using the daily MR images (similar imaging protocol as the one used for simulation). After rigid registration of the GTV, OAR contours were propagated on the daily MR image using deformable image registration. If the OAR contours were not considered optimal, online modifications were made by the physician. The initial plan was then evaluated by the physician and the physicist. If all dose constraints were met, no adaptation was required (nonadapted fractions). If a decrease in tumor coverage or inacceptable OAR dose constraints was observed, the initial plan was optimized on the integrated TPS (adapted fractions). The electron density map (transferred from the CT to the MR images) and the skin contour were checked to ensure correct dose recalculation. Quality assurance of the newly optimized plan was performed by recalculating the plan with a secondary Monte Carlo algorithm before irradiation. Tracking was ensured by following a contrasted structure (usually the prostate) on sagittal images obtained by cine MR. The beam was turned off when more than 5% of the tracked structure was outside the threshold of 3 mm from its initial position.
Clinical assessment, dosimetric evaluation, and endpoints
The primary endpoint was acute toxicities. Secondary endpoints were the effects of the adaptive treatment on the target volume coverage, late toxicities, PSA response, and death from any cause.
All toxicity events were reported according to the Common Terminology Criteria for Adverse Events v5.0 at each clinical examination. The International Prostate Symptom Score (IPSS) was filled in at baseline (ie, at inclusion) and every 6 months after MRgRT end. This tool categorizes symptoms as mild (0-7), moderate (8-19), or severe (20-35). Clinical outcomes and treatment-related toxicity events were assessed and recorded before, on the last day of treatment, and after MRgRT (6 weeks, 3 and 6 months). PSA was measured before and at 3 and 6 months after treatment.
For each adapted fraction delivery, the predicted plan (initial plan on the daily image) and the delivered plan (new plan on the daily image) were compared a posteriori with the initial plan. PTV and GTV coverage values as well as OAR maximum dose and volumetric doses were recorded. Results were analyzed using the paired t test and the Prism software (α = .05).
Results
Patient and treatment characteristics at inclusion
Twenty patients were included from October 21, 2019, to May 13, 2020. The baseline (ie, at inclusion) patient and tumor characteristics are described in Table 1. The median age was 76 years and all patients had a good performance status (Eastern Cooperative Oncology Group score 0 or 1). The median PSA level was 3.7 ng/mL (range, 0.34-34.7). Most patients had no dysuria (n = 16, 80%); 2 patients (10%) reported grade 1 and 2 patients (10%) grade 2 dysuria. The baseline median IPSS score was 3 (1-33).
Table 1.
Baseline patient and treatment characteristics
| Characteristics | N = 20 | % |
|---|---|---|
| Age | ||
| Median (range), in years | 76 (66-83) | |
| ISUP group before the primary treatment | ||
| 1 | 5 | 25 |
| 2 | 6 | 30 |
| 3 | 3 | 15 |
| 4 | 3 | 15 |
| 5 | 2 | 10 |
| Unknown | 1 | 5 |
| Primary treatment | ||
| EBRT or EBRT + ADT | 15 | 75 |
| Brachytherapy | 2 | 10 |
| Prostatectomy + EBRT | 3 | 15 |
| Irradiation dose (Gy) delivered during the primary treatment | ||
| 66 | 2 | 10 |
| 70 | 1 | 5 |
| 74 | 8 | 40 |
| 76 | 1 | 5 |
| 78 | 2 | 10 |
| 80 | 4 | 20 |
| 160 (LDR brachytherapy) | 2 | 10 |
| Primary irradiation areas | ||
| Prostate alone | 12 | 60 |
| Prostate + pelvis | 5 | 25 |
| Prostate bed alone | 1 | 5 |
| Prostate bed + pelvis | 2 | 10 |
| Median interval between primary treatment and MRgRT (range), in months | 123.5 (21-252) | |
| ECOG score before MRgRT | ||
| 0 | 11 | 55 |
| 1 | 9 | 45 |
| PSA concentration (μg/mL) before MRgRT | ||
| Median (range) | 3.7 (0.34-34.7) | |
| Dysuria before MRgRT | ||
| Grade 0 | 16 | 80 |
| Grade 1 | 2 | 10 |
| Grade 2 | 2 | 10 |
| IPSS score and symptom groups before MRgRT | ||
| Median score (range) | 3 (1-33) | 70 |
| Mild (1-7) | 14 | 20 |
| Moderate (8-19) | 4 | 10 |
| Severe (20-35) | 2 | |
| Dose prescription for MRgRT | ||
| 27.5 Gy/5 fractions | 5 | 25 |
| 30 Gy/5 fractions | 12 | 60 |
| 30 Gy/6 fractions | 2 | 10 |
| 38.7 Gy/9 fractions | 1 | 5 |
| Concomitant ADT during MRgRT | ||
| Yes | 4 | 20 |
| No | 16 | 80 |
Abbreviations: ADT = androgen deprivation therapy; EBRT = external beam radiation therapy; ECOG = Eastern Cooperative Oncology Group; IPSS = international prostate symptom score; ISUP = international society of urologic pathology; LDR = low-dose rate; MRgRT = magnetic resonance-guided adaptive radiation therapy; PSA = prostate-specific antigen.
The primary treatment had been EBRT in 15 patients (75%) with or without concomitant ADT, brachytherapy in 2 patients (10%), and EBRT after prostatectomy due to increasing PSA in 3 patients (15%). The prescribed dose (primary treatment) ranged from 66 to 80 Gy for EBRT (median equivalent dose in 2-Gy fractions = 74 Gy) and 160 Gy for low dose rate (LDR) brachytherapy. The median interval between EBRT/brachytherapy and MRgRT was 123.5 months (range, 21-252). Three patients (15%) had a second treatment before inclusion in this trial: high-intensity–focused ultrasound (n = 2) and ADT (n = 1). Four patients (20%) received ADT during MRgRT.
The prescribed dose for MRgRT was 30 Gy in 5 fractions (n = 12, 60%), 27.5 Gy in 5 fractions (n = 5, 25%), 30 Gy in 6 fractions (n = 2, 10%), or 38.7 Gy in 9 fractions (n = 1, 5%).
Initial treatment plans
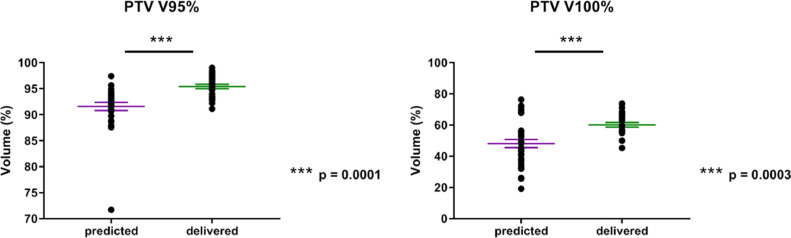
The tumor coverage and OAR dose constraints for each patient are summarized in Table E1. The prescription dose was calculated relative to the D50% (50% of the PTV received 100% of the prescription dose) while ensuring PTV V95% ≥95% (95% of the PTV received at least 95% of the dose prescription). The median GTV volume was 3.5 cm3 (range, 0.88-20.20). All patients met the tumor coverage objectives, with a median PTV V95% of 95.7% (range, 95.0-98.6). The median PTV V100% was 50% (range, 50%-94.2%) because for some patients, the normalization had to be adapted to respect the PTV coverage.
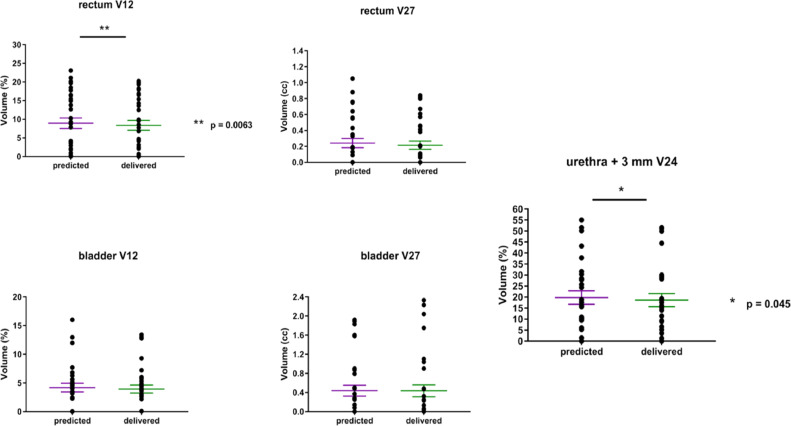
All plans met the rectum and bladder dose constraints. Nine plans (45%) exceeded the urethra dose constraints because of its proximity with the PTV. In 10 patients (50%), the urethra dose constraints were obtained retrospectively (not delineated on the initial plan and considered as a minor deviation of our protocol). The other dose parameters are reported in Tables E2 through E4. All patients included were entirely treated and no treatment interruption was required. The mean time of treatment by fraction was 43 minutes (33-95).
Acute toxicities
At baseline (ie, at inclusion, before MRgRT), 10 patients (50%) reported grade ≤2 genitourinary toxicities (n = 6 polyuria; n = 3 urinary incontinence, n = 4 dysuria) but no gastrointestinal toxicity (Table 2).
Table 2.
Genitourinary and gastrointestinal toxicities according to the CTCAE v5.0 criteria
| Toxicity | Before MRgRT,number of patients (%) | Last day of MRgRT,number of patients (%) | 3 months after MRgRT,number of patients (%) | 6 months after MRgRT,number of patients (%) |
|---|---|---|---|---|
| Dysuria | ||||
| g0 | 16 (80%) | 15 (75%) | 16 (80%) | 16 (80%) |
| g1 | 2 (10%) | 2 (10%) | 3 (15%) | 3 (15%) |
| g2 | 2 (10%) | 3 (15%) | 1 (5%) | 1 (5%) |
| Hematuria | ||||
| g0 | 20 (0%) | 20 (0%) | 19 (95%) | 20 (0%) |
| g1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Urinary incontinence | ||||
| g0 | 17 (85%) | 16 (80%) | 17 (85%) | 15 (75%) |
| g1 | 2 (10%) | 3 (15%) | 3 (15%) | 5 (25%) |
| g2 | 1 (5%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Polyuria | ||||
| g0 | 14 (70%) | 14 (70%) | 16 (80%) | 15 (75%) |
| g1 | 5 (25%) | 5 (25%) | 3 (15%) | 3 (15%) |
| g2 | 1 (5%) | 1 (5%) | 1 (5%) | 2 (10%) |
| Urinary pain | ||||
| g0 | 20 (100%) | 18 (90%) | 20 (100%) | 19 (95%) |
| g1 | 0 (0%) | 2 (10%) | 0 (0%) | 0 (0%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) |
| Diarrhea | ||||
| g0 | 20 (100%) | 19 (95%) | 18 (90%) | 18 (90%) |
| g1 | 0 (0%) | 1 (5%) | 2 (10%) | 2 (10%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rectal bleeding | ||||
| g0 | 20 (100%) | 20 (100%) | 19 (95%) | 19 (95%) |
| g1 | 0 (0%) | 0 (0%) | 1 (5%) | 1 (5%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rectal pain | ||||
| g0 | 20 (100%) | 20 (100%) | 19 (95%) | 19 (95%) |
| g1 | 0 (0%) | 0 (0%) | 1 (5%) | 1 (5%) |
| g2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Abbreviations: CTCAE = Common Terminology Criteria for Adverse Events; g = grade; MRgRT = magnetic resonance-guided radiation therapy.
MRgRT was well tolerated with no grade >2 acute toxicity. At the treatment end, only 1 patient reported an increase of dysuria severity (grade 2 vs grade 1 at baseline), and another patient grade 1 urinary incontinence not present before treatment. Two patients presented grade 1 urinary pain, and 1 patient grade 1 diarrhea (Table 2).
Three months after MRgRT end, no additional urinary toxicity was described, and dysuria and polyuria were reduced compared with baseline. Only 1 additional patient reported grade 1 diarrhea with grade 1 rectal bleeding and rectal pain.
Six months after treatment, 4 additional patients reported genitourinary toxicities (n = 2 grade 1 urinary incontinence; n = 1 grade 2 polyuria; n = 1 grade 2 urinary pain). One patient had grade 1 rectal bleeding. No grade >2 toxicity was observed.
The median IPSS score increased from 3 (at baseline) to 9 at 6 months posttreatment. Seven patients changed IPSS symptom group: 5 (25%) moved from the mild to the moderate symptom group, 1 (5%) from the mild to the severe symptom group, and 1 (5%) from the moderate to the severe symptom group.
Dosimetric benefits of adaptive MRgRT
The initial treatment plans were adapted for 7 patients (35%). Thirty-two fractions (91% of all fractions of these plans) were adapted (only 1 fraction was adapted for 3 patients). In these patients, the mean dosimetric parameters (for all fractions) of the predicted fractions (initial plan based on the daily anatomy) and adapted fractions (new plan based on the daily anatomy) were compared (Table 3). Six patients (patient #5, #10, #12, #16, #19, and #20) received adapted treatments because of insufficient tumor coverage (PTV V95%) (example in Fig 1). PTV coverage was improved in the adapted plans and compared with the predicted plans for 6/7 patients (mean PTV V95% increase of 3.67%, [–0.58% to 6.90%], P = .0001) (Fig 2) without excessive doses to the OARs (Fig 3). One patient (patient #17) received adapted MRgRT because of excessive dose to the rectum (predicted V12 Gy of 20.8%). After adaptation, the rectal V12 Gy decreased below the dose constraints (V12 rectum <20%), and the PTV V95% decreased from 93.5% to 92.9%.
Table 3.
Comparison of the adapted and predicted plans
| Patient #5 |
Patient #10 |
Patient #12 |
Patient #16 |
Patient #17 |
Patient #19 |
Patient #20 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parametern = 7 | Objectives | Mean pred | Mean adapt | Mean pred | Meanadapt | Mean pred | Meanadapt | Mean pred | Meanadapt | Mean pred | Meanadapt | Mean pred | Meanadapt | Mean pred | Meanadapt |
| PTV V100% (%) | ≥50 | 55.19 | 56.75 | 58.58 | 54.89 | 38.34 | 60.53 | 31.34 | 59.61 | 69.91 | 67.52 | 43.59 | 55.49 | 46.44 | 63.71 |
| PTV V95% (%) | ≥95 | 90.64 | 93.90 | 95.24 | 97.50 | 92.14 | 97.80 | 89.06 | 95.96 | 93.51 | 92.93 | 90.30 | 92.78 | 91.33 | 97.00 |
| V27 rectum (cc) | <5 | 0.06 | 0.12 | 0.30 | 0.21 | 0.00 | 0.00 | 0.51 | 0.46 | 0.82 | 0.66 | 0.00 | 0.00 | 0.00 | 0.00 |
| V12 rectum (%) | <20 | 11.41 | 10.80 | 11.57 | 10.95 | 3.33 | 3.77 | 15.80 | 14.48 | 20.81 | 19.16 | 0.00 | 0.00 | 0.69 | 0.71 |
| V27 bladder (cc) | <2 | 1.00 | 0.57 | 1.63 | 2.09 | 0.00 | 0.01 | 0.00 | 0.00 | 0.17 | 0.10 | 0.58 | 0.64 | 0.00 | 0.00 |
| V12 bladder (%) | <20 | 13.63 | 13.17 | 6.73 | 5.48 | 2.56 | 2.83 | 0.01 | 0.02 | 5.38 | 4.35 | 6.23 | 5.75 | 0.00 | 0.00 |
| V24 urethra + 3 mm (%) | <30 | 29.51 | 25.46 | 11.27 | 9.43 | 0.00 | 0.00 | 5.49 | 4.65 | 27.96 | 26.98 | 50.28 | 49.72 | 15.51 | 14.73 |
Abbreviations: Mean pred = mean value for the 5 predicted fractions (ie, before plan adaptation); Mean adapt = mean value for the 5 fractions after plan adaptation; PTV = planning target volume.
Fig. 1.
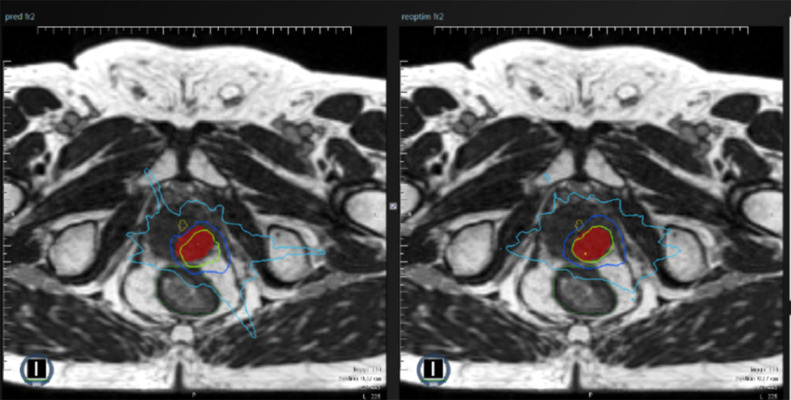
Example of differences between predicted and adapted plans. In this patient, planning target volume (PTV) coverage by the predicted fraction (baseline plan on the daily anatomy, left magnetic resonance [MR] image) was insufficient (PTV V95% = 71.70%). The isodose 95% (green line) did not correctly encompass the PTV (red volume). After adaptation (adapted plan on the daily anatomy, right MR image), PTV coverage was improved (PTV V95% = 96.13%) and the isodose 95% (green line) encompassed the PTV, as planned (red volume).
Fig. 2.
Comparison of planning target volume (PTV) coverage in the predicted fractions (baseline plan on the daily anatomy images) and delivered fractions (new plan on the daily anatomy images).
Fig. 3.
Comparison of the dose to organs at risk in the predicted fractions (baseline plan on the daily anatomy images) and delivered fractions (new plan on the daily anatomy images).
Biochemical response
At month 3 posttreatment, PSA concentration was decreased in 13 of the 16 patients (81%) who did not receive concomitant ADT (median value: 2.75 ng/mL vs 4.20 ng/mL at baseline) and also in the 4 patients with concomitant ADT (median value: 0.34 ng/mL vs 1.2 ng/mL at baseline).
PSA concentration increased in 3 patients (15%) (15.6 ng/mL vs 2.04 ng/mL; 1.66 ng/mL vs 1.44 ng/mL; and 29.98 ng/mL vs 11.37 ng/mL). Choline-based PET imaging at 3 months showed that in these 3 patients, disease was limited within the irradiated site without extraprostatic spread.
At month 6 posttreatment, the biochemical control rate for the whole cohort was 75% (15/20 patients). PSA concentration was decreased in 11 of the 16 patients (69%) who did not receive concomitant ADT (median value: 1.77 ng/mL vs 4.20 ng/mL at baseline) and also in all 4 patients with concomitant ADT (median value: 0.25 ng/mL vs 1.2 ng/mL at baseline).
PSA concentration increased in 5 patients, among whom 2 had a metastatic progression, another an intraprostatic recurrence (outside the reirradiation field), and 2 had no sign of disease recurrence by choline-based PET analysis. In all patients, disease was controlled at the reirradiated site.
Discussion
In the present study, we confirmed the dosimetric feasibility of MRgRT (~30 Gy in 5 fractions) for local relapse of prostate cancer. All patients met the prescription objective (PTV V95% ≥95%), and none of them received rectum or bladder excessive doses. Moreover, treatment was well tolerated with no grade ≥3 genitourinary and gastrointestinal toxicities. Six months after MRgRT end, 5 patients (25%) presented grade 1 to 2 polyuria and 5 (25%) grade 1 urinary incontinence.
To our knowledge, this is the first study on MRgRT for locally recurrent prostate cancer after primary EBRT or brachytherapy. Some studies described the results of salvage irradiation using stereotactic body radiation therapy or brachytherapy for this indication.12 The prescribed dose varied between 100 and 145 Gy with LDR brachytherapy, 19 and 36 Gy (1-5 fractions) with high-dose-rate (HDR) brachytherapy, and between 25 and 36 Gy (5-6 fractions) with hypofractionated EBRT. At the time of the study, the GETUG AFU 31 trial (phase I/II) was the only protocol considered as the “standard” in France by the ethical committees. Therefore, we decided to start with ~30 Gy in 5 fractions because this schedule was considered “acceptable” on the basis of the interim analysis results. We could not include our patients in the GETUG-AFU 31 trial because MRIdian Linac-based treatments were not permitted. Therefore, we decided to wait to have a longer follow-up for our first patients before proposing a dose escalation.
Overall, acute toxicities were rare (25% of grade 1-2 polyuria at 6 months), although the presence of baseline symptoms rendered it more difficult to evaluate the real effect of the second radiation treatment. Only 2 patients (10%) presented acute grade 1 urinary pain at MRgRT end. These results are consistent with other studies on salvage radiation therapy using 30 Gy in 5 fractions or 36 Gy in 6 fractions delivered with other techniques (RapidArc, VERO, and Cyberknife).15, 16, 17 All reported no or few grade 3 toxicities and approximately 20% of acute grade 1 to 2 genitourinary toxicities. In studies that used brachytherapy as salvage treatment for local prostate cancer recurrence,18, 19, 20, 21, 22 acute toxicities were slightly higher than in the present study. Specifically, 29% of patients experienced grade 1 to 2 acute genitourinary toxicities when 30 Gy were delivered in 3 fractions of HDR brachytherapy.18 Acute grade 2 genitourinary toxicities varied between 13% and 93% in the other studies using this technique.19, 20, 21, 22 Salvage LDR brachytherapy showed similar rates of grade 1 to 2 genitourinary toxicities.23, 24, 25 The low toxicity rate observed in our study could also be explained by the reduced volume and lower total dose delivered compared with protocols where the entire prostate received higher doses.26
The 2 benefits of MRgRT compared with other modalities are (1) the possibility of adaptation to the daily anatomy and (2) the tracking of the target (ie, prostate). Both features allow reducing the PTV margins. Therefore, the physician can decide to adapt the dosimetric plan in response to the daily dosimetric results. This decision is based on the comparison of the PTV coverage and OAR dose constraints in the initial and daily plans. Seven patients (35%) received 35 adaptable fractions and 32 (91%) were truly adapted (finally, 3 fractions were delivered according to the initial plan). The adapted plan was chosen when an increase in tumor coverage and/or a decrease in OAR dose was needed. The average tumor coverage increase (PTV V95%) was 3.7% with no excessive increase in OAR dose. The benefit of adapted MRgRT has been dosimetrically demonstrated for different clinical indications.27, 28, 29 Adaptation procedures reduce the dose to the bowel,27 improve lung tumor coverage,28 and are beneficial in 66% of fractions (244/371) in miscellaneous treatments.28 In our study, only 1 patient presented a decrease of the mean PTV coverage (PTV V95%) after adaptation. For this patient, adaptation was based on the rectal dose constraints (V12 >20%). However, the priority given to this constraint did not drastically alter the tumor coverage (PTV V95% from 93.5% to 92.9%).
A longer follow-up is needed to evaluate late toxicities and the potential effect on the quality of life. Recent reports show 10% to 20% of late grade ≥2 genitourinary toxicities and 1% to 5% of grade ≥2 gastrointestinal toxicities.16,30, 31, 32 Most of these genitourinary toxicities are probably related to the urethra dose that resulted in close surveillance of our patients due to potentially more related toxicities over time. Indeed, 1 patient received a high dose to the urethra (V24 urethra + 3 mm = 49.7%), but the decision was to prioritize the PTV coverage. This patient did not report any acute urinary toxicity at the last follow-up visit. Moreover, we decided not to follow the GETUG AFU31 guidelines because we think that the delineation of the urethra in the prostate path better reflects the potential clinical urinary consequences than the delineation on the MR image from the bladder to 2 cm below the prostatic apex. This may also explain the many cases of exceeded dose constraints in our study.
Besides safety, the effect on the disease is another important goal when a new radiation therapy approach is proposed to patients with local prostate cancer recurrence. We evaluated the biochemical response as secondary endpoint. In 85% of our patients, PSA level was decreased at the 3-month follow-up visit, and this PSA reduction was more pronounced at 6 months (in 75% of patients). This result is directly related to MRgRT because only 4 patients concomitantly received ADT. Despite the short follow-up, these preliminary results encourage us to continue patient inclusion for the efficacy study. Nevertheless, we think that reirradiation can improve relapse-free survival with lower toxicity compared with salvage prostatectomy, as recently confirmed by the systematic review and meta-analysis of local salvage therapies after radiation therapy for prostate cancer meta-analysis of local salvage therapies after radiation therapy for prostate cancer that included 150 studies (5-year relapse-free survival rate of 60% after HDR brachytherapy and stereotactic body radiation therapy).33
Our study presents some limitations. First, the small size of our sample limits the generalization of our results. Second, the follow-up was not long enough to reach conclusions on the safety and efficacy of this protocol, but the results are quite reassuring concerning acute toxicities. Third, this single-center study needs to be expanded to other centers to increase the sample size and the follow-up. Finally, heterogeneity of patients, prior treatments, use of ADT, and the reirradiation doses have to be considered for further interpretations of the current results.
Conclusions
MRgRT is feasible for locally recurrent prostate cancer after primary radiation therapy. However, a strict selection of patients is mandatory (MRI, choline-based PET, PSA kinetics +/- biopsy) to avoid unnecessary local treatment in patients with systemic progressive disease. MRgRT offers adaptive possibilities that allow good daily tumor coverage while sparing OARs, and with acceptable acute side effects. A longer follow-up is needed to evaluate the clinical results and late genitourinary and gastrointestinal toxicities.
Acknowledgments
Acknowledgment to Elisabetta Andermarcher for English editing.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors declare that they do not have any conflict of interest related to this work. D.A. is a cofounder of NovaGray but outside the submitted work. ViewRay Inc supports the Montpellier Cancer Institute with an institutional grant for research and development.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2021.100748.
Appendix. Supplementary materials
References
- 1.Ferlay J, Colombet M, Soerjomataram I. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Roach M, Hanks G, Thames H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 4.Brenot-Rossi I. Biochemical recurrence after curative treatment for localized prostate cancer: Performance of choline PET/CT in the assessment of local recurrence. Prog Urol. 2015;25:716–717. doi: 10.1016/j.purol.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Gauthe M, Belissant O, Girard A. PET/CT and biochemical recurrence of prostate adenocarcinoma: Added value of 68Ga-PSMA-11 when 18F-fluorocholine is non-contributive. Prog Urol. 2017;27:474–481. doi: 10.1016/j.purol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Cochet A, Kanoun S, Humbert O. Multimodality MRI and PET for restaging prostate cancer after biochemical failure of the treatment. Cancer Radiother. 2014;18:509–516. doi: 10.1016/j.canrad.2014.07.148. [DOI] [PubMed] [Google Scholar]
- 7.Duchesne GM, Woo HH, Bassett JK. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727–737. doi: 10.1016/S1470-2045(16)00107-8. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi H, Daneshmand S. Androgen deprivation therapy: Evidence-based management of side effects. BJU Int. 2013;111:543–548. doi: 10.1111/j.1464-410X.2012.11774.x. [DOI] [PubMed] [Google Scholar]
- 9.Chade DC, Eastham J, Graefen M. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: A systematic review of the literature. Eur Urol. 2012;61:961–971. doi: 10.1016/j.eururo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Williams AK, Martínez CH, Lu C, Ng CK, Pautler SE, Chin JL. Disease-free survival following salvage cryotherapy for biopsy-proven radio-recurrent prostate cancer. Eur Urol. 2011;60:405–410. doi: 10.1016/j.eururo.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Crouzet S, Blana A, Murat FJ. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int. 2017;119:896–904. doi: 10.1111/bju.13766. [DOI] [PubMed] [Google Scholar]
- 12.Baty M, Crehange G, Pasquier D. Salvage reirradiation for local prostate cancer recurrence after radiation therapy. For who? When? How? Cancer Radiother. 2019;23:541–558. doi: 10.1016/j.canrad.2019.07.125. [DOI] [PubMed] [Google Scholar]
- 13.Mutic S, Dempsey JF. The ViewRay system: Magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Pasquier D, Le Deley M-C, Tresch E. GETUG-AFU 31: A phase I/II multicentre study evaluating the safety and efficacy of salvage stereotactic radiation in patients with intraprostatic tumour recurrence after external radiation therapy-study protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loi M, Di Cataldo V, Simontacchi G. Robotic stereotactic retreatment for biochemical control in previously irradiated patients affected by recurrent prostate cancer. Clin Oncol (R Coll Radiol) 2018;30:93–100. doi: 10.1016/j.clon.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Leroy T, Lacornerie T, Bogart E, Nickers P, Lartigau E, Pasquier D. Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: Preliminary results of the Oscar Lambret Center. Radiat Oncol. 2017;12:95. doi: 10.1186/s13014-017-0833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jereczek-Fossa BA, Rojas DP, Zerini D. Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT) Br J Radiol. 2019;92 doi: 10.1259/bjr.20180494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Łyczek J, Kawczyńska MM, Garmol D. HDR brachytherapy as a solution in recurrences of locally advanced prostate cancer. J Contemp Brachytherapy. 2009;1:105–108. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CP, Weinberg V, Shinohara K. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Radiat Oncol Biol Phys. 2013;86:324–329. doi: 10.1016/j.ijrobp.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Maenhout M, Peters M, van Vulpen M. Focal MRI-guided salvage high-dose-rate brachytherapy in patients with radiorecurrent prostate cancer. Technol Cancer Res Treat. 2017;16:1194–1201. doi: 10.1177/1533034617741797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murgic J, Morton G, Loblaw A. Focal salvage high dose-rate brachytherapy for locally recurrent prostate cancer after primary radiation therapy failure: Results from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2018;102:561–567. doi: 10.1016/j.ijrobp.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 22.Kukiełka AM, Hetnał M, Dąbrowski T. Salvage prostate HDR brachytherapy combined with interstitial hyperthermia for local recurrence after radiation therapy failure. Strahlenther Onkol. 2014;190:165–170. doi: 10.1007/s00066-013-0486-z. [DOI] [PubMed] [Google Scholar]
- 23.Loening SA, Turner JW. Use of percutaneous transperineal 198-Au seeds to treat recurrent prostate adenocarcinoma after failure of definitive radiotherapy. Prostate. 1993;23:283–290. doi: 10.1002/pros.2990230403. [DOI] [PubMed] [Google Scholar]
- 24.Grado GL, Collins JM, Kriegshauser JS. Salvage brachytherapy for localized prostate cancer after radiotherapy failure. Urology. 1999;53:2–10. doi: 10.1016/s0090-4295(98)00492-0. [DOI] [PubMed] [Google Scholar]
- 25.Moman MR, van der Poel HG, Battermann JJ, Moerland MA, van Vulpen M. Treatment outcome and toxicity after salvage 125-I implantation for prostate cancer recurrences after primary 125-I implantation and external beam radiotherapy. Brachytherapy. 2010;9:119–125. doi: 10.1016/j.brachy.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Fuller D, Wurzer J, Shirazi R. Retreatment for local recurrence of prostatic carcinoma after prior therapeutic irradiation: Efficacy and toxicity of HDR-Like SBRT. Int J Radiat Oncol Biol Phys. 2020;106:291–299. doi: 10.1016/j.ijrobp.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Acharya S, Fischer-Valuck BW, Kashani R. Online magnetic resonance image guided adaptive radiation therapy: First clinical applications. Int J Radiat Oncol. 2016;94:394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Finazzi T, Haasbeek CJA, Spoelstra FOB. Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors. Int J Radiat Oncol. 2020;107:270–278. doi: 10.1016/j.ijrobp.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Fischer-Valuck BW, Henke L, Green O. Two-and-a-half-year clinical experience with the world's first magnetic resonance image guided radiation therapy system. Adv Radiat Oncol. 2017;2:485–493. doi: 10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: Preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol. 2015;5:e615–e623. doi: 10.1016/j.prro.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Mbeutcha A, Chauveinc L, Bondiau P-Y. Salvage prostate re-irradiation using high-dose-rate brachytherapy or focal stereotactic body radiotherapy for local recurrence after definitive radiation therapy. Radiat Oncol. 2017;12:49. doi: 10.1186/s13014-017-0789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miszczyk L, Stąpór-Fudzińska M, Miszczyk M, Maciejewski B, Tukiendorf A. Salvage cyberKnife-based reirradiation of patients with recurrent prostate cancer: The single-center experience. Technol Cancer Res Treat. 2018;17 doi: 10.1177/1533033818785496. 1533033818785496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valle LF, Lehrer EJ, Markovic D. A systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer (MASTER) [e-pub ahead of print] Eur Urol. 2020 doi: 10.1016/j.eururo.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.