Abstract
Purpose
Preoperative chemoradiation represents the standard of care in patients with locally advanced rectal cancer. Robustness is often compromised in the setting of proton beam therapy owing to the sensitivity of proton particles to tissue heterogeneity, such as with intestinal gas. The ideal beam arrangement to mitigate the anatomic uncertainty caused by intestinal gas is not well defined.
Methods and Materials
We developed pencil beam scanning plans using (1) 1-beam posteroanterior (PA) plans, (2) 2-beam with right and left posterior oblique (RPO and LPO) plans, (3) 3-beam with PA and opposed lateral plans, and (4) 5-beam with PA, RPO, LPO, and opposed lateral plans. We created 12 plans with robustness optimization and ran a total of 60 plan evaluations for varying degrees of intestinal gas distension to evaluate which plans would maintain clinical goals to the greatest degree.
Results
A single PA beam resulted in considerable loss of target coverage to the clinical target volume prescribed 50 Gy (volume receiving 100% of the prescribed dose [V100%] < 90%) with rectal distension ≥3 cm in diameter in the short axis. In contrast, the other field designs maintained coverage with up to 5 cm of distension. On plans generated based on a 5-cm distended rectum with air medium, the 1-beam, 3-beam, and 5-beam arrangements resulted in loss of target coverage (V100% < 90%) with rectal contraction ≤3 cm, whereas the 2-beam arrangement maintained coverage to as low as 2 cm. On plans generated based on a 3-cm distension of the rectum, both the 2-beam and 3-beam arrangements maintained V100% > 90% even with collapsed rectum to as low as 1 cm, simulating a patient treatment scenario without any rectal gas.
Conclusions
A single PA beam should be avoided when using proton beam therapy for rectal cancer. RPO/LPO and PA/opposed lateral arrangements may both be considered; RPO/LPO is favored to reduce integral dose and avoid beams traversing the hips. In patients for whom the plan CT has rectal distension of ≥3 cm, resimulation or strategies to reduce intestinal gas should be strongly considered.
Introduction
Intensity modulated radiation therapy (IMRT) and proton beam therapy (PBT) are increasingly being used to treat rectal cancer in the preoperative setting.1,2 Although the Radiation Therapy Oncology Group (RTOG) trial 0822 did not show a benefit in toxicity profile using IMRT compared with 3-dimensional conformal radiation therapy (3DCRT), the use of concurrent oxaliplatin in that trial may have masked the marginal benefit of IMRT.3 Accordingly, increasingly conformal treatment with IMRT and even PBT may be appropriate when dose constraints, particularly to the small bowel, cannot be met with 3DCRT. In the setting of reirradiation and in treating younger patients, PBT also has a role.4,5 In younger patients, the lower integral dose afforded by PBT may lead to a lower incidence of radiation-induced second malignancies, although this effect may be muted in part if using short-course irradiation.
Rectal distention with air or fecal matter is a major concern with radiation therapy planning because it may cause positional changes in target volumes and affect the amount of normal tissue subject to irradiation. In prostate cancer, multiple studies have shown an increased risk of biochemical failure in patients simulated with a distended rectum.6, 7, 8 Although patients with rectal cancer are not typically treated with definitive irradiation as are patients with prostate cancer, these studies underscore the fact that variations in rectal distension may be clinically meaningful. When treating rectal cancer with PBT, the penetration depth of the beam depends on the stopping powers of the various regions through which it passes.9 Thus, tissue heterogeneity, such as rectal distension with air, has the potential to dramatically affect the distal range of the treatment beam, potentially compromising target coverage and delivering large amounts of dose into anterior normal tissues. As a result, the most appropriate proton beam arrangement to account for varying tissue homogeneity within the rectal contour is not well-defined.
Historically, a single PA beam was used in this setting, with the assumption that the rectal air content will average out with fractionation.10 More recently, studies of combinations of posteroanterior (PA), opposed lateral, right posterior oblique (RPO), and/or left posterior oblique (LPO) beams have been published.10, 11, 12, 13, 14 A University of Florida study compared 3DCRT, IMRT, and PBT neoadjuvant therapy plans using a PA and opposed lateral arrangement of uniform scanning and passive scattering beams and showed improved small bowel, urinary bladder, and pelvic bone marrow dosimetry with PBT.12 Another study used PA, LPO, and RPO beams using spot scanning PBT and IMPT, similarly showing improved dosimetry to the small bowel, testes, and urinary bladder in patients undergoing neoadjuvant therapy.13 Additional studies from Sweden and the Mayo Clinic used LPO and RPO beam arrangements and showed dosimetric superiority with PBT.14,15 Although collectively these studies supported the use of PBT from a dosimetric standpoint, they all used different field designs.
Given the heterogeneity of previous work, the present study was conceived to more comprehensively explore the optimal beam arrangement in the treatment of rectal cancer with PBT by accounting for variations in intestinal dilation. Using the pencil beam scanning technique, we simulated and compared proton beam arrangements for a wide variety of intestinal gas scenarios to determine which proton beam arrangement would be the most robust. We hypothesized that a single PA beam would be ill-advised and that an RPO/LPO arrangement would provide comparable coverage to 3-beam (PA and opposed laterals) or 5-beam (PA, RPO, LPO, and opposed laterals) arrangements.
Methods and Materials
A patient with rectal cancer with a clinical stage IIIB (cT3, cN1b, cM0), moderately differentiated adenocarcinoma without anatomic variants or artificial hardware was selected for plan optimization and evaluation. The patient's lesion was 6 cm from the external anal verge. The patient underwent 3D computed tomography (CT) simulation with a helical CT scanner (Brilliance Big Bore, Philips Healthcare, Cleveland, OH). An alpha cradle was used for simulation in the supine position. Intravenous iodinated contrast was used for accurate delineation of the nodal basins, although a noncontrast CT was used for proton treatment planning.
Contouring of the target volumes was per RTOG atlas guidelines.16 The primary gross tumor volume (GTV) encompassed the gross disease by physical examination, colonoscopy report, and magnetic resonance imaging (MRI) of the pelvis. The nodal GTV encompassed any positive lymph nodes by MRI of the pelvis or diagnostic CT scan. The high-risk clinical target volume (CTV) included the GTV with a 2-cm superior and inferior margin as well as the mesorectum and presacral space at involved levels. The standard-risk CTV included the high-risk CTV, the presacral space, obturator lymph nodes, internal iliac lymph nodes, entire mesorectum, and rectum. The standard-risk CTV was prescribed 45 Gray equivalents (GyE) in 25 fractions, and the high-risk CTV received 50 GyE via a simultaneous integrated boost. The entire rectum was contoured and observed to have a maximal diameter of approximately 2 cm on the short axis. To simulate rectal contraction, the rectal contour was isotropically reduced in size by 0.5 cm to generate a contour with a maximal diameter of approximately 1 cm. The rectal contour was then isotropically expanded in size by 0.5 cm, 1 cm, and 1.5 cm to generate contours with diameters of 3 cm, 4 cm, and 5 cm, respectively. Care was taken so that the artificial contours did not extend into rigid structures, such as the piriformis muscle or bone. The contours also did not extend into the ischiorectal fossa or otherwise outside the mesorectum. Normal structures were contoured as per RTOG guidelines.17 Of note, individual loops of small bowel were contoured as opposed to a bowel bag. The internal genitalia contour comprised the penile bulb and the base of the penis, with all else denoted external genitalia. All contours were completed by a physician specializing in gastrointestinal radiation oncology.
A single-field uniform dose plan was generated if a single beam was used. Multifield uniform dose plans using the single-field uniform dose technique were generated otherwise, based on pencil beam scanning technique. Multifield optimization was avoided owing to a lack of robustness to anatomic uncertainties. Planning was completed with RayStation, version 6 (RaySearch Laboratories AB, Stockholm, Sweden). Monte Carlo dose calculations were performed with robust optimization parameters: 3.5% for range uncertainty and 5 mm isotropically for setup uncertainties. All evaluations in this analysis were done with nominal planning. To improve plan robustness, intestinal air was overridden as water to create an “overshooting at worst-case scenario” condition in the planning CT scan. Clinical goals and dose constraints were institutional but based on RTOG 0822 (Table 1). Plans were optimized such that the volume receiving 100% of the prescribed dose (V100%) was greater than 97% for CTVs. For plan evaluation, V100% < 90% was considered to represent significant degradation of the treatment plan. First, plans were generated and optimized based on the planning CT simulation scan in which the rectum was not distended using the following 4 beam arrangements: (1) PA, (2) RPO (140o) and LPO (220o), (3) PA and opposed lateral beams, and (4) PA, RPO, LPO, and opposed lateral beams. For multibeam plans, the extent of beam overlap was minimized to reduce possible skin toxicity. To assess how an overdistended rectum could perturb dose distribution, the plans were then evaluated on the 1 cm, 2 cm, 3 cm, 4 cm, and 5 cm rectal contours assigning air medium to the rectal contour. Thus, there were a total of 20 plan evaluations for the different setups. Subsequently, plans were generated and optimized for all 4 beam arrangements based on a 5-cm rectal contour. Evaluations were then run on 4 cm, 3 cm, 2 cm, and 1 cm rectal contours with air medium as well as the planning CT scan to assess the effect of an underdistended rectum on the dose distribution. This afforded an additional 20 plan evaluations for the different beam setups. Finally, plans were optimized based on the 4-cm and 3-cm rectal contours and then run on the other rectal contours as well as the planning CT for the 2- and 3-beam arrangements. This generated an additional 20 plan evaluations. In total, 60 plan evaluations were run and evaluated. Dosimetric parameters for the target volumes, small bowel, bladder, external genitalia, internal genitalia, and femoral heads were evaluated and compared.
Table 1.
Institutional clinical goals and dose constraints based on RTOG 0822
| Target/Organ at risk | Dose constraint | Clinical goal |
|---|---|---|
| CTV45 | V100% | >95-97% |
| CTV50 | V100% | >95-97% |
| CTV50 | Dmax | <110% |
| Small bowel | V40Gy | <70 cm3 |
| V35Gy | <300 cm3 | |
| V30Gy | <350 cm3 | |
| Dmax | <52 Gy | |
| Bladder | V40Gy | <50% |
| V30Gy | <60% | |
| Genitalia | V30Gy | <35% |
| V20Gy | <50% | |
| Femoral heads | V45Gy | <5% |
| V40Gy | <30% | |
| V30Gy | <35% |
Abbreviations: CTV45 = clinical target volume prescribed 45 GyE; CTV50 = clinical target volume prescribed 50 GyE; Dmax = maximum dose to 0.03 cubic centimeters; RTOG = Radiation Therapy Oncology Group; V100% = volume receiving 100% of the prescribed dose; V40Gy = percentage of volume receiving 40 Gy.
Results
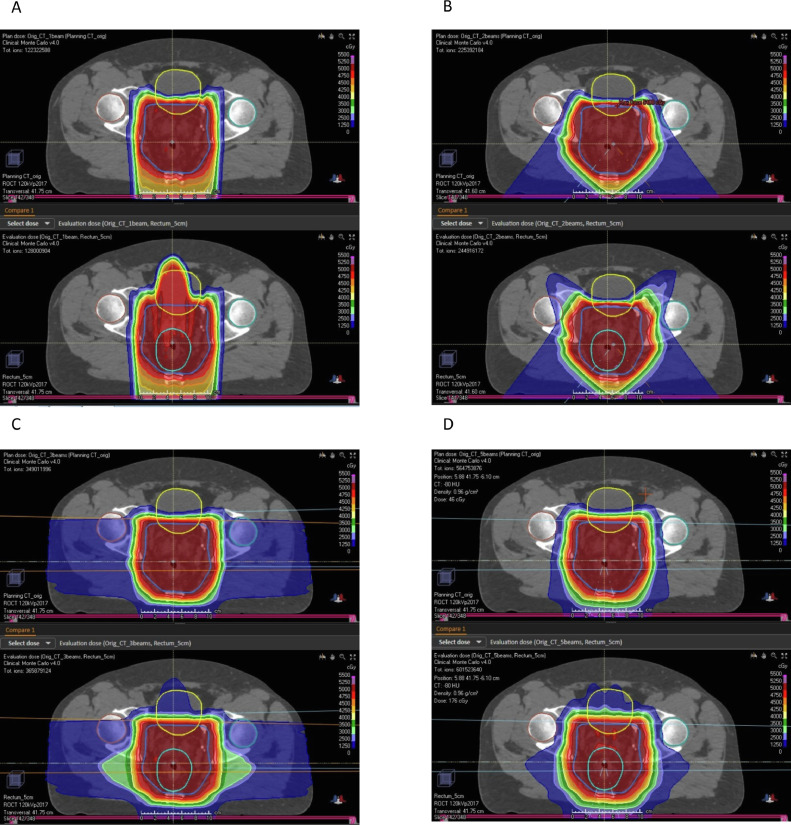
Dosimetric parameters are presented in Table 2 for the plans optimized to the planning CT scan and then evaluated based on rectal diameters of 1 cm, 2 cm, 3 cm, 4 cm, and 5 cm for each of the 4 beam arrangements. In addition, the percentage of volume receiving 30 Gy (V30Gy) and V20Gy were evaluated for external genitalia and V45Gy, V40Gy, and V30Gy were evaluated for the femoral heads for each of the plan evaluations. In all cases, these values were less than 2% and so are not displayed for conciseness. With a single PA beam arrangement, a V100% > 90% to the high-risk CTV prescribed 50 Gy (CTV50Gy) was lost with a relatively small rectal distension of ≥3 cm (for 3 cm, V100% = 88.8%). In addition, dose constraints to the bladder (V30Gy < 60 and V40Gy < 50) were violated with distension of ≥3 cm (for 3 cm, V30Gy = 61.4% and V40Gy = 50.4%) and to the internal genitalia with ≥2 cm (V30Gy). The 2-beam, 3-beam, and 5-beam arrangements maintained V100% > 95% for the CTV50Gy even with 5-cm rectal distension. Relative to the 2-beam arrangement, the 3-beam and 5-beam arrangements had modest increases in small bowel parameters that exceeded dose constraints. In particular, for the small bowel, the 3-beam and 5-beam arrangements had V40Gy > 70 cm3, whereas the 2-beam arrangement had V40Gy < 70 cm3. Despite the opposed lateral configuration included in the 3-beam and 5-beam arrangements, dose constraints to the femoral heads were not exceeded. Figure 1 represents representative slices of plans for (A) 1-beam, (B) 2-beam, (C) 3-beam, and (D) 5-beam arrangements based on the planning CT (above) and with maximal rectal distension to 5 cm (below), showing the characteristic altered dose distributions with increased air medium with a single PA beam arrangement. In contrast, differences in the target coverage between 2-beam, 3-beam, and 5-beam arrangements were not overt when looking at this dose distribution.
Table 2.
Dosimetric parameters for the 4 beam arrangements optimized to the planning CT and iterated across rectal diameter sizes
| Dosimetric parameter | Beam arrangement |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 beam (PA) |
3 beam (PA, opposed lateral) |
||||||||||||
| Plan CT | 1 cm | 2 cm | 3 cm | 4 cm | 5 cm | Plan CT | 1 cm | 2 cm | 3 cm | 4 cm | 5 cm | ||
| CTV45 | V100% (%) | 99.0 | 98.8 | 99.1 | 99.1 | 99.0 | 99.0 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 |
| CTV50 | V100% (%) | 98.5 | 96.0 | 93.0⁎ | 88.8⁎ | 83.0⁎ | 78.4⁎ | 100.0 | 99.9 | 99.9 | 99.8 | 99.7 | 98.8 |
| CTV50 | Dmax (%) | 52.6 | 53.5 | 53.4 | 53.6 | 53.3 | 53.8 | 52.4 | 52.8 | 52.8 | 53.0 | 53.1 | 52.9 |
| Small bowel | V40Gy (%) | 68.2 | 68.2 | 71.0⁎ | 75.7⁎ | 81.8⁎ | 88.8⁎ | 75.3⁎ | 75.5⁎ | 76.0⁎ | 76.8⁎ | 77.5⁎ | 78.0⁎ |
| V35Gy (%) | 88.7 | 89.1 | 92.3 | 97.6 | 104.1 | 112.4 | 98.6 | 98.8 | 99.7 | 100.9 | 102.0 | 103.0 | |
| V30Gy (%) | 105.6 | 106.0 | 109.6 | 115.1 | 122.2 | 130.1 | 116.5 | 116.8 | 118.1 | 119.7 | 121.0 | 122.4 | |
| Dmax (Gy) | 49.3 | 49.3 | 49.5 | 49.5 | 49.2 | 49.5 | 47.7 | 47.7 | 47.6 | 47.6 | 47.6 | 47.8 | |
| Bladder | V40Gy (%) | 23.0 | 26.9 | 36.7 | 50.4⁎ | 65.9⁎ | 81.6⁎ | 24.8 | 25.5 | 26.5 | 27.5 | 28.7 | 29.4 |
| V30Gy (%) | 31.0 | 36.3 | 47.3 | 61.4⁎ | 76.7⁎ | 88.9⁎ | 34.4 | 36.0 | 38.2 | 40.7 | 42.7 | 44.2 | |
| Internal genitalia | V30Gy (%) | 14.7 | 25.2 | 35.1⁎ | 40.8⁎ | 41.1⁎ | 41.2⁎ | 15.2 | 17.3 | 18.1 | 18.3 | 18.1 | 18.3 |
| V20Gy (%) | 19.2 | 31.6 | 41.9 | 46.1 | 46.5 | 46.3 | 21.6 | 25.6 | 27.6 | 28.2 | 28.2 | 28.1 | |
| 2 beam (RPO, LPO) | 5 beam (PA, RPO, LPO, opposed lateral) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTV45 | V100% (%) | 100.0 | 100.0 | 100.0 | 100.0 | 99.9 | 99.9 | 99.5 | 99.5 | 99.5 | 99.5 | 99.5 | 99.4 |
| CTV50 | V100% (%) | 100.0 | 100.0 | 100.0 | 99.9 | 99.9 | 99.9 | 100.0 | 100.0 | 99.9 | 99.7 | 99.1 | 97.6 |
| Dmax (%) | 54.1 | 54.0 | 54.0 | 54.3 | 54.0 | 53.8 | 52.2 | 52.6 | 52.8 | 52.7 | 52.5 | 52.5 | |
| Small bowel | V40Gy (%) | 68.6 | 68.5 | 68.6 | 68.8 | 69.0 | 69.6 | 78.1⁎ | 78.3⁎ | 79.0⁎ | 79.4⁎ | 80.2⁎ | 81.0⁎ |
| V35Gy (%) | 84.4 | 84.6 | 84.5 | 84.6 | 85.3 | 85.9 | 102.0 | 102.3 | 103.8 | 105.0 | 106.4 | 108.3 | |
| V30Gy (%) | 97.9 | 98.0 | 98.0 | 98.3 | 98.7 | 99.5 | 122.4 | 122.8 | 124.7 | 126.5 | 128.4 | 130.7 | |
| Dmax (Gy) | 49.1 | 49.0 | 48.9 | 49.0 | 48.9 | 48.9 | 46.9 | 46.8 | 46.8 | 47.0 | 46.9 | 47.0 | |
| Bladder | V40Gy (%) | 22.3 | 22.3 | 22.2 | 22.3 | 22.4 | 22.9 | 26.5 | 27.7 | 29.7 | 31.0 | 32.6 | 33.9 |
| V30Gy (%) | 26.7 | 26.8 | 26.8 | 26.9 | 27.0 | 27.6 | 35.6 | 38.3 | 40.3 | 43.4 | 46.8 | 50.9 | |
| Internal genitalia | V30Gy (%) | 19.6 | 19.8 | 19.9 | 20.4 | 20.7 | 20.6 | 17.1 | 19.6 | 21.5 | 24.1 | 24.7 | 24.7 |
| V20Gy (%) | 25.8 | 25.9 | 25.9 | 26.5 | 26.9 | 27.0 | 23.6 | 27.3 | 31.5 | 37.4 | 38.4 | 38.3 | |
Abbreviations: CT = computed tomography; CTV45 = clinical target volume prescribed 45 GyE; CTV50 = clinical target volume prescribed 50 GyE; Dmax = maximum dose to 0.03 cubic centimeters; LPO = left posterior oblique; PA = posteroanterior; RPO = right posterior oblique; V100% = volume receiving 100% of the prescribed dose; V40Gy = percentage of volume receiving 40 Gy.
Values did not meet institutional dose constraints.
Fig. 1.
Plan comparison for (A) posteroanterior (PA) field arrangement; (B) right posterior oblique (RPO) / left posterior oblique (LPO); (C) PA/opposed lateral; and (D) RPO/LPO/PA/opposed lateral on plan CT (above) and with 5-cm rectal distension (below). With PA field arrangement, there was a high-dose region extending anteriorly to the bladder with maximal rectal distension. In contrast, with maximal rectal distension, target coverage perturbations in dose distributions were much more modest for the other beam arrangements.
Next, plans were generated and optimized to the rectal contour of 5 cm and were then evaluated based on rectal diameters of 4 cm, 3 cm, 2 cm, 1 cm, and planning CT for each of the 4 beam arrangements (Table 3). Again, all external genitalia and femoral head values did not approach institutional constraints and are not included in Table 3. In addition, there were minor violations of V40Gy to the small bowel for the 3-beam and 5-beam arrangements (77.2% and 77.1%, respectively). With rectal contraction to 3 cm, V100% > 90% was lost to the CTV50Gy for the 1-beam (87.5%), 3-beam (84.8%), and 5-beam arrangements (85.7%) but upheld for the 2-beam arrangement (94.2%). Nonetheless, with further rectal contraction to 2 cm, V100% > 90% to the CTV50Gy was lost for the 2-beam arrangement as well (81.3%).
Table 3.
Dosimetric parameters for the 4 beam arrangements optimized to the 5-cm rectal diameter and iterated across rectal diameter sizes including plan CT
| Dosimetric parameter | Beam arrangement |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 beam (PA) |
3 beam (PA, opposed lateral) |
||||||||||||
| 5 cm | 4 cm | 3 cm | 2 cm | 1 cm | Plan CT | 5 cm | 4 cm | 3 cm | 2 cm | 1 cm | Plan CT | ||
| CTV45 | V100% (%) | 99.9 | 98.1 | 94.2⁎ | 89.0⁎ | 84.1⁎ | 81.5⁎ | 100.0 | 99.7 | 97.9 | 90.1⁎ | 79.0⁎ | 72.5⁎ |
| CTV50 | V100% (%) | 97.6 | 94.7⁎ | 87.5⁎ | 77.1⁎ | 67.0⁎ | 63.4⁎ | 99.8 | 98.2 | 84.8⁎ | 61.2⁎ | 41.4⁎ | 34.7⁎ |
| CTV50 | Dmax (%) | 55.6⁎ | 56.5⁎ | 58.9⁎ | 60.9⁎ | 58.9⁎ | 56.6⁎ | 53.9 | 53.8 | 54.4 | 55.5⁎ | 56.6⁎ | 54.7 |
| Small bowel | V40Gy (%) | 68.6 | 64.7 | 62.6 | 61.9 | 61.9 | 61.8 | 77.2⁎ | 75.5⁎ | 73.8⁎ | 72.9⁎ | 72.6⁎ | 72.4⁎ |
| V35Gy (%) | 85.9 | 81.4 | 78.6 | 77.5 | 77.2 | 77.1 | 100.3 | 98.1 | 96.4 | 95.3 | 94.7 | 94.6 | |
| V30Gy (%) | 102.0 | 96.4 | 93.0 | 91.7 | 91.5 | 91.3 | 119.5 | 117.1 | 115.1 | 114.1 | 113.6 | 113.6 | |
| Dmax (Gy) | 50.6 | 50.4 | 51.0 | 50.6 | 50.7 | 50.3 | 48.1 | 48.0 | 48.1 | 48.1 | 48.1 | 48.1 | |
| Bladder | V40Gy (%) | 24.0 | 8.4 | 2.4 | 1.8 | 1.8 | 1.8 | 26.0 | 19.9 | 14.4 | 7.9 | 6.6 | 6.6 |
| V30Gy (%) | 36.0 | 17.0 | 6.5 | 4.7 | 4.7 | 4.7 | 36.0 | 31.3 | 27.5 | 25.6 | 25.0 | 25.0 | |
| Internal genitalia | V30Gy (%) | 23.6 | 23.3 | 20.0 | 13.0 | 5.7 | 2.1 | 17.8 | 17.7 | 17.5 | 16.4 | 14.6 | 12.6 |
| V20Gy (%) | 33.2 | 32.7 | 29.6 | 21.8 | 13.1 | 6.3 | 26.4 | 26.3 | 26.1 | 23.7 | 21.8 | 20.3 | |
| 2 beam (RPO, LPO) | 5 beam (PA, RPO, LPO, opposed lateral) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTV45 | V100% (%) | 99.9 | 99.9 | 99.0 | 94.8⁎ | 87.7⁎ | 82.0⁎ | 99.9 | 99.7 | 97.9 | 91.6⁎ | 81.8⁎ | 74.4⁎ |
| CTV50 | V100% (%) | 99.9 | 99.8 | 94.2⁎ | 81.3⁎ | 67.2⁎ | 58.5⁎ | 99.6 | 99.0 | 85.7⁎ | 61.6⁎ | 40.9⁎ | 33.1⁎ |
| Dmax (%) | 54.9 | 54.3 | 55.0⁎ | 55.0⁎ | 55.1⁎ | 55.5⁎ | 53.4 | 53.6 | 54.4 | 55.6⁎ | 56.0⁎ | 54.8 | |
| Small bowel | V40Gy (%) | 68.9 | 67.9 | 67.5 | 67.3 | 67.0 | 67.0 | 77.1⁎ | 74.9⁎ | 72.5⁎ | 70.7⁎ | 70.0⁎ | 69.8 |
| V35Gy (%) | 85.4 | 84.1 | 83.3 | 83.2 | 83.0 | 82.9 | 99.6 | 96.7 | 94.0 | 91.9 | 91.2 | 91.2 | |
| V30Gy (%) | 99.1 | 98.1 | 97.4 | 96.9 | 96.9 | 97.0 | 118.9 | 115.4 | 112.5 | 110.6 | 109.4 | 109.4 | |
| Dmax (Gy) | 49.2 | 48.7 | 48.7 | 48.7 | 48.8 | 48.6 | 47.7 | 47.6 | 47.5 | 47.6 | 47.5 | 47.4 | |
| Bladder | V40Gy (%) | 21.3 | 20.4 | 19.9 | 19.5 | 19.4 | 19.5 | 26.9 | 19.3 | 11.0 | 3.7 | 2.3 | 1.9 |
| V30Gy (%) | 26.3 | 25.1 | 24.6 | 24.5 | 24.4 | 24.4 | 37.8 | 30.1 | 24.2 | 19.5 | 16.9 | 16.5 | |
| Internal genitalia | V30Gy (%) | 15.2 | 15.1 | 14.4 | 11.8 | 10.4 | 10.1 | 19.8 | 19.6 | 18.6 | 15.8 | 13.0 | 9.7 |
| V20Gy (%) | 21.9 | 21.9 | 21.3 | 19.6 | 18.9 | 18.8 | 29.1 | 20.1 | 27.3 | 23.9 | 21.4 | 18.4 | |
Abbreviations: CT = computed tomography; CTV45 = clinical target volume prescribed 45 GyE; CTV50 = clinical target volume prescribed 50 GyE; Dmax = maximum dose to 0.03 cubic centimeters; LPO = left posterior oblique; PA = posteroanterior; RPO = right posterior oblique; V100% = volume receiving 100% of the prescribed dose; V40Gy = percentage of volume receiving 40 Gy.
Values did not meet institutional dose constraints.
Finally, plans optimized to the rectal contours of 4 cm and 3 cm were then evaluated based on all other rectal diameters and the planning CT for the 2-beam and 3-beam arrangements (Table 4). This evaluation was completed based on the observation that with the plans optimized based on a 5-cm contour, plan degradation was observed with modest rectal contraction. With the 4-cm plans, V100% > 90% to the CTV50Gy was met for the 2-beam arrangement with rectal contraction to 2 cm (92.4%), whereas it was 89.4% for the 3-beam arrangement. With the 3 cm plans, V100% > 90% was met with both the 2-beam and 3-beam arrangements with rectal contraction all the way to 1 cm (93.1% and 91.6%, respectively), simulating a patient with no rectal gas.
Table 4.
Dosimetric parameters for the 2 and 3 beam arrangements optimized to the 4-cm rectal diameter and 3-cm rectal diameter iterated across rectal diameter sizes including plan CT
| Dosimetric parameter | Beam arrangement |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 beam (RPO, LPO), plan 4 cm |
2 beam (RPO, LPO), plan 3 cm |
||||||||||||
| 5 cm | 4 cm | 3 cm | 2 cm | 1 cm | Plan CT | 5 cm | 4 cm | 3 cm | 2 cm | 1 cm | Plan CT | ||
| CTV45 | V100% (%) | 100.0 | 99.9 | 99.9 | 99.0 | 94.5⁎ | 88.9⁎ | 100.0 | 100.0 | 100.0 | 100.0 | 99.4 | 95.5 |
| CTV50 | V100% (%) | 99.9 | 99.9 | 99.7 | 92.4⁎ | 78.8⁎ | 69.0⁎ | 99.9 | 100.0 | 100.0 | 99.7 | 93.1⁎ | 82.6⁎ |
| CTV50 | Dmax (%) | 53.9 | 54.3 | 54.5 | 54.6 | 54.8 | 55.5⁎ | 54.3 | 54.3 | 54.3 | 54.2 | 54.5 | 55.5⁎ |
| Small bowel | V40Gy (%) | 69.5 | 68.5 | 67.8 | 67.7 | 67.7 | 67.4 | 70.4⁎ | 70.0 | 69.6 | 69.6 | 69.3 | 69.3 |
| V35Gy (%) | 85.3 | 84.4 | 83.6 | 83.6 | 83.2 | 83.4 | 86.3 | 85.8 | 85.3 | 85.2 | 85.0 | 85.0 | |
| V30Gy (%) | 98.8 | 98.0 | 97.6 | 97.3 | 97.1 | 97.1 | 100.1 | 99.3 | 98.7 | 98.5 | 98.6 | 98.5 | |
| Dmax (Gy) | 48.4 | 48.3 | 48.4 | 49.8 | 50.0 | 50.0 | 48.5 | 48.4 | 48.4 | 48.4 | 48.5 | 48.5 | |
| Bladder | V40Gy (%) | 21.4 | 20.8 | 20.6 | 20.5 | 20.5 | 20.4 | 21.6 | 21.3 | 21.1 | 21.1 | 21.0 | 21.0 |
| V30Gy (%) | 27.1 | 26.3 | 25.9 | 25.7 | 25.7 | 25.6 | 26.9 | 26.4 | 26.1 | 26.0 | 26.0 | 26.0 | |
| Internal genitalia | V30Gy (%) | 15.2 | 15.2 | 14.5 | 12.7 | 11.3 | 11.1 | 16.9 | 16.9 | 16.5 | 15.1 | 14.4 | 14.1 |
| V20Gy (%) | 22.5 | 22.5 | 22.1 | 20.6 | 20.2 | 20.1 | 24.4 | 24.4 | 23.9 | 22.9 | 22.6 | 22.4 | |
| 3 beam (PA, opposed lateral), plan 4 cm | 3 beam (PA, opposed lateral), plan 3 cm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTV45 | V100% (%) | 100.0 | 99.9 | 99.8 | 98.5 | 93.5⁎ | 85.6⁎ | 100.0 | 100.0 | 100.0 | 99.8 | 99.0 | 95.1 |
| CTV50 | V100% (%) | 99.9 | 99.9 | 99.4 | 89.4⁎ | 70.4⁎ | 56.9⁎ | 99.9 | 100.0 | 99.9 | 99.6 | 91.6⁎ | 77.0⁎ |
| Dmax (%) | 53.8 | 54.4 | 54.4 | 54.1 | 56.5⁎ | 55.0 | 54.3 | 53.8 | 53.5 | 53.7 | 54.3 | 54.2 | |
| Small bowel | V40Gy (%) | 72.9⁎ | 71.5⁎ | 69.9 | 68.4 | 68.0 | 67.9 | 74.3⁎ | 73.2⁎ | 71.5⁎ | 69.7 | 69.1 | 69.0 |
| V35Gy (%) | 93.0 | 90.7 | 88.4 | 86.6 | 85.9 | 85.9 | 95.0 | 92.9 | 90.2 | 88.1 | 87.1 | 87.1 | |
| V30Gy (%) | 110.0 | 107.4 | 105.0 | 103.1 | 102.4 | 102.4 | 112.2 | 109.8 | 106.9 | 104.4 | 103.4 | 103.3 | |
| Dmax (Gy) | 48.7 | 48.7 | 48.7 | 48.9 | 48.7 | 48.5 | 48.2 | 48.3 | 48.4 | 48.1 | 48.2 | 48.4 | |
| Bladder | V40Gy (%) | 27.1 | 23.3 | 18.5 | 14.5 | 10.1 | 9.4 | 28.6 | 26.6 | 23.2 | 19.5 | 16.6 | 14.8 |
| V30Gy (%) | 38.7 | 34.2 | 28.7 | 25.3 | 23.6 | 23.4 | 40.5 | 37.8 | 33.1 | 28.9 | 26.2 | 25.5 | |
| Internal genitalia | V30Gy (%) | 21.2 | 21.2 | 20.1 | 18.5 | 16.4 | 15.2 | 21.2 | 21.3 | 20.8 | 19.2 | 16.9 | 14.6 |
| V20Gy (%) | 28.5 | 28.5 | 27.7 | 26.0 | 24.3 | 23.5 | 29.5 | 29.5 | 28.7 | 26.8 | 25.0 | 23.5 | |
Abbreviations: CT = computed tomography; CTV45 = clinical target volume prescribed 45 GyE; CTV50 = clinical target volume prescribed 50 GyE; Dmax = maximum dose to 0.03 cubic centimeters; LPO = left posterior oblique; PA = posteroanterior; RPO = right posterior oblique; V100% = volume receiving 100% of the prescribed dose; V40Gy = percentage of volume receiving 40 Gy.
Values did not meet institutional dose constraints.
Discussion
To our knowledge, this is the first study evaluating the optimal proton therapy field design in the treatment of patients with rectal cancer. Based on a total of 60 plan evaluations, our analysis suggests that a single PA beam arrangement should be avoided. In addition, both RPO/LPO and PA/opposed lateral beam arrangements appear to be reasonable choices, although the RPO/LPO approach intuitively offers less integral dose and avoids beams that traverse the femoral heads. The addition of RPO/LPO beams to the PA/opposed lateral arrangement did not appear to afford any marginal benefit and is not indicated. Finally, the study's results suggest that for patients with a maximal rectal diameter >3 cm on the short axis on the planning CT, either the patient should be resimulated or noninvasive measures to reduce intestinal gas should be pursued before treatment based on the possibility that rectal contraction may severely compromise target coverage with subsequent treatments. Noninvasive gas-reducing measures may include a prophylactic bowel regimen, a daily enema, or rectal catheters.
Previous preclinical planning studies evaluating the dosimetric benefits of PBT in patients with rectal cancer have generally acknowledged but not accounted for perturbations in intestinal gas. Colaco et al compared plans generated with 3DCRT, IMRT, and PBT in 8 patients undergoing preoperative therapy.12 The PBT plans were generated with uniform scanning and passive scattering and a 3-beam arrangement of PA and opposed lateral beams, with heavier weight of the PA beam (3.1-1). To account for rectal distension with air, the Hounsfield units were overridden for the air-filled portion of the rectum. Wolff et al generated proton, RapidArc, IMRT, and 3DCRT plans for 25 consecutive patients undergoing preoperative treatment, using spot scanning protons in a 3-beam arrangement with PA, RPO (135o), and LPO (225o) beams, but did not mention accounting for intestinal gas.13 Radu et al compared IMRT and PBT in patients with locally advanced (cT4a), unresectable rectal cancer, with simultaneous integrated boosts to 62.5 Gy in 25 fractions.14 For the proton plans, spot scanning was used with RPO (140o) and LPO (220o) beams. In 2 plans, an additional PA beam was added owing to a shallow target. Intestinal gas was contoured and the plans recalculated with water-equivalent material. Of interest, when the gas was replaced with water, the targets were no longer covered by the 95% isodose line in all proton plans. In addition, the Mayo Clinic reported on their experience treating 11 patients with preoperative short-course PBT with pencil beam scanning to 25 Gy in 5 fractions.15 They used an RPO/LPO field arrangement with a hinge angle of 80o. Rectal gas was contoured and assigned Hounsfield units of –450 for optimization. Collectively, these studies highlight the heterogeneity with which beam arrangements are selected and bowel gas is treated and underscore that air content is an important obstacle in the treatment of rectal cancer with proton therapy.
Whereas tissue inhomogeneity modulates the intensity of a photon-based treatment beam, it results in both range uncertainty and intensity with proton radiation therapy. Accordingly, traversing a particle beam through tissues that have inconsistent densities must be performed with care. In the present study, the single PA beam plans deteriorated with intestinal gas variations. The single PA beam field design is not practical because severe variations in intestinal gas distension may lead to a larger beam range, thus resulting in loss of target coverage with the dose instead spread to the anterior organs at risk, including the small bowel, internal genitalia, and bladder. This was evident in our analysis in the plans optimized to the planning CT and iterated across rectal diameter sizes, where with distension of the rectum to ≥3 cm, the V100% > 90% of the CTV50Gy was lost and dose constraints to the bladder and internal genitalia were compromised. Similarly, on plans optimized based on a 5-cm rectal contour, the V100% > 90% of the CTV50Gy was lost, with rectal contraction of ≤3 cm owing to insufficient range.
The current analysis supports an RPO/LPO arrangement over a PA/opposed lateral arrangement. Parallel opposed beams were historically favored with PBT because this arrangement is least affected by proton range uncertainty, but it has higher scatter and wider dose penumbra owing to greater target depth.18 With the advent of robust optimization,19, 20, 21 other beam arrangements have gained favor owing to potential improved dosimetric benefits, particularly for prostate cancer.22 In addition, opposed lateral beams should be avoided in patients with hip prosthesis because the range is uncertain. When comparing the RPO/LPO design with the PA/opposed lateral field design in the present study, the 2-field design was favored. For both the 2- and 3-beam designs, target coverage was maintained across rectal diameters for plans optimized to the planning CT, but V40Gy to the small bowel was slightly higher for the 3-beam design. On plans optimized to a rectal contour diameter of 5 cm, both designs were subject to severe target coverage loss with rectal contraction, although the 2-beam arrangement maintained V100% > 90% to a rectal diameter as low as 3 cm, whereas the 3-beam arrangement only maintained this coverage to 4 cm. In addition, the V40Gy to the small bowel was higher for the 3-beam arrangement. We concluded that based on slightly superior robustness of target coverage in the face of varying rectal distension, less dose to the small bowel, and less integral dose, the 2-beam arrangement was superior to the 3-beam arrangement. As an aside, we concluded that the RPO/LPO/PA/opposed lateral field design (5 beams) should generally be avoided, because its target coverage was similar to that of the PA/opposed lateral arrangement and it resulted in a higher integral dose.
Clinicians should, in addition, strongly reconsider resimulation or noninvasive measures to reduce intestinal gas when the maximal bowel diameter exceeds 3 cm on the short axis. This is based on the severe loss of target coverage observed with all field designs in patients optimized to 5-cm rectal contours with contraction beyond 3 cm. Based on this observation, we optimized plans to 4-cm and then 3-cm rectal contours for the 2- and 3-beam plans. With the plans optimized to 4 cm, there was still loss of V100% > 90% with rectal contraction to 1 cm for both plans. In contrast, with plans optimized to 3 cm, coverage was maintained for contraction to as low as 1 cm for both the 2- and 3-beam plans, simulating a patient without any rectal gas. If resimulation is not pursued, minimally invasive interventions to reduce intestinal gas, such as rectal catheters and dietary changes, should be strongly considered if contours exceed 3 cm in diameter on the short axis. In addition, cone beam CT imaging or verification quality assurance CT imaging should be used when treating rectal or anal cancer with proton therapy, given the varying degrees of rectal air content.
This study has several important limitations. First, all of the treatment plans and plan evaluations were generated based on a single patient. As the goal of the present study was to show the perturbations in dose distribution and plan quality and robustness based on intestinal dilation, we believe that the results are readily generalizable even with the use of only 1 patient. In addition, the patient was simulated in supine positioning to improve setup reproducibility and patient comfort. We acknowledge that many centers routinely use prone positioning in the treatment of rectal cancer.
In conclusion, our analysis of 60 plan evaluations suggests that an RPO/LPO field design is the ideal beam arrangement in the treatment of rectal cancer with PBT with pencil beam scanning to best account for internal anatomic variations owing to intestinal gas and that single PA beam arrangements should be avoided. Resimulation or other gas-reducing measures should be undertaken when the maximal bowel diameter exceeds 3 cm on the short axis.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
References
- 1.Reyngold M, Niland J, Ter Veer A. Trends in intensity modulated radiation therapy use for locally advanced rectal cancer at National Comprehensive Cancer Network centers. Adv Radiat Oncol. 2018;3:34–41. doi: 10.1016/j.adro.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaios EJ, Wo JY. Proton beam radiotherapy for anal and rectal cancers. J Gastrointest Oncol. 2020;11:176–186. doi: 10.21037/jgo.2019.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong TS, Moughan J, Garofalo MC. NRG Oncology Radiation Therapy Oncology Group 0822: A phase 2 study of preoperative chemoradiation therapy using intensity modulated radiation therapy in combination with capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2015;93:29–36. doi: 10.1016/j.ijrobp.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman AT, Both S, Sharkoski T. Proton reirradiation of recurrent rectal cancer: Dosimetric comparison, toxicities, and preliminary outcomes. Int J Part Ther. 2014;1:2–13. [Google Scholar]
- 5.Quinn TJ, Kabolizadeh P. Rectal cancer in young patients: Incidence and outcome disparities. J Gastrointest Oncol. 2020;11:880–893. doi: 10.21037/jgo-20-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Crevoisier R, Tucker SL, Dong L. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–973. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Engels B, Soete G, Verellen D, Storme G. Conformal arc radiotherapy for prostate cancer: Increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys. 2009;74:388–391. doi: 10.1016/j.ijrobp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Heemsbergen WD, Hoogeman MS, Witte MG, Peeters ST, Incrocci L, Lebesque JV. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: Results from the Dutch trial of 68 GY versus 78 Gy. Int J Radiat Oncol Biol Phys. 2007;67:1418–1424. doi: 10.1016/j.ijrobp.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, SU-E-T-481 Zheng Y. Dosimetric effects of tissue heterogeneity in proton therapy: Monte Carlo simulation and experimental study using animal tissue phantoms. Med Phys. 2012;39:3815–3816. doi: 10.1118/1.4735570. [DOI] [PubMed] [Google Scholar]
- 10.Tatsuzaki H, Urie MM, Willett CG. 3-D comparative study of proton vs. x-ray radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 1992;22:369–374. doi: 10.1016/0360-3016(92)90056-n. [DOI] [PubMed] [Google Scholar]
- 11.Isacsson U, Montelius A, Jung B, Glimelius B. Comparative treatment planning between proton and X-ray therapy in locally advanced rectal cancer. Radiother Oncol. 1996;41:263–272. doi: 10.1016/s0167-8140(96)01851-8. [DOI] [PubMed] [Google Scholar]
- 12.Colaco RJ, Nichols RC, Huh S. Protons offer reduced bone marrow, small bowel, and urinary bladder exposure for patients receiving neoadjuvant radiotherapy for resectable rectal cancer. J Gastrointest Oncol. 2014;5:3–8. doi: 10.3978/j.issn.2078-6891.2013.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff HA, Wagner DM, Conradi LC. Irradiation with protons for the individualized treatment of patients with locally advanced rectal cancer: A planning study with clinical implications. Radiother Oncol. 2012;102:30–37. doi: 10.1016/j.radonc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Radu C, Norrlid O, Braendengen M, Hansson K, Isacsson U, Glimelius B. Integrated peripheral boost in preoperative radiotherapy for the locally most advanced non-resectable rectal cancer patients. Acta Oncol. 2013;52:528–537. doi: 10.3109/0284186X.2012.737022. [DOI] [PubMed] [Google Scholar]
- 15.Jeans EB, Jethwa KR, Harmsen WS. Clinical implementation of preoperative short-course pencil beam scanning proton therapy for patients with rectal cancer. Adv Radiat Oncol. 2020;5:865–870. doi: 10.1016/j.adro.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myerson RJ, Garofalo MC, El Naqa I. Elective clinical target volumes for conformal therapy in anorectal cancer: A radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gay HA, Barthold HJ, O'Meara E. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trofimov A, Nguyen PL, Coen JJ. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: A treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Unkelbach J, Trofimov A. Including robustness in multi-criteria optimization for intensity-modulated proton therapy. Phys Med Biol. 2012;57:591–608. doi: 10.1088/0031-9155/57/3/591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unkelbach J, Chan TC, Bortfeld T. Accounting for range uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2007;52:2755–2773. doi: 10.1088/0031-9155/52/10/009. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Lim GJ, Lee A. Uncertainty incorporated beam angle optimization for IMPT treatment planning. Med Phys. 2012;39:5248–5256. doi: 10.1118/1.4737870. [DOI] [PMC free article] [PubMed] [Google Scholar]