Abstract
Objectives
The present study aims to evaluate the healing effect of a new topical paste formulation on mucosal wounds.
Methods
This study was conducted in 40 adult male rabbits. The animals were divided into two groups: a control group containing rabbits that were treated with only Orabase paste (comprising olive oil and beeswax) and an experimental group that included rabbits that were treated with Orabase paste containing hyaluronic acid, rosemary extract, and metronidazole. Each of these groups was randomly divided into four groups according to the observational period (post-treatment days 1, 3, 7, and 15). Further, biopsy samples for histological examination were obtained from the animals’ oral mucosal defects.
Results
We found that the new formulation (Orabase paste containing hyaluronic acid, rosemary extract, and metronidazole) had a stronger healing effect. The inflammation and re-epithelization scores at 3–7 days for the experimental group appeared superior in which (P-values were 0.038 and 0.034 for inflammation and 0.046 and 0.025 for re-epithelization) respectively. Furthermore, the re-epithelization score on the 15th day for the new formulation was significant (P ≤ 0.05), with the inflammatory response being milder.
Conclusions
Topical application of new paste formulation provided good therapeutic results. The new formulation was effective in resolving inflammation during specific phases of mucosal healing and significantly advanced wound healing at different stages. It also afforded a better healing response in terms of reduced wound contraction.
Keywords: Hyaluronic acid, Metronidazole, Orabase formula, Rosemary extract, Wound healing
الملخص
أهداف البحث
تهدف الدراسة الحالية إلى تقييم التأثير العلاجي لتركيبة معجون موضعي جديد على جروح الغشاء المخاطي.
طرق البحث
أجريت هذه الدراسة على ٤٠ أرنبا ذكرا بالغا، وتم تقسيمهم إلى مجموعتين: مجموعة ضابطة عولجت بمعجون أورابيز (يتكون من زيت الزيتون وشمع العسل) فقط ومجموعة البحث التي تحتوي على المواد الفاعلة المضافة إلى معجون الأورابيز ذي الصيغة الجديدة التي تم استحداثها بإضافة مستحضرات من المواد العشبية (خلاصة إكليل الجبل وحامض الهايلورونيك وعقار الميترونيدازول). كما تم تقسيم كل مجموعة بشكل عشوائي إلى أربع مجموعات فرعية مقسمة حسب المدة الزمنية لملاحظة التئام الجروح التي تمت متابعتها (اليوم الأول، والثالث، والسابع، واليوم الخامس عشر بعد الجراحة). تمت التضحية بالحيوانات في فترات الشفاء المحددة والحصول على الخزعات لفحصها نسيجيا من عيوب الغشاء المخاطي للفم لجميع الأرانب في جميع المجموعات.
النتائج
أظهرت النتائج قوة شفاء أكثر للعامل العلاجي الجديد. حيث فاقت المجموعة التجريبية بالنسبة لدرجة الالتهاب وإعادة التكوير الظهاري في اليوم الثالث والسابع على التوالي. بينما في اليوم الخامس عشر، كان هناك فرق معنوي للمجموعة المعالجة بالمعجون المستحدث من حيث إعادة التجلد مع استجابة التهابية أقل إذا ما قورنت بالمجموعة الضابطة.
الاستنتاجات
كان للمعجون الجديد أثر كبير في إيقاف الالتهاب خلال فترات محددة من التئام الغشاء المخاطي مع تقدم فاعل في التئام الجروح في مراحل مختلفة، وبالرغم من ذلك فإنه من الممكن أن يحقق استجابة شفاء أفضل عن طريق التقليل من تقلص الجرح.
الكلمات المفتاحية: حمض الهايلورونيك, عقار الفلاجيل, صيغة القاعدة الاساسية للمركب, مستخلص اكليل الجبل, التئام الجروح
Introduction
Functional, active wound dressings can establish a moist environment for wounds, protect against secondary infections, eliminate wound exudation, promote tissue regeneration, as well as increase wound healing capacity. Hyaluronic acid (HA) it has been demonstrated to take apart in proving tissue regeneration and healing process.1 HA or hyaluronan is a substance which naturally present in the human body. It is one of the essential elements of the extracellular tissue matrix of connective tissue, synovial fluid, and many other tissues.2 It existence throughout all mammals suggests that HA is a biomolecule of considerable importance.3 Naturally, hyaluronic acid is a polysaccharide macromolecule (straight-chain glycosaminoglycan), from a biochemical viewpoint.4 Many studies showed that HA may enhance wound repair by reserve the damaged tissue through its anti-inflammatory properties.3, 4, 5 Moreover, HA is currently available in the market in gel or liquid form at different concentrations and under the commercial name Hyadent® for application in dentistry for treating dental diseases such as gingivitis and periodontitis.6
HA is typically present in the bloodstream at low concentrations. Nevertheless, HA levels are rising increasingly, which occurred via platelets and even endothelial cells injured. At high altitudes, HA around initial injury sites is degraded in a series of catalytic steps modulated by hyaluronidases and reactive oxygen species (ROS). Owing to the heterogeneity of HA, HA polymers are unique, with mostly size-dependent functions.7 The wound-healing process restores the integrity of damaged tissue and its normal functioning. Free radicals develop at the injury site and slow the wound-healing process gradually. Antioxidant therapy can aid in the wound-healing process. Rosemary extract has numerous antioxidant components.8 Natural antimicrobial agents derived from plant essential oils can be used to counter bacterial growth and facilitate wound healing.9 One of the major herbs with several medical benefits is rosemary (Rosmarinus officinalis L.). It main contains 1,8-cineole, camphor, α-pinene, and α-terpineol, which are antimicrobial in nature. The antioxidant properties of rosemary are attributable to several components. Three phenolic diterpenes, including carnosic acid, carnosol, and rosmarinic acid, are principally responsible for the antioxidant effects of rosemary. The antioxidant and antimicrobial properties of rosemary appear to be beneficial for wound healing.10,11 Metronidazole is a synthetic composite derivative of the antibiotic nitroimidazole, which inhibits cell death due to bacterial DNA synthesis.12 It has been established as a medication that may be used for the management of infection with anaerobic microorganisms and recommended for systemic or local administration in support of conventional periodontal therapy.13
Therefore, we hypothesised that the application of HA combined with rosemary extract and pure metronidazole in a new paste formulation could improve wound healing. No similar evidence from in vivo or clinical studies on the same components is available in the literature. Thus, the aim of the present study is to investigate wound healing of mucosal soft tissue in a rabbit model treated with a topical mixture of HA gel (Hyadent®), rosemary (Rosmarinus officinalis L.) oil, and metronidazole powder as a new paste formulation, by evaluating histological parameters on post-treatment days 1, 3, 7, and 15.
Materials and Methods
Experimental section
Preparation of the base material
A new paste formulation was prepared by the investigators to test its efficacy against oral mucosal lesions. The experimental oral topical paste consisted of beeswax (from NDI) and unpurified olive oil (obtained from the local market) the exact product of control Orabase paste. In order to eliminate any bias in result the experimental product, HA gel (Hyadent®, BioScience GmbH, Dümmer, Germany), rosemary (Rosmarinus officinalis L.) oil, and pure metronidazole powder (Flagyl) (State Company for Drug Industries and Medical Appliances [SDI], Samarra, Iraq) were added to Orabase paste.
Preparation of the rosemary extract
Dried leaves of rosemary, from Morocco, were purchased from Alrayan Medical Herbal Centre (Erbil, Iraq), with vegetal authentication as Rosmarinus officinalis L. To obtain the oil extract, the dried leaves were washed with water and subjected to hydrodistillation for 3 h in Clevenger apparatus. Five grams of the oil extract was poured into a sterile container, which was closed and stored at −10 °C until use.14,15
Constituents and preparation of the Orabase paste
To prepare 100 g of the control product, Orabase paste (comprising 1 g of carboxymethylcellulose, 10 g of pectin, 0.18 g of methylparaben, and 0.02 g of propylparaben) was mixed with 12 g of beeswax and 77.8 g of unpurified olive oil. Beeswax was immersed in a water bath. Olive oil was weighed and then added to the melted wax while blending gently to achieve homogenisation. Thereafter, the powdered materials were mixed and added gradually to the mixture of beeswax and olive oil with continuous mixing, while the mixture was still immersed in the water bath until the paste became homogenised. Finally, this prepared mixture was poured into a sterile container and stored at room temperature until use within a few days after preparation.16
The experimental product was prepared immediately before its application. The active ingredients added to the Orabase paste consisted of 0.25 mL of HA (commercially available in a sterile 1.2-mL syringe as a hydrogel for dental use [Hyadent®, BioScience GmbH, Dümmer, Germany]), 25 μL of rosemary (R.osmarinus officinalis L.) extract oil, and 0.04 g of pure metronidazole powder (SDI). The ingredients were mixed well to obtain a homogenised paste 5 min before the application of the experimental product. The concentrations of the chosen materials were established based on the results of previous in vitro studies (not displayed).
Experimental model and housing
Forty male adult mixed-breed rabbits were used. The animals were purchased from a local supplier and weighed 1.5 ± 0.5 kg on average. They were divided into the following two groups of 20 animals each according to the product applied: a control group, which was treated with only the Orabase paste, and the experimental group, which was treated with the new paste formulation. Each group was further randomly divided into four subgroups, with five animals in each subgroup, based on the observation period (1, 3, 7, and 15 post-treatment days). The rabbits were housed in an animal house at a constant temperature of 24 °C under a 12-h light–-dark cycle with good ventilation; they were provided fresh vegetables. They were monitored by a veterinarian throughout the study period and were in good health. The animals were randomly selected, and intramuscular doses of 4 mg/kg ketamine hydrochloride in 50 mg/mL (Gracure Pharmaceuticals Ltd., Bhiwadi, India) and xylazine base 5 mg/kg in 20 mg/mL (Interchemi Co, Holland) were administered in the thigh muscle of each animal. To determine effective anaesthesia, the loss of ear pinch reflex was tested after 10–15 min.
Treatment procedure
Once anaesthesia was induced, the animal was placed in a prone position. To induce a typical oral mucosal wound (approximately 0.8 cm), the tissue was punched with a locally made device. The wound was created by pushing the device against the disinfected oral mucosal area on the right cheek (Figure 1). Both the control product (0.5 g of plain Orabase/excision wound for the control group) and the experimental product (0.5 g of plain Orabase + 0.25 mL of Hyadent + 25 μL of rosemary oil + 0.04 gm of metronidazole powder for the experimental group) were applied topically to the wound once a day for three continuous days. On recovery from anaesthesia, the treated animals were caged separately. During the first 24 h after the procedure, the animals were closely observed, particularly with regard to feeding and physical activity. Within 3–4 h of the treatment procedure, all animals started their ordinary activities. At the specified healing times, the animals were sacrificed, and biopsy samples were obtained from all rabbits' oral defects. The samples were fixed in freshly prepared formalin (at a concentration of 10%) for 48 h. The samples were sectioned and stained with haematoxylin and eosin to examine the histology of the wound area.
Figure 1.
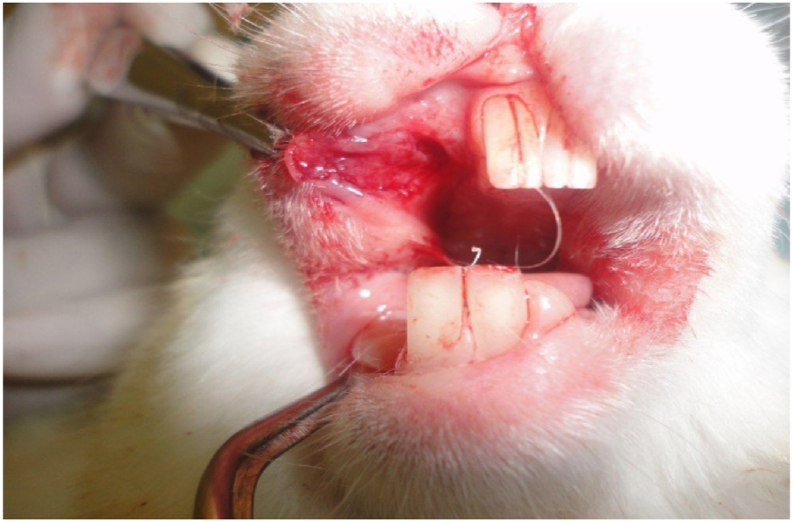
0.8cm round oral mucosal defect.
Specimen collection and scoring
As mentioned previously, the animals in each group were divided into the following four subgroups of five rabbits each depending on the time of sacrifice.
-
•
G1: Animals were sacrificed for specimen collection 1 day after the treatment procedure.
-
•
G2: Animals were sacrificed for specimen collection 3 days after the treatment procedure.
-
•
G3: Animals were sacrificed for specimen collection 7 days after the treatment procedure.
-
•
G4: Animals were sacrificed for specimen collection 15 days after the treatment procedure.
Inflammation scoring
Inflammation and repair were evaluated as follows as described previously.17,18
Score 1: Presence of acute inflammation.
Score 2: Predominance of granulation tissue.
Score 3: Predominance of chronic inflammation (fibroblasts began to proliferate).
Score 4: Resolution of inflammation and cicatrisation (decrease or absence of chronic inflammation).
Re-epithelization scoring
The scoring was performed as follows.
Score 0: Re-epithelization at the edge of the wound.
Score 1: Re-epithelization over less than half of the wound area.
Score 2: Re-epithelization over more than half of the wound area.
Score 3: Re-epithelization of irregular thickness over the entire wound area.
Score 4: Re-epithelization of normal thickness over the entire wound area.
Statistical analysis
All data were statistically analysed using SPSS 19.0 software package. Regarding statistical variances, mean values between the groups were compared using the paired Wilcoxon signed-rank test. Statistical differences among all periods of healing were investigated using the Kruskal–Wallis test. The exact time at which the study product exerted its effect was determined using the Mann–Whitney test. A P-value of up to 0.05 was considered to indicate significance.
Results
The results of the statistical analysis of the differences between the experimental and control groups regarding inflammation and re-epithelization at specific healing time points are described below. Regarding the study product, its maximum effect was noted on the third day and continued up to 7 days, after which it decreased until day 15 (Figure 2, Figure 3, Figure 4). The paired Wilcoxon signed-rank test showed variable discrepancies at all the healing time points. The inflammation and re-epithelization scores on days 3 and 7 appeared superior for experimental group in which (P-values were 0.038 and 0.034 for inflammation and 0.046 and 0.025 for re-epithelization) respectively, while in 15 days’ period histopathological score appeared significant regarding re-epithelization (0.046) with an exception for inflammation in which P-value was (0.157) as shown in Table 1. As shown in Table 2 and Figure 5, Figure 6, the effect of the study product was noted at all healing time points. Inflammatory cells started to appear in the first 3 days and continued to be recruited until day 7. Monocytes started to appear on day 3 and then matured to macrophages. On the other hand, fibroblasts started to form new granulation tissue and thus new epithelium as shown in Table 3.
Figure 2.
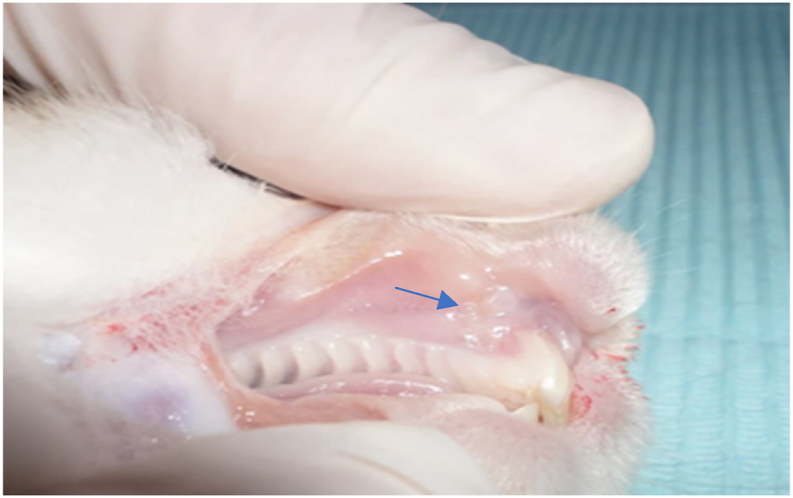
Complete healing of mucosa at 15 days in study experimental group.
Figure 3.
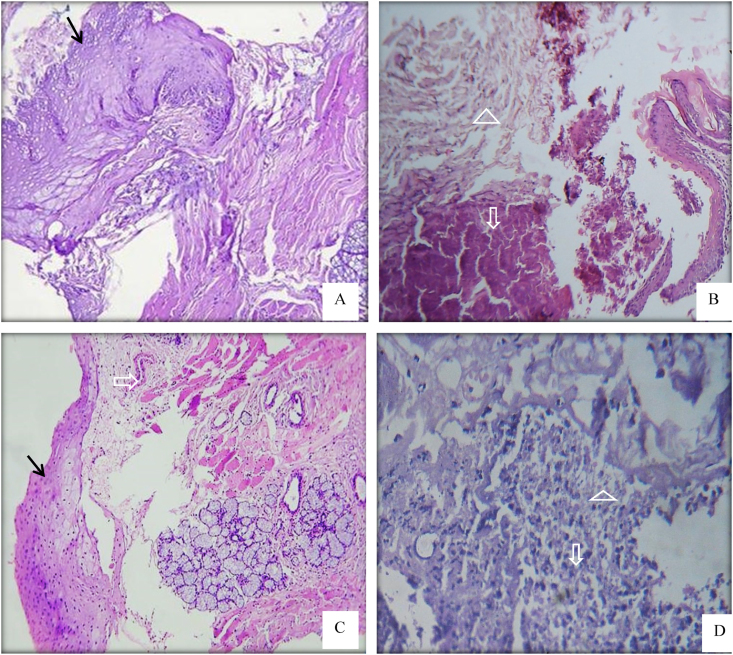
A Histopathological section of oral mucosal wound at day 1. A,B; for control group showed thickening of mucosal tissue at the edges of the wound with numerous blood cells filled the mucosal defect. C,D; sections of treated group with neo-orabase paste demonstrated thickening of the mucosa next to the wound edge and the oral mucosal epithelium extended into the wound cavity with fibrin strands and numerous inflammatory cells were seen in the submucosal wound. The mucosal layer indicated by solid arrow, the inflammatory and blood cells by open arrow, the fibrin strands by open triangle. H& E, ×100 & ×400.
Figure 4.
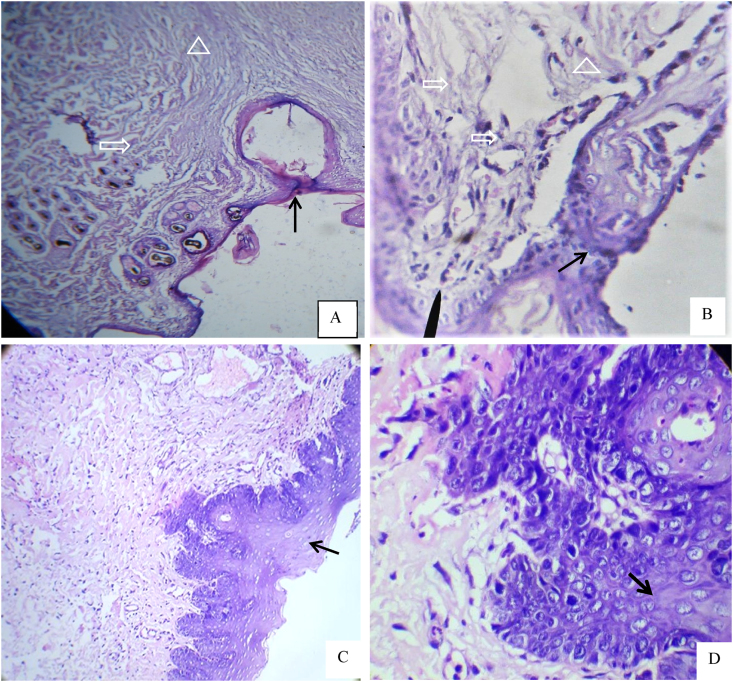
A Histological section of oral mucosal wound at day 3. A,B; for control group showed thin mucosa covered the wound with dense irregular arrangement of granulation tissue. C,D; represented treated group showed complete re-epithelialization over the wound edge which consist of several cells thickness and the submucosal layer composed from well vascularized granulation tissue contains numerous blood vessels, fibroblast cells and collagen fiber with less inflammatory infiltrated cells. The mucosal layer indicated by solid arrow, the inflammatory and blood cells by open arrow, granulation tissue by open triangle. H& E, ×100 & ×400.
Table 1.
A histological readings between groups in the same periods of mucosal healing for inflammation and reepithelization done by Paired Wilcoxon signed-rank test.
| Healing parameters | P - value |
|||
|---|---|---|---|---|
| Healing period | ||||
| 1 day | 3 day | 7 days | 15 days | |
| Inflammation | 0.564 | 0.038 | 0.034 | 0.157 |
| Re-epithelization | 1.000 | 0.046 | 0.025 | 0.046 |
p ≤ 0.05.
Table 2.
Kruskal–Wallis Test for histopathological readings of study material among all healing periods of mucosal healing for inflammation and reepithelization.
| Healing parameters | P-values among all healing time |
|---|---|
| inflammation | 0.000 |
| Reepithelization | 0.001 |
Figure 5.
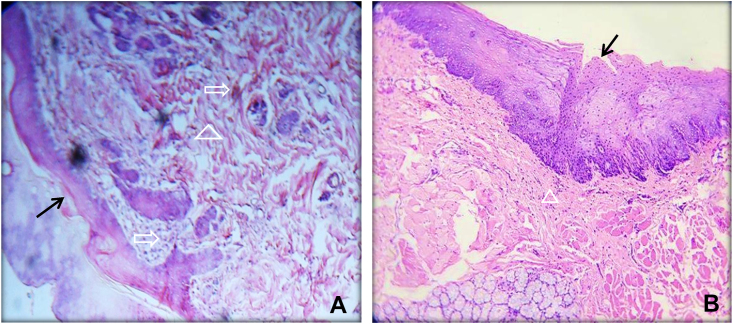
A Histological section of oral mucosal wound at day 7. A; represent control group showed the scab still attached to underlining thin mucosa covering the wound and the submucosa filled with fibrous tissue. B; treated group showed complete thick epithelium covered the wound with minimal amount of granulation tissue. The mucosal layer indicated by solid arrow, the inflammatory cell and fibroblasts cell by open arrow, granulation tissue by open triangle. H& E, ×100.
Figure 6.
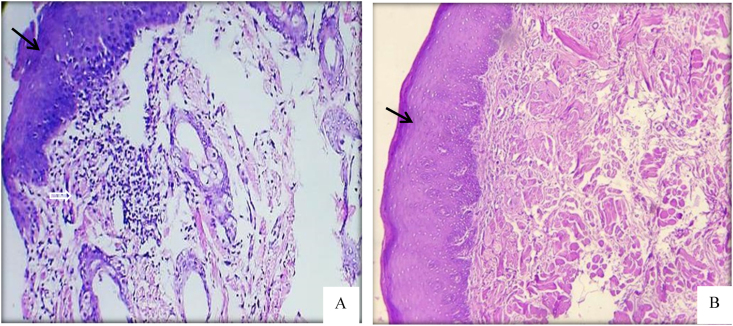
A Histological section of oral mucosal wound at day 15. A; there is complete reepithelialization over the wound and there is little amount of granulation tissue for control group. B; treated group with neo-formulated paste showed normal epithelial thickness and appearance covering the whole wound side with difficulty to recognized the wound line and complete absence of inflammation. The mucosal layer indicated by solid arrow, the inflammatory cell and fibroblasts cell by open arrow. H& E, ×100.
Table 3.
Mann–Whitney test for histopathological readings of study material to determining exact period in which material affect.
| Healing periods | Healing parameters |
|
|---|---|---|
| Inflammation | Reepithelization | |
| 1–2 | 0.008 | 0.008 |
| 1–3 | 0.008 | 0.008 |
| 1–4 | 0.008 | 0.008 |
| 2–3 | 1.000 | 0.690 |
| 2–4 | 1.000 | 0.032 |
| 3–4 | 1.000 | 0.032 |
1 = 1 day, 2 = 3 day, 3 = 7 day, 4 = 15 day.
Discussion
The results of the application of a new paste formulation in this study were determined by a histopathological evaluation of the healing process in rabbits in which wounds were induced on the buccal mucosa of the cheek. All animals regained their normal activity within a few hours after the treatment procedure. The healing time points (days 1, 3, 7, and 15) were chosen because the inflammation and re-epithelization phases completed accordingly. The major challenge encountered was how to maintain contact between the study product and the wound for a long time and prevent it from washing away and removal during food consumption. Therefore, it was necessary to apply the study product for three days to increase the contact time between the product and the wound, maintain product concentration, and maximise the benefit of the product. Recently, a topical mixture of chemical and mechanical treatments such as metronidazole and HA, which has been described as a supplementary therapeutic measure for periodontitis, was reported to afford strong recovery.19 HA is naturally present in the connective tissue of humans. It is involved in the healing process when it apply topically as agel wherein it reduces bleeding, gingival crevicular fluid, and lysosomal enzymes (hyaluronidase and chondroitinase) without triggering immunological reactions as adverse side effects.20 HA, the simplest glycosaminoglycan, is involved in many major biological processes, including cell signalling, adhesion, proliferation and regulation, and differentiation. HA depends on the molecular weight (MW) and concentration of the effect on cell proliferation and differentiation. HA is drawn attention in the field of tissue engineering and for disease treatments owing to its high viscosity, high elasticity, extremely negative charge, biocompatibility, biodegradability, and non-immunogenicity.4 Regarding this study, we found that the new paste formulation had effects superior to control treatment (control group) by promoting the healing process and enhancing the release of inflammatory cytokines, which in turn increased the differentiation and proliferation of cells. Compared to untreated wounds, the wounds treated with the new formulation paste showed maximum resolution within the acute inflammatory phase during the first week. All wounds completely healed by the end of the second post-treatment week. The effect of HA on wound healing in cheek pouches in rats has been investigated.21 It was found that the application of exogenous HA enhanced microcirculatory perfusion and facilitated wound closure at the tissue repair site. The results of this study showed that the newly formed paste-induced areas of granulation tissue formation were invaded by large, engorged blood vessels. This finding may be explained by the fact that HA may assist in the physical preservation of the matrix during the inflammatory response phase in the blood clots in the wound. This also promotes the recruitment and migration of cells and regulates fibrin degradation. HA–fibrin matrix degradation products function as regulators of wound-healing molecules.22 The histopathological score appeared significant regarding re-epithelization, and the formation of granulation tissue continued until day 15 and the pattern appeared to be better than that in the control group. This may be attributed to various biological effects exerted through the induction of a variety of growth factors present in the α-granules of platelets. Most of these factors may affect multiple cell and tissue types. Among these factors, the epithelial growth factor stimulates angiogenesis, endothelial chemotaxis, epithelial and mesenchymal cell mitogenesis, and collagen synthesis.23 The degree of inflammation in this study appeared low, probably due to the presence of rosemary in our new paste formulation paste, which acts as an effective antioxidant and anti-inflammatory agent that decreases the inflammatory rate. Such findings coming straightforward with many studies in which rosemary (R.osmarinus officinalis L.) essential oil has been proven to have antioxidant, antibacterial, antifungal, and anticancer effects24,25 and may have a better effect on wound healing. The anti-inflammatory and antibacterial effects of rosemary extract are primarily attributable to its flavonoid and terpene constituents, which reduce the rates of inflammatory cytokine release and promote wound-healing processes.26, 27, 28 Topical application of metronidazole powder has been reported to decrease or eradicate wound infection, advance wound appearance, reduce surrounding cellulitis, eliminate tissue necrosis, and reduce pain.29
To preserve the wound from turning into a chronic wound and suppressing anaerobic bacteria from resulting in pus formation and bad odour in the wound area with tissue liquefaction, a metronidazole antibiotic was added to the paste, which decreased the chance of exacerbation to a chronic wound by eliminating both aerobic and anaerobic bacteria.30 This helps maintain a moist wound environment and is effective in tackling the infection-associated odour.31,32 However, wound healing is a complex process that requires efficient vascularization and the formation of a new collagen matrix. Many studies have considered the single application of certain natural products with anti-inflammatory activity, such as HA or antimicrobial, antibacterial, and antioxidant drugs may also play a role in the acceleration of wound healing. The results obtained for the synergic mixture of HA and rosemary extract, as an antioxidant, could protect against peroxidative damage due to free radicals generated at the wound site. It could also boost cellular activity to support wound healing33,34 and facilitate the deposition of more collagen fibres by fibroblasts.35 On the other hand, the addition of topical metronidazole in the new paste formulation was evaluated to verify whether wound healing was promoted. This may be related to the elimination of anaerobic and aerobic bacteria and a significant reduction in wound colonisation. In addition, the formulation decreased exudate volume and pain, which in turn improved wound appearance.19,32 The promising synergistic mix formulation has been derived from local etiological elements to eradicate local inflammation and accelerate tissue healing. The present study showed an obvious synergistic effect of HA, rosemary oil, and metronidazole to achieve better wound healing. Thus, this new paste formulation could be a good candidate for enhancing wound contraction and healing without any complications.
Conclusion
The topical application of our novel paste formulation comprising a mixture of HA, rosemary (R.osmarinus officinalis) oil extract, and pure metronidazole powder afforded good therapeutic results. It resulted in the inhibition of inflammation in the 3- and 7-day subgroups and achievement of faster re-epithelialization. Furthermore, all animals treated with this formulation showed better healing response in terms of reduction of wound contraction, with interesting outcomes mostly in the 15-day subgroup.
Recommendation
The study provides a valuable clinical option for the treatment of mucosal wounds. This was confirmed by the results afforded by the application of the new paste formulation containing HA, rosemary extract, and metronidazole for the management of deep contaminated wounds. The study also suggests the usability of this formulation for clinical application in future studies.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All animal procedures were conducted in compliance with the National Institutes of Health guidelines for laboratory animals and animal welfare (NIH publication no. 8023, revised 1978). Furthermore, the authors declare that they have obtained the appropriate institutional approval for this study.
Authors’ contributions
WKA conceived and designed the study, conducted the literature review search, provided research materials, and collected and organised the data. AIN analysed and interpreted the data. WKA and AIN wrote the initial and final drafts of the article and provided logistical support. All authors critically reviewed and approved the final draft and are responsible for the contents and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Prestwich G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Contr Release. 2011;155:193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falcone S.J., Palmeri D.M., Berg R.A. Rheological and cohesive properties of hyaluronic acid. J Biomed Mater Res. 2006;76:721–728. doi: 10.1002/jbm.a.30623. [DOI] [PubMed] [Google Scholar]
- 3.Kessiena L. Aya, Robert S. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen. 2014;22(5):579–593. doi: 10.1111/wrr.12214. [DOI] [PubMed] [Google Scholar]
- 4.Ningbo Zhao X.W., Lei Qin, Min Zhai, Jing Yuan, Ji Chen, Dehua Li. Effect of hyaluronic acid in bone formation and its applications in dentistry. Review Article. J Biomed Mater Res A. 2016;104A:1560–1569. doi: 10.1002/jbm.a.35681. [DOI] [PubMed] [Google Scholar]
- 5.Hahn S.K., Jelacic S., Maier R.V., Stayton P.S., Hofman A.S. Anti-inflammatory drug delivery from hyaluronic acid hydrogels. J Biomater Sci Polym Ed. 2004;15:1111–1119. doi: 10.1163/1568562041753115. [DOI] [PubMed] [Google Scholar]
- 6.Awartani F.A., Tatakis D.N. Interdental papilla loss: treatment by hyaluronic acid gel injection: a case series. Clin Oral Invest. 2016;20:1775–1780. doi: 10.1007/s00784-015-1677-z. [DOI] [PubMed] [Google Scholar]
- 7.Stern R., Asari A.A., Sugahara K.N. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Javad A., Kuchaki E., Amirreza S., Mansour N. Properties of wound healing activities of rosemary extract. J Biol Act Prod Nat. 2012;2(4):218–224. [Google Scholar]
- 9.Khezri K., Farahpour M.R., Mounesi Rad S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif Cells Nanomed Biotechnol. 2019;47(1):980–988. doi: 10.1080/21691401.2019.1582539. [DOI] [PubMed] [Google Scholar]
- 10.Pintore G., Usai M., Bradesi P., Juliano C., Boatto G., Tomi F. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica, Flavour Fragrance J. 2002;17(1):15–19. [Google Scholar]
- 11.Jiang Y., Wu N., Fu Y.J., Wang W., Luo M., Zhao C.J. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ Toxicol Pharmacol. 2011;32:63–68. doi: 10.1016/j.etap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Ang C.W., Jarrad A.M., Cooper M.A., Blaskovich M.A. Nitroimidazoles: molecular fireworks that combat a broad spectrum of infectious diseases. J Med Chem. 2017;60:7636–7657. doi: 10.1021/acs.jmedchem.7b00143. [DOI] [PubMed] [Google Scholar]
- 13.Miani P.K., do Nascimento C., Sato S., Filho A.V., da Fonseca M.J.V., Pedrazzi V. In vivo evaluation of a metronidazole-containing gel for the adjuvant treatment of chronic periodontitis: preliminary results. Eur J Clin Microbiol Infect Dis. 2012;31:1611–1618. doi: 10.1007/s10096-011-1484-7. [DOI] [PubMed] [Google Scholar]
- 14.Jennifer TCdA., Flavia dOP., Paula S.F., Núbia P.L.T., Andressa V.P., Thaysa B.M.T. Effect of essential oil of rosmarinus officinalis L. (Rosemary) on the healing of cutaneous lesions in mice. J Chem Pharmaceut Res. 2017;9(5):381–386. [Google Scholar]
- 15.Boutekedjiret C., Bentahar F., Belabbes R., Bessiere J.M. Extraction of rosemary essential oil by steam distillation and hydrodistillation. Flavour Fragrance J. 2003;18:481–484. [Google Scholar]
- 16.Al – Nema Z.M. College of Pharmacy, University of Mosul; Iraq: 2000. A substitute preparation for" triamcinolone acetonide in orabase" for the treatment of recurrent oral aphthous ulceration. MSc Thesis. [Google Scholar]
- 17.Naser A.I. University of Mosul College of Dentistry; 2012. Evaluation of chitosan as accelerator for skin wound healing. (an experimental study) p. 64. MSc Thesis Oral and Maxillofacial Surgery. [Google Scholar]
- 18.Fathi W.K. The effect of hyaluronic acid and platelet -rich plasma on soft tissue wound healing: an Experimental Study on Rabbits. Al–Rafidain Dent J. 2012;12(1):115–125. [Google Scholar]
- 19.Mahmood A.A., Abdul-Wahab G.A., Al-Karawi S.I. Effect of hyaluronan and metronidazole gels in management of chronic periodontitis. J Int Oral Health. 2019;11(3):158–163. [Google Scholar]
- 20.Jentsch H., Pomowski R., Kundt G., Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontol. 2003;30:159–164. doi: 10.1034/j.1600-051x.2003.300203.x. [DOI] [PubMed] [Google Scholar]
- 21.King S.H.W., Proctor K., Newsome A. Benefical actions of exogenous hyaluronic Acid on wound healing. Surgery. 1991;109(1):76–84. [PubMed] [Google Scholar]
- 22.Prathiba V., Gupta P.D. Cutaneous Wound healing: significance of proteoglycans in scar formation. Curr Sci. 2000;78(6):1–5. [Google Scholar]
- 23.Nikolidakis D., Jansen J. The biology of platelet– rich plasma and its application in oral surgery: literature Review. Tissue Eng B Rev. 2008;14(3):249–258. doi: 10.1089/ten.teb.2008.0062. [DOI] [PubMed] [Google Scholar]
- 24.Oluwatuyi M.K.G., Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65:3249–3254. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Leal P.F., Braga M., Sato D.N., Carvalho J.E., Marques M.O., Meireles M.A. Functional properties of spice extracts obtained via supercritical fluid extraction. J Agric Food Chem. 2003;51:2520–2525. doi: 10.1021/jf0260693. [DOI] [PubMed] [Google Scholar]
- 26.Gad A.S., Sayd A.F. Antioxidant properties of rosemary and its potential uses as natural antioxidant in dairy products-a review. Food Nutri Sci. 2015;6:179–193. [Google Scholar]
- 27.Montenegro L., Pasquinucci L., Zappalà A., Chiechio S., Turnaturi R., Puglia C. Rosemary essential oil-loaded lipid nanoparticles: in vivo topical activity from gel vehicles. Pharmaceutics. 2017;9(4):48. doi: 10.3390/pharmaceutics9040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira J.R., Camargo S.E.A., de Oliveira L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J Biomed Sci. 2019;26(1):1–22. doi: 10.1186/s12929-019-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul J., Pieper B. Topical metronidazole for the treatment of wound odor: a review of the literature. Ostomy/Wound Manag. 2008;54(3):18–27. [PubMed] [Google Scholar]
- 30.Löfmark S., Edlund C., Nord C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;1:S16–S23. doi: 10.1086/647939. 50(Suppl 1) [DOI] [PubMed] [Google Scholar]
- 31.Kavitha K.V., Tiwari S., Purandare V.B., Khedkar S., Bhosale S.S., Unnikrishnan A.G. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(4):546–556. doi: 10.4239/wjd.v5.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ousey K. The role of topical metronidazole in the management of infected wounds. Wounds UK. 2018;14(5):78–83. [Google Scholar]
- 33.Hasani-Ranjbar S., Larijani B., Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy - Drug Targets. 2009;8(1):2–10. doi: 10.2174/187152809787582561. [DOI] [PubMed] [Google Scholar]
- 34.Francetti L., Dellavia C., Corbella S., Cavalli N., Moscheni C., Canciani E. Morphological and molecular characterization of human gingival tissue overlying multiple oral exostoses. Case Rep Dent. 2019;2019:31263605. doi: 10.1155/2019/3231759. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canciani E., Sirello R., Pellegrini G., Henin D., Perrotta M., Toma M. Effects of vitamin and amino acid-enriched hyaluronic acid gel on the healing of oral mucosa: in vivo and in vitro study. Medicina. 2021;57:285. doi: 10.3390/medicina57030285. [DOI] [PMC free article] [PubMed] [Google Scholar]