Abstract
Purpose
Oligoprogression, defined as limited sites of progression on systemic therapy, in patients with metastatic renal cell carcinoma (mRCC) is not uncommon, possibly because of inter- and intratumoral heterogeneity. We evaluated the effect of stereotactic ablative radiation therapy (SAbR) for longitudinal control of oligoprogressive mRCC.
Methods and Materials
Patients with extracranial mRCC were included in this retrospective analysis if they progressed in ≤3 sites on systemic therapy while demonstrating response/stability at other sites and received SAbR to all progressing sites without switching systemic therapy. Our primary endpoint was modified progression-free survival (mPFS), which we calculated from the start of SAbR to the start of a subsequent systemic therapy, death, or loss to follow-up.
Results
We identified 36 patients with a median follow-up of 20.4 months (interquartile range, 10.9-29.4). Forty-three sites were treated with SAbR with a median dose of 36 Gy (range, 18-50) in 3 fractions (range, 1-5). Median time to SAbR from the start of systemic therapy was 11.4 months (interquartile range, 6.1-17.1). Median mPFS was 9.2 months (95% confidence interval [CI], 5.9-13.2). Patients receiving SAbR while on immunotherapy exhibited a longer median mPFS (>28.4 months, log-rank P = .0001) than patients not on immunotherapy (9.2 months). Median overall survival from SAbR administration was 43.4 months (95% CI, 21.5-not Reached). The 1-year local control rate was 93% (95% CI, 78.7-97.5). Most SAbR-related toxicities were grade 1 to 2 (33% of patients), with one grade 5 hemoptysis event possibly related to SAbR or disease progression.
Conclusions
SAbR has the potential to extend the the duration of current systemic therapy for selected patients with mRCC, preserving subsequent therapies for later administration possibly enabling longer treatment duration.
Introduction
Over one-third of patients with renal cell carcinoma (RCC) develop metastasis (mRCC) and their outcome remains poor.1 The standard of care for patients with mRCC is systemic therapy.2 Combination frontline therapy yields a median progression-free survival (PFS) of over 1 year,3,4 but complete responses are few, and most patients develop resistance. Upon progression, the standard of care is to switch systemic therapies, but each subsequent line typically yields a shorter PFS.2,5
The causes of resistance are not fully understood. In many tumors, resistance to targeted therapies results from mutations in the target. Bypass pathways and cell plasticity also drive resistance. In RCC, an example has been reported of a mutation in hypoxia-inducible factor 2α rendering resistance to its inhibitor.6 RCC is considered a paradigm for both histologic7 and molecular tumor heterogeneity. Genomic studies of multiple regions of primary and matched metastases show inter- and intratumoral mutational heterogeneity.8 Both branched and parallel evolution of clonally accumulated mutational drivers have been reported.8, 9, 10, 11 This reveals Darwinian selection of the fittest clones.8,9 The tumor microenvironment may also influence resistance.12
Resistance to systemic therapy manifests as generalized or focal disease progression. The underlying mechanisms probably differ. In some patients, resistance involves limited sites of metastases. This suggests that changes at those sites likely drove their progression. When systemic therapy is well tolerated, targeting those sites with focal therapies may be reasonable. Herein, we retrospectively report our institutional experience using stereotactic ablative radiation therapy (SAbR), which has shown excellent disease control rates with minimal toxicity in mRCC,13, 14, 15, 16, 17, 18, 19, 20, 21 to eradicate limited sites of progressing metastases and delay switching systemic therapy.
Methods and Materials
Patients and SAbR treatments
With institutional review board approval, we retrospectively reviewed patients with mRCC treated with SAbR between 2007 and 2017. Oligoprogression was defined as disease progression or disease causing new pain (2 patients) at 1 to 3 extracranial metastatic sites regardless of overall metastatic burden. We excluded patients who had brain metastases at the time of oligoprogression because of historically poorer outcomes.22 We included patients who demonstrated some response or stability on systemic therapy for mRCC of any histology, developed progression in 1 to 3 extracranial sites, and received curative-dose SAbR to all sites of progression without changing systemic therapy. Imaging scans were required to document tumor progression. Patients not eligible for SAbR due to location of sites of progression or who were treated with conventionally fractionated or moderately hypofractionated radiation therapy were excluded. Risk stratification was based on International Metastatic RCC Database Consortium criteria.23 Biological effective dose was calculated with an α/β of 2.63 (Table E1).24 Patients received subsequent SAbR courses at the treatment team's discretion. Radiation simulation, planning, and dose constraints were previously described.20,25 Tyrosine kinase inhibitors (TKIs) were typically withheld during the administration of SAbR. The treating medical and radiation oncologists and a multidisciplinary team including urologists, radiologists, and pathologists made treatment decisions together.
Outcome evaluation
Patients were followed up with clinical examinations and imaging (computed tomography or magnetic resonance imaging [MRI]) every 2 to 5 months. Our primary endpoint was modified progression-free survival (mPFS), calculated from the start of SAbR to the start of a subsequent systemic therapy, death, or loss to follow-up, with censoring at last follow-up. PFS was calculated from the start of the ongoing systemic therapy (during which SAbR was administered) to the start of a subsequent line of therapy, death, or loss to follow-up, with censoring at last follow-up. Overall survival (OS) was calculated from the start of SAbR to death or loss to follow-up, with censoring at last follow-up. Toxicities were graded by the Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
We provide statistics as means, standard deviations, and ranges for continuous variables; and frequencies and percentages for categorical variables. Dosing parameters are summarized as medians and modes. Cox regression models for hazard ratios and log-rank tests for Kaplan-Meier curves determined differences in OS, PFS, and mPFS. All statistics were calculated using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient characteristics
Thirty-six patients met the criteria for oligoprogression with definitive SAbR administration. Table 1 shows patient and treatment characteristics. The median follow-up for all patients was 20.4 months (interquartile range, 10.9-29.4). Most patients (35 patients, 97%) had clear cell RCC, presented with localized disease (27 patients, 75%), and were in the favorable/intermediate risk categories per International Metastatic RCC Database Consortium criteria (32 patients, 89%). Most patients had >5 total metastatic sites at time of SAbR (23 patients, 64%), and most progressed in 1 (30 patients, 83%) or 2 (5 patients, 14%) sites. At time of SAbR, 16 patients (44%) were on frontline therapy, 15 (42%) were on second-line, and 5 (14%) were on third- through fifth-line systemic therapy. Pazopanib (39%) was the most common systemic therapy at time of SAbR. Most patients (30 patients, 83%) had only 1 metastasis treated. Five patients (14%) had 2 sites treated, and 1 patient had 3 sites treated with SAbR. Twenty-three patients were treated for existing lesions that had progressed; 12 were treated for new lesions; 1 patient was treated for both. Forty-three lesions were treated with SAbR with a median dose of 36 Gy (range, 18-50), a median of 12 Gy per fraction (range, 6-40), and a median of 3 fractions (range, 1-5). Of these lesions, the most common sites were bone (46.5%), lung (14%), liver (12%), and soft tissue (12%). After the initial SAbR course at oligoprogression, 11 patients received additional SAbR to 11 lesions before initiating a new line of systemic therapy.
Table 1.
Patient and treatment characteristics
| Frequency (%) (N = 36 patients) |
|
|---|---|
| Mean age ± SD (range) | 67.3 ± 8.9 (46-84) |
| Gender | |
| Female | 12 (33.3%) |
| Male | 24 (66.7%) |
| Ethnicity | |
| Hispanic | 5 (13.9%) |
| Non-Hispanic | 31 (86.1%) |
| Race | |
| Asian | 2 (5.6%) |
| White | 33 (91.7%) |
| Missing | 1 (2.8%) |
| Risk score (IMDC) | |
| 0, Favorable | 10 (27.8%) |
| 1-2, Intermediate | 22 (61.1%) |
| 3-6, Unfavorable | 2 (5.6%) |
| Missing | 2 (5.6%) |
| Mean primary tumor diameter, cm | 8.2 ± 3.4 |
| Histology | |
| RCC, NOS | 1 (2.8%) |
| ccRCC | 35 (97.2%) |
| Fuhrman grade | |
| 1 | 0 (0%) |
| 2 | 9 (25.0%) |
| 3 | 18 (50.0%) |
| 4 | 4 (11.1%) |
| Missing | 5 (13.9%) |
| Nephrectomy | |
| No | 3 (8.3%) |
| Yes | 33 (91.7%) |
| pT | |
| pT1 | 6 (16.7%) |
| pT2 | 4 (11.1%) |
| pT3 | 21 (58.3%) |
| Missing | 5 (13.9%) |
| pN | |
| pN0 | 9 (25.0%) |
| pN1 | 3 (8.3%) |
| pNX | 12 (33.3%) |
| Missing | 12 (33.3%) |
| M | |
| 0 | 27 (75.0%) |
| 1 | 9 (25.0%) |
| Number of lines of systemic therapy | |
| 1 | 16 (44.4%) |
| 2 | 15 (41.7%) |
| 3 | 4 (11.1%) |
| 4 | 0 (0%) |
| 5 | 1 (2.8%) |
| Systemic therapy on SAbR | |
| Axitinib | 3 (8.3%) |
| Bevacizumab | 1 (2.8%) |
| Cabozantinib + nivolumab | 1 (2.8%) |
| Everolimus | 1 (2.8%) |
| Nivolumab | 5 (13.9%) |
| Pazopanib | 14 (38.9%) |
| Sunitinib | 9 (25.0%) |
| Temsirolimus | 2 (5.6%) |
| Number of mets present at SAbR | |
| ≤5 metastases | 13 (36.1%) |
| >5 metastases | 23 (63.9%) |
| Number of SAbR mets treated initially | |
| 1 | 30 (83.3%) |
| 2 | 5 (13.9%) |
| 3 | 1 (2.8%) |
| Sites treated at first SAbR | (n = 43 lesions) |
| Adrenal | 2 (4.7%) |
| Bone | 20 (46.5%) |
| Kidney | 2 (4.7%) |
| Liver | 5 (11.6%) |
| Lymph node | 2 (4.7%) |
| Lung | 6 (14.0%) |
| Pancreas | 1 (2.3%) |
| Soft tissue | 5 (11.6%) |
| SAbR fractionation | |
| 1 fraction | 14 (32.6%) |
| 3 fractions | 13 (30.2%) |
| 5 fractions | 16 (37.2%) |
| Median/mode dose/fractionation (range) | |
| 1 fraction | 20.5/20 (20-40) |
| 3 fractions | 12/12 (6-15) |
| 5 fractions | 8/8 (6-10) |
Abbreviations: ccRCC = clear cell renal cell carcinoma; IMDC = International Metastatic RCC Database Consortium; mets = metastasis sites; NOS = not otherwise specified; pT = pathologic stage; RCC = renal cell carcinoma; SAbR = stereotactic ablative radiation therapy; SD = standard deviation.
SAbR's effect on systemic therapy duration
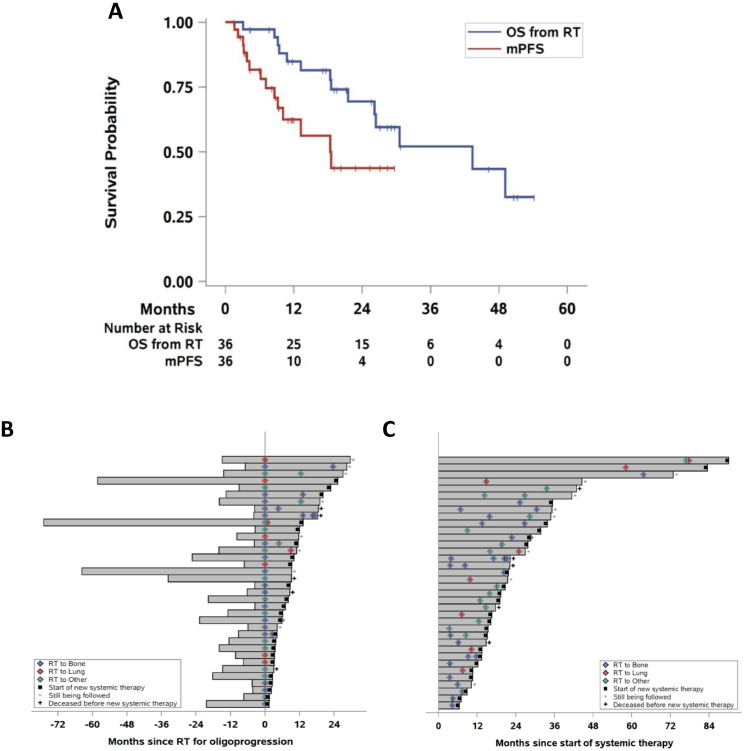
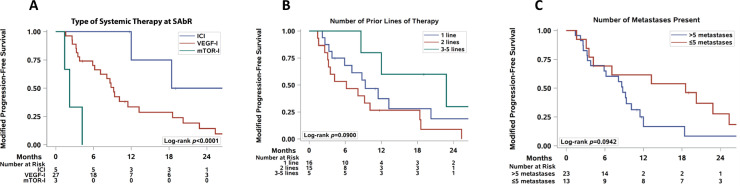
Figure 1 shows Kaplan-Meier curves (Fig 1A) of OS and mPFS for all patients and swimmer's plots (Fig 1B, 1C) demonstrating the temporal relationships among systemic therapies, SAbR, and follow-up for all 36 patients. The median time from starting the ongoing systemic therapy to SAbR was 11.4 months (interquartile range, 6.1-17.1). Median mPFS was 9.2 months (95% confidence interval [CI], 5.9-13.2). One- and 2-year mPFS for all patients were 36% (95% CI, 20.4-52.1) and 17% (95% CI, 5.8-32.8), respectively. Patients receiving immune checkpoint inhibitors (ICI) at the time of SAbR exhibited a longer median mPFS (not reached by 28.4 months, Cox P = .0017, log-rank P = .0001; 5 patients) than patients who received vascular endothelial growth factor inhibitors (9.2 months, 27 patients) or mechanistic target of rapamycin (mTOR) inhibitors (2.2 months, 3 patients; Table 2 and Fig 2A). The number of previous lines of therapy did not affect mPFS (Cox P = .11, log-rank P = .090, Table 2 and Fig 2B). Patients with 5 or fewer metastases at the time of SAbR had a longer mPFS than those with more than 5 (10.1 vs 8.4 months), but the difference was not statistically significant (Cox P = .099, log-rank P = .094, Table 2 and Fig. 2C). Other factors analyzed, including risk group, staging, number of metastases treated, and site of metastasis treated, did not affect mPFS upon univariate analysis.
Figure 1.
Survival and swimmer plots of patient outcomes. (A) Kaplan-Meier estimate of overall survival (OS) and modified progression-free survival (mPFS). (B) Swimmer plot of systemic therapy centered on time of first stereotactic ablative radiation therapy (SAbR). (C) Swimme plot from onset of the systemic therapy during which SAbR was delivered. Diamonds, SAbR-treated lesions with numbers representing treatment sites and colors referring to location. Black squares, start of new systemic therapy. Asterisks, follow-up ongoing. Plus sign (+), patients deceased before new line of systemic therapy. RT, radiation therapy is SAbR.
Table 2.
Univariate mPFS analysis
| Median mPFS | 1-year mPFS (95% CI) | HR 95% CI | Cox P | |
|---|---|---|---|---|
| Risk group | ||||
| Favorable | 9.2 (1.4-18.4) | 45.7% (14.3-73.0) | Reference | .40 |
| Intermediate | 6.6 (3.2-11.5) | 20.5% (6.6-39.6) | 1.29 (0.54, 3.12) | |
| Unfavorable | 22.8* | 100% | 0.35 (0.04, 2.88) | |
| Number of prior lines | ||||
| 1 line | 9.3 (3.8-20.2) | 37.4% (13.4-61.8) | Reference | .11 |
| 2 lines | 6.2 (2.6-10.1) | 26.7% (8.3-49.6) | 1.74 (0.79, 3.85) | |
| 3-5 lines | 22.8 (8.6-*) | 60.0% (12.6-88.2) | 0.50 (0.14, 1.82) | |
| Type of systemic tx before RT† | ||||
| Immune checkpoint inhibitor | Not reached | 75.0% (12.8-96.1) | Reference | .0017 |
| VEGF inhibitor | 9.2 (5.9-13.2) | 33.5% (16.1-51.9) | 3.58 (0.84, 15.3) | |
| mTOR inhibitor | 2.2 (1.4-4.2) | 0% | 29.73 (4.27-207.2) | |
| Type of systemic tx on RT† | ||||
| Immune checkpoint inhibitor | Not reached | 75.0% (12.8-96.1) | Reference | .068 |
| VEGF or mTOR inhibitor | 8.6 (4.2-11.5) | 30.1% (14.5-47.5) | 3.84 (0.90, 16.36) | |
| pT | ||||
| pT1 | 5.9 (1.6-18.4) | 44.4% (6.6-78.5) | Reference | .26 |
| pT2 | Not reached | 75.0% (12.8-96.1) | 0.16 (0.02, 1.43) | |
| pT3 | 8.8 (3.5-11.5) | 22.9% (7.8-42.5) | 0.77 (0.28, 2.12) | |
| M | ||||
| M0 | 11.5 (3.5-18.6) | 41.6% (22.4-59.8) | Reference | .79 |
| M1 | 8.8 (3.2-*) | 22.2% (3.4-51.3) | 1.13 (0.47, 2.68) | |
| Number of mets at RT | ||||
| ≤5 | 18.6 (3.5-25.3) | 61.5% (30.8-81.8) | Reference | .099 |
| >5 | 8.8 (3.8-11.5) | 16.7% (3.6-38.2) | 2.02 (0.88, 4.67) | |
| Number of mets treated | ||||
| 1 | 10.1 (5.9-18.4) | 40.0% (21.9-57.6) | Reference | .54 |
| 2-3 | 8.4 (1.6-*) | 16.7% (0.8-51.7) | 1.36 (0.51, 3.61) | |
| Site of metastasis, bone | ||||
| Bone | 10.1 (4.2-18.6) | 36.4% (14.1-59.4) | 1.03 (0.49, 2.19) | .93 |
| Non-bone | 9.2 (3.3-22.8) | 35.1% (14.6-56.6) | Reference | |
| Site of metastasis, lung | ||||
| Lung | 17.3 (3.2-*) | 50.0% (11.1-80.4) | Reference | .35 |
| Non-lung | 8.8 (5.9-13.2) | 33.4% (16.8-50.9) | 1.67 (0.57, 4.88) |
Abbreviations: CI = confidence interval; HR = hazard ratio; mets = metastasis sites; mPFS = modified progression-free survival; mTOR = mechanistic target of rapamycin; pT = pathologic stage; RT = radiation therapy; tx = therapy; VEGF = vascular endothelial growth factor.
Limit unable to be estimated.
The single patient receiving combination therapy was excluded.
Figure 2.
Subset survival analysis of oligoprogressive patients. (A) Kaplan-Meier estimate of modified progression-free survival (mPFS) based on type of systemic therapy during stereotactic ablative radiation therapy (SAbR), including immune checkpoint inhibitors (ICIs), vascular endothelial growth factor (VEGF) inhibitors (VEGF-I), and mechanistic target of rapamycin inhibitors (mTOR-I). (B) Kaplan-Meier estimate of mPFS based on number of prior lines of systemic therapy. (C) Kaplan-Meier estimate of mPFS according to number of metastases present at time of radiation.
SAbR's effect on PFS and OS
Median PFS (from the start of systemic therapy where SAbR was given) was 21.7 months (95% CI, 16.3-31.8). One- and 2-year PFS for all patients were 86% (95% CI, 69.4-93.9) and 43% (95% CI, 25.9-58.1), respectively. No factors predicted better PFS upon univariate analysis (Table 3). Median OS was 43.4 months (95% CI, 21.5-not reached). One- and 2-year OS for all patients were 85% (95% CI, 67.3-93.4) and 69% (95% CI, 48.8-83.1), respectively.
Table 3.
Univariate PFS analysis
| Median PFS | 1-year PFS (95% CI) | HR 95% CI | Cox P | |
|---|---|---|---|---|
| Risk group | ||||
| Favorable | 27.8 (14.8-90.2) | 100% | Reference | .22 |
| Intermediate | 16.4 (12.3-22.2) | 77.2% (53.7-89.8) | 2.10 (0.84, 5.30) | |
| Unfavorable | 31.8* | 100% | 0.82 (0.10, 6.88) | |
| Number of prior lines | ||||
| 1 line | 20.8 (13.5-90.2) | 87.1% (57.3-96.6) | Reference | .47 |
| 2 lines | 21.7 (10.7-27.8) | 80.0% (50.0-93.1) | 1.54 (0.68, 3.49) | |
| 3-5 lines | 31.8 (14.8-*) | 100% | 0.86 (0.23, 3.17) | |
| Type of systemic tx before RT† | ||||
| Immune checkpoint inhibitor | Not reached | 100% | Reference | .082 |
| VEGF inhibitor | 22.2 (15.2-33.8) | 84.9% (64.5-94.0) | 2.08 (0.48, 8.95) | |
| mTOR inhibitor | 13.4 (6.5-21.7) | 66.7% (5.4-94.5) | 6.98 (1.14, 42.91) | |
| Type of systemic tx on RT† | ||||
| Immune checkpoint inhibitor | Not reached | 100% | Reference | .26 |
| VEGF or mTOR inhibitor | 20.8 (14.8-31.8) | 83.1% (64.0-92.6) | 2.30 (0.54, 9.82 | |
| pT | ||||
| pT1 | 22.3 (8.9-90.2) | 83.3% (27.3-97.5) | Reference | .21 |
| pT2 | Not reached | 100% | 0.28 (0.03, 2.47) | |
| pT3 | 17.8 (13.4-27.8) | 81.0% (56.9-92.4) | 1.55 (0.52, 4.62) | |
| M | ||||
| M0 | 22.3 (15.4-33.8) | 85.0% (64.9-94.1) | Reference | .46 |
| M1 | 16.6 (10.7-35.4) | 88.9% (43.3-98.4) | 1.39 (0.58, 3.33) | |
| Number of mets at RT | ||||
| ≤5 | 31.8 (13.4-90.2) | 84.6% (51.2-95.9) | Reference | .15 |
| >5 | 20.8 (14.8-27.8) | 86.7% (64.3-95.5) | 1.87 (0.80, 4.35) | |
| Number of mets treated | ||||
| 1 | 22.2 (16.6-33.8) | 89.8% (71.5-96.6) | Reference | .24 |
| 2-3 | 13.6 (7.1-*) | 66.7% (19.5-90.4) | 1.80 (0.67, 4.80) | |
| Site of metastasis, bone | ||||
| Bone | 22.2 (12.3-33.8) | 81.9% (53.8-93.8) | 1.36 (0.63, 2.96) | .44 |
| Non-bone | 20.8 (16.3-42.8) | 89.5% (64.1-97.3) | Reference | |
| Site of metastasis, lung | ||||
| Lung | 50.0 (10.7-83.5) | 83.3% (27.3-97.5) | Reference | .59 |
| Non-lung | 21.7 (15.4-31.8) | 86.5% (68.0-94.7) | 1.34 (0.45, 3.97) |
Abbreviations: CI = confidence interval; HR = hazard ratio; mets = metastasis sites; mTOR = mechanistic target of rapamycin; VEGF = vascular endothelial growth factor; PFS = progression-free survival; pT = pathologic stage; RT = radiation therapy; tx = therapy.
Limit unable to be estimated.
The single patient receiving combination therapy was excluded.
Patients who switched systemic therapy within 3 months of receiving SAbR
Five patients switched systemic therapy within 3 months of receiving radiation to their progressing site(s). Two of these patients had SAbR to 2 sites of progression. Three of these patients had at least one bony metastasis treated. The median time on current systemic therapy (from initiation to SAbR) for these patients was 7.5 months, which is significantly shorter than the 11.4 months for the entire cohort. All switches in systemic therapy were prompted by progressive disease that was previously stable or newly found metastasis on interim scans.
Local control and toxicity
Three of 43 lesions treated had local failure, all within 1 year, and 2 additional sites could not be assessed due to lack of follow-up imaging after SAbR. Of the 3 failures, 1 was a lung lesion treated with 36 Gy in 3 fractions, the second was a rib lesion treated with 20 Gy in 1 fraction, and the third was a liver lesion treated with 42 Gy in 3 fractions. Overall, the 1-year local control rate was 93% (95% CI, 78.7-97.5). Of 36 patients treated, 13 (36%) had documented toxicity related to SAbR and/or systemic therapy. Six of these were acute grade 1 toxicities, including myositis, pneumonitis, fatigue, diarrhea, nausea, and vomiting (Table 4). Seven patients experienced late toxicity from radiation, including grade 1 to 2 radiation-induced neuropathic pain, grade 2 bone toxicity, radiation-induced myositis, grade 2 pneumonitis, and grade 2 gastric ulcer. There were no grade 3 or 4 toxicities.
Table 4.
Treatment-related toxicity
| Grade 1-2 | Grade 3-4 | Grade 5 | |
|---|---|---|---|
| Acute | |||
| Myositis | 1 | - | - |
| Pneumonitis | 1 | - | - |
| Fatigue | 2 | - | - |
| Nausea | 3 | - | - |
| Diarrhea | 1 | - | - |
| Vomiting | 1 | - | - |
| Late | |||
| Myositis | 1 | - | - |
| Pneumonitis | 2 | - | - |
| Neuropathy | 2 | - | - |
| Bone | 1 | - | - |
| Gastric ulcers | 1 | - | - |
| Hemoptysis | - | - | 1* |
SAbR contribution suspected, but uncertain.
There were 5 deaths in this cohort. One patient died of hemoptysis 8 months after receiving SAbR to the lung hilum. Initially, he was on second-line therapy with nivolumab. After SAbR, he developed a chronic cough, which was attributed to post-radiation pneumonitis. Eight months after SAbR, he was admitted to an outside hospital with presumptive community-acquired pneumonia, developed hemoptysis after discharge, opted for hospice care, and died within a few days. The contribution of the pneumonia, radiation therapy, and possibly disease progression to the grade 5 hemoptysis and death is unclear. Four additional patients died after SAbR and before starting a new systemic therapy. One patient had deteriorating health while on axitinib as third-line systemic therapy and opted for palliative care/hospice 8 months after SAbR. Another patient was admitted with multiple large brain metastases 2 months after receiving SAbR to an ischio-anal mass and died shortly after discharge while on third-line therapy. Another patient on second-line therapy developed end-stage renal disease, went on dialysis, and was lost to follow-up. Finally, one patient was on nivolumab for 9 months as second-line therapy after SAbR, but it was withheld due to concerns for optic neuritis. She remained off therapy for approximately a year but was admitted with disease progression and altered mentation and was discharged to hospice care; she died shortly thereafter from complications of progressing disease.
Discussion
mRCC remains largely incurable, and as RCC progresses, systemic therapy is re-evaluated and changed as necessary. Widespread disease progression, especially soon after initiating systemic therapy, suggests innate resistance. In contrast, limited progression after a prolonged period on systemic therapy suggests focally acquired resistance. Using a local therapy like SAbR is an attractive option to eradicate the few progressing sites while patients remain on the same systemic therapy, particularly if the systemic therapy is active overall and well tolerated. Our study shows that, in patients with oligoprogressive mRCC, SAbR may increase the PFS from the time of radiation to the next systemic therapy by a median of 9.2 months.
SAbR is increasingly being used to treat mRCC beyond intracranial and bone metastases.20,21 Multiple studies have evaluated its use in various settings, including oligometastasis and oligoprogression.13, 14, 15, 16, 17, 18, 19, 20, 21,26, 27, 28 Although most of these studies are retrospective and limited by patient numbers, a heterogeneous patient population, and short follow-up, local control of irradiated lesions is typically 78% to 98% at 1-3 years, and grade 3 to 4 adverse events after SAbR are generally <5%. For example, one multi-institutional retrospective study by the Genitourinary Group reviewed 188 patients with mRCC who received SAbR to 252 sites, including a heterogeneous population of oligometastasis and oligoprogression, at central nervous system and non-central nervous system sites.14 They had 101 patients with oligoprogressive disease, but only 7 of them were treated with SAbR after partial response to systemic therapy. More recently, a meta-analysis of the safety and survival of patients with oligometastatic cancer treated with SAbR revealed 1.2% acute grade 3 to 5 toxicity, 1.7% late grade 3 to 5 toxicity, and 94.7% 1-year local control,29 consistent with our findings. Our study extends this body of literature by focusing on SAbR's effects in a select group of patients with RCC who demonstrated some response to systemic therapy with limited oligoprogressive disease, and it shows that SAbR can control the progressing sites while delaying changes in systemic therapy. For some patients, SAbR can also be considered as a longitudinal strategy, with several rounds of SAbR administered over time if only a few sites progress and the disease appears to remain sensitive to the ongoing systemic therapy. Using SAbR for longitudinal disease control is similar to what we reported recently for patients with oligometastatic RCC.21 A prospective phase II study investigating SAbR treatment of 37 patients with oligoprogressive RCC was recently reported, and revealed a 9.6 months mPFS, which is comparable to our findings (NCT02019576).30
One challenge to deploying SAbR for oligoprogression is identifying the patient population most likely to benefit. In our study, oligoprogressive patients treated with SAbR while on an ICI-containing regimen appeared to have better mPFS than those receiving either vascular endothelial growth factor or mTOR inhibitors. These patients may have received additional synergistic benefits from SAbR's antigen presenting properties and immune cell recruitment.31 Several trials are investigating the combination of ICI and radiation in kidney cancer (including NCT03065179, NCT02781506, and NCT03115801). In addition to therapy before SAbR, sites of metastatic disease may inform about the aggressiveness of the cancer.32, 33, 34 RCC commonly metastasizes to the lung, lymph nodes, bone, liver, and brain.35 Tumors that metastasize to the bone, liver, and brain have been shown to be associated with worse OS.35 In our study, we determined that patients with metastases to the bone or lung did not exhibit a different mPFS or PFS. However, this may be due to the limited number of patients in this study. RCC that metastasize to the pancreas may have a more indolent course,35,36 but with only one patient with a pancreatic lesion treated, we were unable to assess whether this patient population may benefit from SAbR to oligoprogressive disease.
SAbR was generally well tolerated, with 33% of patients experiencing grade 1 to 2 adverse events, one patient hospitalized for a gastric ulcer (no operative or endoscopic intervention), and one patient who developed fatal hemoptysis 8 months after lung SAbR, where SAbR's contribution was unclear. How SAbR should be optimally integrated with systemic therapy remains uncertain. Several studies retrospectively evaluated treatment-related toxicities in patients receiving TKI therapy and SAbR. One study that investigated toxicity rates in patients on TKI therapy undergoing SAbR to spinal metastases showed no grade 3 or greater toxicities.37 Another study showed that 4 of 56 patients receiving SAbR to oligoprogressive lesions while on TKI therapy experienced grade 3 toxicities, including radiation dermatitis, neuropathy, and anemia.26 Given these limited data, caution should be exercised when combining SAbR with TKI and mTOR inhibitors, particularly when radiosensitive structures are close to the targeted lesion. In such instances, holding systemic therapy is reasonable. Holding ICI during SAbR is unlikely to affect toxicity given the long half-life of antibodies. Concurrent administration of SAbR and ICI may be safe, as reported previously.38, 39, 40
Our study has several limitations. First, it is a retrospective study from a single institution, and it involves a cohort of highly selected patients. Patient selection for this report was based on specific, objective criteria (including number of progressive sites, SAbR treatment to all, and continuation of existing systemic therapy) and did not necessarily include provider intent to use SAbR for oligoprogression. This merits consideration, as nearly 50% of the SAbR-treated lesions were in bone, where SAbR is standard of care. Second, the absence of a control group precludes determining SAbR's specific contribution to extending systemic therapy . Third, although it makes sense that extending the duration of systemic therapy and overall disease control should benefit patients, this lacks formal evaluation. Fourth, some of the patients may have had more indolent cancer given prolonged disease control before SAbR, which may have led to longer PFS and/or mPFS in the cohort. Also, our patient population was treated between 2007 and 2017, during which time immunotherapy became approved in the front line. Lastly, the median follow-up of 20 months is still too short to assess the long-term control of SAbR-treated metastases.
Conclusions
Select patients with oligoprogressive mRCC may benefit from receiving SAbR to progressing sites, which may increase the duration of the ongoing systemic therapy while preserving other therapies for the future . Patients who are tolerating systemic therapy well, with control in most sites and limited progression amenable to SAbR, may benefit from this approach. Prospective validation better delineating the patient population benefiting from this approach is warranted. There are multiple clinical trials evaluating whether SAbR increases mPFS in oligoprogressive patients (NCT03696277, NCT03693014).
Acknowledgments
We would like to thank Dr. Jonathan Feinberg for his help while preparing this manuscript.
Footnotes
Sources of support: J.B. and A.C. are funded by P50CA196516. R.H. is funded by American Cancer SocietyRSG-16-004-01-CCE.
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.adro.2021.100692.
Contributor Information
James Brugarolas, Email: Brugarolas@UTSouthwestern.edu.
Raquibul Hannan, Email: Raquibul.Hannan@utsouthwestern.edu.
Appendix. Supplementary materials
References
- 1.Noone AM HN, Krapcho M, Miller D. National Cancer Institute; Bethesda, MD: April 2018. SEER Cancer StatisticsReview, 1975-2015.https://seer.cancer.gov/csr/1975_2015/ Available at: Accessed May 5, 2021. [Google Scholar]
- 2.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. New Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Plimack ER, Stus V. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, Tomczak P. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 6.Courtney KD, Ma Y, Diaz de Leon A. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin Cancer Res. 2020;26:793–803. doi: 10.1158/1078-0432.CCR-19-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Q, Christie A, Rajaram S. Ontological analyses reveal clinically-significant clear cell renal cell carcinoma subtypes with convergent evolutionary trajectories into an aggressive type. EBioMedicine. 2020;51 doi: 10.1016/j.ebiom.2019.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlinger M, Rowan AJ, Horswell S. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turajlic S, Xu H, Litchfield K. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell. 2018;173 doi: 10.1016/j.cell.2018.03.057. 581-594 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turajlic S, Xu H, Litchfield K. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell. 2018;173 doi: 10.1016/j.cell.2018.03.043. 595-610 e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell TJ, Turajlic S, Rowan A. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx renal. Cell. 2018;173 doi: 10.1016/j.cell.2018.02.020. 611-623 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzese C, Franceschini D, Di Brina L. Role of stereotactic body radiation therapy in the management of oligometastatic renal cell carcinoma. J Urol. 2018;201:70–76. doi: 10.1016/j.juro.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Meyer E, Pasquier D, Bernadou G. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: A study of the Getug group. Eur J Cancer. 2018;98:38–47. doi: 10.1016/j.ejca.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Stenman M, Sinclair G, Paavola P, Wersall P, Harmenberg U, Lindskog M. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005-2014. Radiother Oncol. 2018;127:501–506. doi: 10.1016/j.radonc.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Wersall PJ, Blomgren H, Lax I. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88–95. doi: 10.1016/j.radonc.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Zelefsky MJ, Greco C, Motzer R. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744–1748. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svedman C, Sandstrom P, Pisa P. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 19.Kothari G, Foroudi F, Gill S, Corcoran NM, Siva S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol. 2015;54:148–157. doi: 10.3109/0284186X.2014.939298. [DOI] [PubMed] [Google Scholar]
- 20.Wang CJ, Christie A, Lin MH. Safety and efficacy of stereotactic ablative radiation therapy for renal cell carcinoma extracranial metastases. Int J Radiat Oncol Biol Phys. 2017;98:91–100. doi: 10.1016/j.ijrobp.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Schoenhals J, Christie A. Stereotactic ablative radiation therapy (SABR) used to defer systemic therapy in oligometastatic renal cell cancer. Int J Radiat Oncol Biol Phys. 2019;105:367–375. doi: 10.1016/j.ijrobp.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuch B, La Rochelle JC, Klatte T. Brain metastasis from renal cell carcinoma: Presentation, recurrence, and survival. Cancer. 2008;113:1641–1648. doi: 10.1002/cncr.23769. [DOI] [PubMed] [Google Scholar]
- 23.Heng DY, Xie W, Regan MM. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning S, Trisler K, Wessels BW, Knox SJ. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997;80(12 Suppl):2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Kim DWN, Medin PM, Timmerman RD. Emphasis on repair, not just avoidance of injury, facilitates prudent stereotactic ablative radiotherapy. Semin Radiat Oncol. 2017;27:378–392. doi: 10.1016/j.semradonc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 26.He L, Liu Y, Han H. Survival outcomes after adding stereotactic body radiotherapy to metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. Am J Clin Oncol. 2020;43:58–63. doi: 10.1097/COC.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaorsky NG, Lehrer EJ, Kothari G, Louie AV, Siva S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis of 28 studies. Eur Urol Oncol. 2019;2:515–523. doi: 10.1016/j.euo.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Correa RJM, Louie AV, Zaorsky NG. The emerging role of stereotactic ablative radiotherapy for primary renal cell carcinoma: A systematic review and meta-analysis. Eur Urol Focus. 2019;5:958–969. doi: 10.1016/j.euf.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer EJ, Singh R, Wang M. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: A systematic review and meta-analysis. JAMA Oncol. 2021;7:92–106. doi: 10.1001/jamaoncol.2020.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung P. ASCO Virtual Scientific Program; 2020. A phase II multicenter study of stereotactic radiotherapy (SRT) for oligoprogression in metastatic renal cell cancer (mRCC) patients receiving tyrosine kinase inhibitor (TKI) therapy. [DOI] [PubMed] [Google Scholar]
- 31.Marciscano AE, Haimovitz-Friedman A, Lee P. Immunomodulatory effects of stereotactic body radiation therapy (SBRT): Preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. 2019;110:35–52. doi: 10.1016/j.ijrobp.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 32.Ryan C, Stoltzfus KC, Horn S. Epidemiology of bone metastases. Bone. 2020 doi: 10.1016/j.bone.2020.115783. [DOI] [PubMed] [Google Scholar]
- 33.Horn SR, Stoltzfus KC, Lehrer EJ. Epidemiology of liver metastases. Cancer Epidemiol. 2020;67 doi: 10.1016/j.canep.2020.101760. [DOI] [PubMed] [Google Scholar]
- 34.Singh R, Stoltzfus KC, Chen H. Epidemiology of synchronous brain metastases. Neurooncol Adv. 2020;2:vdaa041. doi: 10.1093/noajnl/vdaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudani S, de Velasco G, Wells JC. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singla N, Xie Z, Zhang Z. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight. 2020;5:e134564. doi: 10.1172/jci.insight.134564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JA, Balagamwala EH, Angelov L. Spine stereotactic radiosurgery with concurrent tyrosine kinase inhibitors for metastatic renal cell carcinoma. J Neurosurg Spine. 2016;25:766–774. doi: 10.3171/2016.4.SPINE16229. [DOI] [PubMed] [Google Scholar]
- 38.Mohamad O, Diaz de Leon A, Schroeder S. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1440168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang C, Welsh JW, de Groot P. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral t cells. Clin Cancer Res. 2017;23:1388–1396. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sha CM, Lehrer EJ, Hwang C. Toxicity in combination immune checkpoint inhibitor and radiation therapy: A systematic review and meta-analysis. Radiother Oncol. 2020;151:141–148. doi: 10.1016/j.radonc.2020.07.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.