Abstract
The outbreak of COVID-19 was recognized in December 2019 in China and as of October5th, the pandemic was swept through 216 countries and infected around 34,824,108 individuals, thus posing an unprecedented threat to world's health and economy. Several researchers reported that, a significant mutation in membrane proteins and receptor binding sites of preceding severe acute respiratory syndrome coronavirus (SARS-CoV) to turned as novel SARS-CoV-2 virus and disease was named as COVID-19 (Coronavirus disease 2019). Unfortunately, there is no specific treatment available for COVID-19 patients. The lessons learned from the past management of SARS-CoV and other pandemics, have provided some insights to treat COVID-19. Currently, therapies like anti-viral treatment, immunomodulatory agents, plasma transfusion and supportive intervention etc., are using to treat the COVID-19. Few of these were proven to provide significant therapeutic benefits in treating the COVID-19, however no drug is approved by the regulatory agencies. As the fatality rate is high in patients with comorbid conditions, we have also enlightened the current in-line treatment therapies and specific treatment strategies in comorbid conditions to combat the emergence of COVID-19. In addition, pharmaceutical, biological companies and research institutions across the globe have begun to develop thesafe and effective vaccine for COVID-19. Globally around 170 teams of researchers are racing to develop the COVID-19 vaccine and here we have discussed about their current status of development. Furthermore, recent patents filed in association with COVID-19 was elaborated. This can help many individuals, researchers or health workers, in applying these principles for diagnosis/prevention/management/treatment of the current pandemic.
Keywords: Covid-19, Vaccines, Inline treatment, Patents, Clinical trials
Graphical abstract
Highlights
-
•
Reporting patents related information on COVID related aspects.
-
•
This manuscript has special focus on Treatment strategies for COVID 19 with Comorbidities.
-
•
Clinical trials of inline treatment approaches were also added.
-
•
COVID Management in comorbidities.
1. Introduction
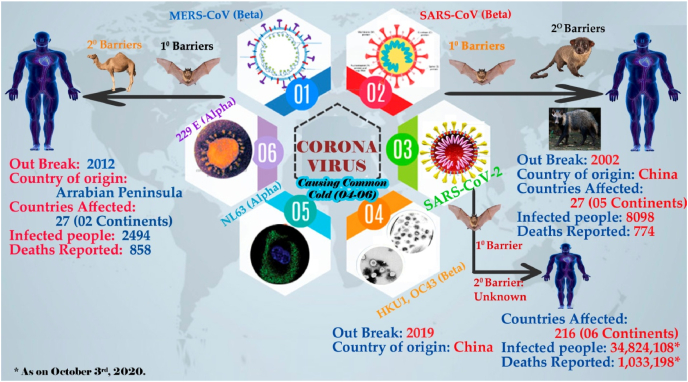
The coronavirus, believed to be originated 800 years before from the bats, as confirmed by many researchers (Y. chen et al., 2020, Chen et al., 2020a; Paules et al., 2020). On basis of genetic features, family coronaviridae is categorized as α, β, λ, and δ, where alpha (α) and beta (β) coronavirus are proven as pathogenic to humans and mammalians. Coronaviruses are a form of positive strand non segmented RNA viruses that can cause serious respiratory and neurological illness (Weiss and Leibowitz, 2011). Six different types of coronaviruses can cause disease among the humans, two of them can cause middle east respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV), while the rest four (HKU1, 229E,NL63 and OC43) are less pathogenic will cause only common cold (Cui et al., 2019; S. Su et al., 2016). MERS-CoV and SARS-CoV outbreak was happened in Middle east (2012) and China (2002) respectively (Zaki et al., 2012; Zhong et al., 2003). Again, in December 2019, few patients who are linked with seafood market in Wuhan of China, were shown pneumonia like symptoms. Consequently, about 20,000 sequences from each sample were obtained to match the genome. Genome matches confirmed 85% similarity with SARS-like β-coronavirus (N. F. C. Zhu et al., 2020). Further investigations on isolated virus conforms the new strain of Coronavirus and named this new virus as SARS-CoV 2 (Fig. 1).
Fig. 1.
Types of coronaviruses and their outbreaks.
Based on its severity and rapid spread across the World, World Health Organization (WHO) declared COVID-19 (coronavirus disease-2019) as a global pandemic disease. Previously, MERS became endemic in majority of the Middle Eastern countries. Diversity, rapid animal to human spread and broad genetic variation makes coronaviruses to emerge periodically in future also. Since COVID-19 emerged, the face of research and development, health care systems and social containment measures were completely reorganized to manage the mounting number of patients. At the time of this review writing (October 4, 2021), more than 235.7 Million cases and 4.8 Million deaths were recorded throughout the world (COVID-19 related analytics, graphs, and charts | Corona Tracker, 2020). Unfortunately, there are no medications that are approved for treating the coronavirus infections. However, as per the existing evidence and based on clinical trial data anti-viral drugs like Remdesivir, Favipiravir; anti-inflammatory drug dexamethasone; anti coagulants along with multi vitamin and mineral therapy showing promising results to attenuate the COVID 19 disease. Currently, drug repositioning therapies are successfully employed in treating the SARS infections. drug repositioning, refers to the application of existing drugs to treat the diseases other than its intended use (Amy Maxmen, 2020). This strategy is found its application owing to, lower costs and comparatively less time to reach the market In contrast to this, drug repositioning strategy has few limitations like, patent barriers, lack of funding opportunities, heterogeneity of population and complexity in regulatory approval process (Mercorelli et al., 2018; Pushpakom et al., 2018).
Among the deaths reported, majority of patients are having high prevalence of concomitant diseases like hypertension, cardio-vascular complications, diabetes, renal failure, and COPD (chronic obstructive pulmonary disease). The fatality rate is high in elder patients, with foresaid comorbidities (W. Guan, Liang, et al., 2020; Sanyaolu et al., 2020).
Consequently, the researchers emphasised more on the development of vaccine. Globally, more than 170 teams of researchers are working to develop a potent vaccine for mitigating the coronavirus infections.
Patent is a form of intellectual joy, that gives its inventor the legal right to prohibit others from using, selling, or making the same invention for a limited period of time. In many jurisdictions, patent rights fall under the private law and in some industries, it is considered as a competitive advantage. Quite a few patents were filed related to coronavirus management or treatment or prevention. The present context emphasis on current inline treatment, treatment strategies in comorbid conditions, progress of vaccine development and additionally, patents filed related to COVID-19. Patent related information further helps the researchers to control or manage or treat the present pandemic situation.
2. Inline treatment
As on date there is no specific approved treatment for either complete prevention or treatment of the most prevailing pandemic COVID-19. The daily rise of positive cases led to the urgent need of medication. Hence, scientists and researchers initiated and conducting intense search for the application of existing drugs in combating the present worse condition through repurposing in addition to discovery trials for new chemical entities. National and international collaborations are witnessed in this pandemic time to fight against the COVID-19. Such combined efforts have led to the process of exploring potential areas like treatment strategies through small molecule drugs, immunotherapies, convalescent plasma, cell based therapies, antibodyand interferon therapies (G. Li and De Clercq, 2020).
Another key point of specific attention is the swift genomic sequencing of COVID-19 which became a challenge for researchers. However, highly conserved regions involving enzymes or proteins among the related viruses like SARS-CoV, MERS-CoV and SARS-CoV-2 has given scope to propose some treatment options (G. Li and De Clercq, 2020; Lythgoe and Middleton, 2020).
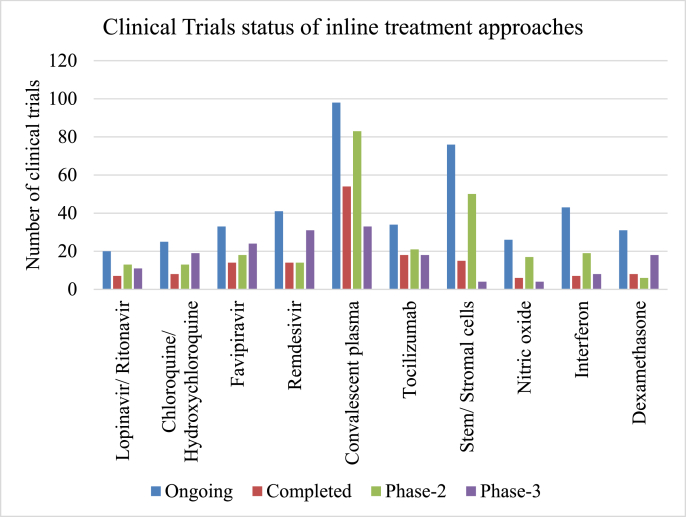
As per the present guidelines or preclinical and clinical outcomes, there are several proposed inline treatment strategies to combat the existing disease condition to possible extent. In this review a brief outline of such potential treatment strategies will be given in addition to the clinical trials status across the globe (As on October 30, 2021) (Fig. 2). A special focus is further given on treatment strategies for comorbidities like diabetes, hypertension, obesity, cardiovascular disease, etc.
Fig. 2.
Status of clinical trials of inline treatment approaches(Source: clinicaltrials.gov accessed on 30-09-2021).
2.1. Antivirals
As the present pandemic is of viral origin, the primary focussed repurposing of drugs is in the area of antivirals and the rapid trials are going on with antiviral drugs.
2.1.1. Lopinavir/ritonavir
As on date, 20 clinical trials are ongoing with combination of lopinavir/ritonavir as per the statistics at clinicaltrials.gov. Among them 13trials reached phase-2 and 11 trials were reached to phase-3. The priority for these drugs is due to their efficacy against earlier viruses SARS-CoV, MERS-CoV and HIV (Lythgoe and Middleton, 2020). Both of these drugs are protease inhibitors. Lopinavir has insufficient oral bioavailability due to the rapid catabolism by the specific enzyme, 3A4 isoenzyme which is a part of Cytochrome P450 enzyme system (Sham et al., 1998).In order to overcome this loss and significantly enhance the half-life of lopinavir, the concomitant administration of ritonavir is followed to inhibit the enzymatic catabolism of lopinavir. The combined efficacy of lopinavir/ritonavir has been proved against SARS-CoV during 2004 (Chu et al., 2004). However, yet no significant data is available to propose this combination against SARS-CoV-2 as trials are ongoing (Cao et al., 2020a).
Few earlier studies revealed that the use of lopinavir/ritonavir combination therapy has shown reduction in viral loads, fever, and pulmonary damage (Meini et al., 2020).
Cao et al. reported the results of a randomized controlled open-label clinical study where 99 severe COVID-19 patients received lopinavir/ritonavir and 100 patients received standard care for a duration of 2 weeks. The results of this study are statistically non-significant between the two groups, but shown more clinical improvement with the lopinavir/ritonavir patients compared with standard care (Cao et al., 2020b; Meini et al., 2020).
2.1.2. Remdesivir
A novel nucleotide analogue, Remdesivir, having antiviral effect against Ebola, Marburg, SARS-CoV and MERS-CoV has been in clinical trials to evaluate its effect against COVID-19 (Sheahan et al., 2017; M. Wang et al., 2020; Warren et al., 2016). Remdesivir is found to have a therapeutic benefit against COVID-19 based on the recovery rate in USA in remdesivir treated patients (Holshue et al., 2020; Lythgoe and Middleton, 2020). As on date there are 41 ongoing clinical trials to check the efficacy of remdesivir against COVID-19. CYP3A inhibitors are potential boosters of other antivirals as well, in particular remdesivir (Shytaj et al., 2021; Xie and Wang, 2021).
With a significant benefit of reducing the mortality of COVID-19 patients, USFDA has issued emergency use authorization for emergency use of remdesivir in COVID-19 positive patients (Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients With Moderate COVID-19, 2020; Samudrala et al., 2020). It has been reported that treatment with remdesivir and chloroquine has shown effective response against recently emerged coronavirus (Khan et al., 2020; Samudrala et al., 2020; M. Wang et al., 2020). However, still additional scientific data is necessary to be developed to establish the treatment strategy and dosage regimen.
However, in another study the use of remdesivir has not shown statistically significant improvement in clinical benefits, however shown reduction in recovery time of COVID patients in clinical trials and non-significant reduction in mortality rate (Davies et al., 2020; Yeming Wang et al., 2020). As there is no other available treatment option at this emergency situation, USFDA has re-issued the emergency use authorization for use of this drug (COVID-19 Update: FDA Broadens Emergency Use Authorization for Veklury (remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19 | FDA, 2020; USFDA, 2020).
2.1.3. Favipiravir
Favipiravir is found effective against wide range of viruses like resistant influenza, and able to inhibit RNA viruses like arena-, bunya- and filo-viruses (Delang et al., 2018; Jiancheng Zhang et al., 2020). Favipiravir has also shown potential benefit against SARS-CoV-2 (Cai et al., 2020).As a further attempt, a combination of favipiravir with interferon-α and also with baloxavirmarboxil are under clinical trials (Khan et al., 2020).
Based on the reports of Ministry of Science and Technology, China, favipiravir came into treatment strategy for COVID-19 based on the positive results in a clinical trial in Shenzhen and Wuhan cities. The symptoms like fever and severity of infections are managed with the application of favipiravir. However, a still confident and scientific data need to be assessed in this regard for its use in the treatment of COVID-19 patients with advanced stages (Samudrala et al., 2020).
2.1.4. Other antivirals
Antiviral drugs that are effective against influenza subtypes and other RNA viruses are also in investigation for their efficacy against COVID-19. Studies are also conducting on other antivirals like oseltamivir, ribavirin, umifenovir, triazavirin, danoprevir, azvudine, sofosbuvir, ledipasvir, sofosbuvir, daclatasvir, darunavir, cobicistat, emtricitabine, and tenofovir to test the efficacy of these drugs. (Lythgoe and Middleton, 2020).
EIDD-2801 is an antiviral drug that is providing potential results against SARS-CoV-2. EIDD-2801 is comparatively more preferred than remdesivir as the former is meant for oral administration whereas the latter must rely on intravenous administration. EIDD-1931 is another analogue, that is found to be effective against several types of viruses like SARS-CoV, MERS-CoV, and even the newly emerging SARS-CoV-2 based on cell based assays (Jiancheng Zhang et al., 2020).
2.2. Corticosteroids
Corticosteroids are known to treat acute respiratory distress disease and sepsis due to their anti-inflammatory, antifibrotic effects and suppression of collagen deposition. Use of dexamethasone for COVID-19 therapy has been proposed with the reported advantages of minimum fatality rate and hospital stays of SARS infect patients (R. C. Chen et al., 2006).
However, adverse effects associated with high doses of corticosteroids like delayed viral clearance, secondary infections and viral resistance need to be considered while testing their application in treatment of COVID-19 patients. A low to moderate doses are found to be beneficial in seriously ill patients suffering with COVID-19 pneumonia as per the guidelines of WHO(Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19), 2020).
The use of corticosteroids for SARS-CoV and MERS-CoV has reported detrimental effects with increased mortality and nosocomial infections for influenza and delayed virus clearance (J. W. Yang et al., 2020). Corticosteroids therapy has offered controversial implications in COVID-19. Even though there is an improvement in the symptoms and oxygenation with severe COVID-19 patients, there are also cases with increased fatality rate in observational studies. Hence, a more randomized controlled trials are to be conducted for better scientific understanding of the use of corticosteroids (J. W. Yang et al., 2020).
Early COVID-19 pneumonia was reported to be managed well with low-dose and short-term use of methylprednisolone (Yin et al., 2020). ARDS patients has shown decreased rate of mortality in a study with the use of methylprednisolone (C. Wu et al., 2020).
2.3. Anticoagulants
COVID-19 patients with high platelet count of elevated D-dimer may get beneficial effects with anticoagulant therapy [39]. Heparin is found to be showing better prognosis in patients affected with severe COVID-19 [40].The role of heparin is found to be promising as Microft-West et al. reported the interaction between heparin and SARS-CoV-2 spike S1 protein receptor binding domain (Mycroft-West et al., 2020). Another study results are suggesting that heparin is found to be effective in COVID-19 management beyond anticoagulant effect through its pleiotropic activity (Gozzo et al., 2020). However, studies with large number of subjects are necessary to confirm the benefits of heparin in management of COVID-19.
2.4. Antimalarials
2.4.1. Chloroquine and hydroxychloroquine
Chloroquine is a well-known drug for treating malaria and autoimmune diseases which is also known for its broad-spectrum antiviral action. In addition to the earlier reports on effect of chloroquine against SARS-CoV(Keyaerts et al., 2004; Savarino et al., 2006; Yiwu Yan et al., 2013), it has also been found effective against SARS-CoV-2 in improving the disease conditions like pneumonia, and pulmonary lesions (Gao et al., 2020; M. Wang et al., 2020). Chloroquine is known to decrease the severity and duration of disease (Jiancheng Zhang et al., 2020).In addition to chloroquine, its analogue and a relatively low toxic agent, hydroxychloroquine has been found to be another potential outcome for dealing with SARS-CoV-2 patients (J. Liu et al., 2020).
Chloroquine show antiviral effect by inhibiting the glycosylation of S-protein of angiotensin converting enzyme-2 and also by increasing the pH of acidic cellular organelles thereby avoiding the fusion of virus into the host cells (Savarino et al., 2003, 2006).These antimalarial agents are also shown to cause decreased expression of cytokines thereby minimizing the intensity of COVID-19 (Huang et al., 2020).Their low cost and high availability in addition to potency against COVID-19 has made them prominent for the treatment of COVID-19. Gautret et al. reported that a combination of hydroxychloroquine and azithromycin has shown significant reduction in viral load in COVID-19 patients (Gautret et al., 2020).
Later reports revealed that a short term use of hydroxychloroquine is safe and also the combination of chloroquine with azithromycin could develop other side effects like cardiovascular complications, angina, chest pain and heart failure (Lane et al., 2020). However, there are earlier reports as well as recovery trial stating that chloroquine and hydroxychloroquine were not effective against viral treatments like SARS-CoV-2 and no significant benefit over mortality rate (Group, 2020; Savarino, 2011).
2.5. Monoclonal antibodies
2.5.1. Tocilizumab and sarilumab
COVID-19 patients are known to invoke a hyperinflammatory state with multiple calls and mediators like interleukins (IL-1, IL-6, IL-12, IL-18), tumor necrosis factor alpha (TNFα) etc. Seriously ill patients infected with COVID-19 are found to have cytokine dysregulation majorly involved with IL-6. Hence, IL-6 inhibitors are proposed in the treatment strategies for COVID-19 (Cao et al., 2020b).
COVID-19 patients with cytokine storm, particularly IL-6 are found to be responding with IL-6 receptor antagonists namely tocilizumab and sarilumab. Numerous clinicals are going on with tocilizumab and sarilumab (Mehta et al., 2020).
Tocilizumab and sarilumab, the targeted monoclonal antibodies are found to reduce the evident features of cytokine driven hyperinflammation like dyspnoea, persistent fever and elevated markers showing improved clinical outcomes through decreased cell proliferation, differentiation, oxidative stress and exudation (Cao et al., 2020b). These antibodies are included in the international guidelines for management of severe of critically ill patients. Numerous clinical trials are going on for increased interest in testing the clinical implications of these drugs. Tocilizumab is reported to be reducing the likelihood of advancement to death in COVID-19 patients (Salama et al., 2020).
2.5.2. Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody that was approved by USFDA for several types of cancers like metastatic colorectal cancer, lung cancer, renal cancer, cervical cancer, ovarian cancer, etc. As it acts against vascular endothelial growth factor (VEGF), bevacizumab is proposed to be used in treatment of COVID-19 because VEGF is responsible for acute respiratory distress, pulmonary edema, dyspnea, and acute lung injury. Clinical trials are initiated on this drug for confirming its application in clinical improvement of COVID-19 cases (Samudrala et al., 2020).Bevacizumab is well known to treat acute respiratory distress syndrome which is highly associated complication in COVID-19 ill patients (Jiancheng Zhang et al., 2020).
2.6. Ivermectin
In vitro studies revealed that the anti-parasitic agent, ivermectin could effectively act against SARS-CoV-2 growth in cell cultures (Caly et al., 2020).Further studies are necessary to prove its efficacy and safety for managing SARS-CoV-2 in animals or humans. Melo et al. reported that their study supports the use of immunomodulatory drugs like ivermectin to improve the clinical condition of COVID-19 infected patients (Melo et al., 2021).
2.7. Convalescent plasma therapy
Plasma therapy has shown improved signs of relief and treatment. Here, in this treatment convalescent plasma collected from the recovered COVID-19 patients and it has been approved by several regulatory bodies for treatment (Roback and Guarner, 2020). USFDA has granted clinical permission for the application of convalescent plasma to the treatment of critically ill patients affected by COVID-19 (Investigational COVID-19 Convalescent Plasma-Emergency INDs Frequently Asked Questions, 2020).Several studies reported that, recovery from the infection was observed approximately after a week of transfusion of convalescent plasma.
2.8. Vitamins and mineral
Vitamin C and D are found to be beneficial in controlling the disease condition of COVID-19. Grant et al. reported the importance of vitamin D in reducing the viral replication and risk of respiratory tract infections in COID-19 patients (Grant et al., 2020).Zinc supplementation is thought to combat the loss of taste and smell symptoms in COVID-19 patients. In addition, the role of zinc as potential inhibitor of various RNA viruses such as SARS-CoV has initiated the clinical trials to test its effect against COVID-19 (Keyhan et al., 2020; Jiancheng Zhang et al., 2020).
2.9. Janus kinase inhibitors
A highly selective janus kinase (JAK) inhibitor, Baricitinib is found to be useful in the management of COVID-19 disease by impeding the hyperimmune responses and also by inhibiting the invasion of host cells with SARS-CoV-2 virus (Richardson et al., 2020).On the other hand, this agent may also cause inhibition of production of interferons which is detrimental in patients suffering from SARS-CoV-2. Hence, the use of baricitinib should be done very cautiously preferably in ill patients with abnormal biomarkers to yield some clinical benefits (Jiancheng Zhang et al., 2020).
2.10. Mesenchymal stem cells
A recent study revealed that transplantation of mesenchymal stem cells has shown improved functional outcomes at significant level with noticeable adverse effects in COID-19 patients during a study held at Beijing YouAn Hospital (Leng et al., 2020).
2.11. Other treatment strategies
The use of inhaled nitric oxide in SARS infected patients has shown improved arterial oxygenation and reduced the need of ventilation. Hence, clinical trials are going on for testing the application of inhaled nitric oxide in COVID-19 patients suffering with acute respiratory distress syndrome (L. Chen et al., 2004).
Interferon alpha-1a, alpha-1b, beta-1a, beta-1b are known to show beneficial effects in COVID-19 patients and hence numerous ongoing clinical trials are there in this area (Jiancheng Zhang et al., 2020).Intravenous immunoglobulins especially purified IgG products are meant for benefit in respiratory syndromes and hence clinical trials are initiated to test their application in COVID-19, however their numerous adverse effects like myalgia, thromboembolism, and renal failure need to be addressed.
Natural killer cell therapy, CYNK-001 comprising from placental hematopoietic stem cells has obtained investigational new drug application approved by USFDA (Celularity Announces FDA Clearance of IND Application for CYNK-001 in Coronavirus, First in Cellular Therapy, 2020).This therapy has found to show potential inhibition against SARS-CoV-2 via direct killing of infected host cells as well as indirect induction of immune response. However, the efficacy and safety of this therapy need to be confirmed by ongoing clinical trials (Jiancheng Zhang et al., 2020).Αlpha-lipoic acid is an antioxidant that is reported to handle the oxidative stress which is playing a major role in coronavirus infections (Khan et al., 2020).
3. Treatment strategies for COVID-19 with comorbidities
It is reported that COVID-19 infected patients with comorbidities are at higher risk than others (Fang et al., 2020). Table 1 depicts the literature that represents the impact of comorbid diseases towards COVID-19 severity.
Table 1.
Percent of comorbid cases in COVID-19 positive cases.
| Comorbid disease | Percent reported | Patients population | Reference |
|---|---|---|---|
| Cerebrovascular diseases | 22% | 52 patients | (X. Yang et al., 2020) |
| Diabetes | 22% | ||
| Hypertension | 23·7% | 1099 patients | (W. Guan, Ni, et al., 2020) |
| Diabetes mellitus | 16·2% | ||
| Coronary heart diseases | 5·8% | ||
| Cerebrovascular disease | 2·3% | ||
| Hypertension | 30% | 140 patients | (Jin jin Zhang et al., 2020) |
| Diabetes | 12% | ||
| Case-fatality rate cardiovascular disease | 10.5% | 1023 deaths among 44,672 confirmed cases | (Z. Wu and McGoogan, 2020) |
| Diabetes | 7.3% | ||
| Chronic respiratory disease | 6.3% | ||
| Hypertension | 6.0% | ||
| Cancer | 5.6% | ||
| Diabetes | 8.9% | 146 patients | Fadini et al. (2020) |
| Diabetes | 8.2% | 1590 patients | (W. J. Guan et al., 2020) |
| Diabetes | 34.7%; 9.7% | 4103 patients (hospitalized vs non-hospitalized) | Petrilli et al. (2020) |
| Obesity | 39.5%; 30.8% | ||
| Diabetes | 24% | 258 patients | (Y. Zhang et al., 2020) |
| Diabetes | 9.8% | 16003 patients | Kumar et al. (2020) |
| Diabetes | 2.79% | 1382 patients | Roncon et al. (2020) |
| Diabetes | 19% | 191 patients | Zhou et al. (2020) |
| Diabetes | 13% | 7337 patients | (L. L. Zhu et al., 2020) |
| Diabetes | 25% | 193 patients | Yongli Yan et al. (2020) |
| Diabetes | 44% | 59 patients | Sardu et al. (2020) |
| Diabetes | 4.8% | 61414470 patients | Barron et al. (2020) |
It is observed that major proportion of COVID-19 patients are having comorbidities and it might be giving a claim that patients suffering from diseases like diabetes, hypertension are more prone to infection by SARS-CoV-2. One of the reasons might be the over expression of angiotensin-converting enzyme 2 (ACE2)in these diseases (Gracia-Ramos, 2020).
The pathogenic viruses SARS-CoV and SARS-CoV-2 bind to the host cells through ACE-2 receptors which is expressed on different organs like lungs, kidneys, blood vessels and intestine (Wan et al., 2020). Patients (Type-1, type-2 diabetes, and hypertension patients) taking ACE inhibitors and angiotensin II type-I receptor blockers (ARBs) are known to show upregulation of ACE2 (X. C. Li et al., 2017). Thiazolidinediones and ibuprofen usage are also meant for over expression of ACE2.
These factors, in turn favour the infection of coronavirus in humans due to the overexpression of ACE2. However, this hypothesis may need strong scientific assessment with more clinical investigation. In addition, there is a conflict with ACE2 pharmacological effects and its polymorphic effects. COVID-19 patients treated with medicaments that increase ACE2 levels are at still higher risk due to comorbidities like diabetes, hypertension, and cardiac diseases (Fang et al., 2020).However, the prevalence of comorbid diseases varies from country to country based on the age factors and living styles. For example, China has highest prevalence of diabetes, hypertension, and cardiovascular diseases in their population (Du et al., 2019; IDF Diabetes Atlas 9th edition 2019, 2019; Z. Wang et al., 2018; Y. Xu et al., 2013).
Since non-steroidal anti-inflammatory drugs are commonly used in elderly patients, this may result in worsening of the condition of COVID-19 patients with adverse effects like renal failure, heart problems, and gastrointestinal toxicity. Hence, a proper management of the treatment should be done (Wongrakpanich et al., 2018).Overall, the severity of the diseaseis high in older population and the population who had more significant number of comorbid conditions than others. These results may suggest that age and comorbidities are risk factors for COVID-19 (J. Yang et al., 2020).
3.1. Diabetes
Diabetes is one of the most prevalent comorbidities that worsen COVID-19 to severe levels and even lead to mortality. Treatment for COVID-19 patients associated with diabetes is a challenging task as multiple implications need to be managed. Adequate glycaemic control is another challenge in addition to the goal of reducing the severity of coronavirus load. It needs an extensive team effort to manage the complications in COVID-19 patients with diabetes (Apicella et al., 2017).
The coronavirus on binding to the ACE2 receptors, especially on pancreatic beta-cells leads to acute loss of insulin secretory capacity in addition to cytokine storm which may lead to rapid metabolic deterioration. This lead to development of diabetic ketoacidosis or hyperglycaemic hyperosmolar syndrome (Hamming et al., 2004).
On August 26, 2020, Ministry of Health and Family Welfare, Govt. of India has released a document for clinical guidance on diabetes management at COVID-19 (Government of India & Ministry of Health & Family Welfare, n.d.).This guidance document has clearly given the instructions to screen every COVID-19 patient for hyperglycaemia for 1 pre-meal and 1 post-meal capillary blood glucose values. If found diabetic, every patient should be kept on diabetic diet as per the proposed diet chart following the timing and quantity of diet advised.The pre-meal and post-meal glucose values are specified as <140 and < 180 mg/dL respectively for continuing the existing oral anti-diabetic drugs if the patient is conscious, COVID symptoms are mild, kidney and liver function tests are normal.
A follow up capillary blood glucose testing over next 24 h is advised for those showing pre-meal and post-meal glucose values ≥ 140 or ≥180 mg/dL respectively. In case the values of pre-meal and post-meal are in the range of 140–150 and 180–200 mg/dL respectively, then diabetic meal is advised. If there is a significant elevation of values of pre-meal ≥150 and post-meal ≥200 mg/dL or the initial testing shows any value ≥ 200 mg/dL, then oral anti-diabetics or insulin are to be initiated along with diabetic diet (Government of India & Ministry of Health & Family Welfare, n.d.).
If the COVID-19 patient is proposed to be on steroids treatment of any drugs with potential effect on glycaemic or else the severity of COVID-19 is increasing which may lead to stress glycaemia, then there should be a repeated monitoring of blood glucose levels. At any point of testing if the blood glucose level is ≥ 250 mg/dL, urine/blood ketone levels are to be tested and if found positive, then an endocrinologist need to be consulted immediately. At any point, if the blood glucose level of pre-meal is ≥ 300 mg/dL and/or post-meal is ≥ 400 mg/dL, then an endocrinologist needs to be consulted immediately irrespective of ketone levels in order to initiate the insulin infusion. In the follow up testing on later days, fasting plasma glucose ≥126 mg/dL and/or HbA1c ≥ 6.5% are considered to be as diabetic mellitus positive.
In case the patient has no earlier diabetes, history but found to be diabetic during these tests with pre-meal blood glucose value 150–180 mg/dL and/or post-meal blood glucose value 200–250 mg/dL, then an endocrinologist needs to be consulted for initiating the oral anti-diabetic drug treatment. In emergency cases to avoid delay in treatment, metformin and gliptin are advised. In case of excess values, insulin infusion can be initiated as advised by an endocrinologist.
Earlier reports reveal that administration of chloroquine or hydroxychloroquine may lead to hypoglycaemia especially in patients taking insulin or sulfonylureas, because of their effect on insulin secretion and degradation (Ünübol et al., 2011). Also, antiviral drugs proposed for COVID-19 like lopinavir and ritonavir are known to cause hyperglycaemia and cause imbalance in the glycaemic control (Paengsai et al., 2019). These antiviral agents can also cause hepatic and muscular toxicity when used along with statins in patients with fatty liver disease (Bruno et al., 2006). Pharmacokinetic interactions are noticeable with antivirals against anti-diabetic drugs (Liverpool COVID-19 Interactions, 2020). Use of glucocorticoids in COVID-19 patients with severe ARDS can worsen insulin resistance, glycaemic control, causing sever hyperglycaemia and sustained gluconeogenesis. This might be due to reduced insulin sensitivity and insulin secretion exerted by glucocorticoids and also by interfering with GLP-1 effects along with enhanced production of glucagon.
3.2. Hypertension
Hypertension is another most frequent comorbidity observed in COVID-19 patients (J. Yang et al., 2020). Use of ACE inhibitors or ARBs in COVID-19 patients with hypertension has shown a lower rate of severe disease and a lower inflammatory response (Meng et al., 2020). ACE inhibitors are found to be unlikely to account for the connection of hypertension with COVID-19 (Reynolds et al., 2020).De Abajo et al. reported a case-population study with 1139 adult COVID-19 positive patients taking antihypertensive drugs. In these patients, they used predominantly ACE inhibitors and ARBs; in few patients they have given aldosterone antagonists or renin inhibitors. Comparing with other antihypertensive agents, the use of RAAS inhibitors were found to be not associated with increased risk of COVID-19 that need hospital admission (odds ratio 0.94, 95% CI 0.77–1.15) or increased risk of severity in complications with COVID-19 that needs intensive care or leading to a fatal outcome (1.08, 0.80–1.47). These observations are not influenced by age, sex or background cardiovascular risk (de Abajo et al., 2020; Williams and Zhang, 2020). Use of ACE inhibitors in hypertensive patients and also associated with type 2 diabetes patients especially those with poor control over blood glucose may increase severity of COVID-19 (Cure and Cumhur Cure, 2020).
3.3. Obesity
Obesity associated COVID-19 lead to severe illness and death (Cariou et al., 2020; Shi et al., 2020).Possible proposed mechanisms include detrimental restrictive ventilatory effect of abdominal fat, impaired lung perfusion due to intravascular disseminated coagulation, deep venous thrombosis, immune dysregulation, and chronic inflammation causing organ failure in severe COVID-19 cases (Connors and Levy, 2020; Sattar et al., 2020; Simonnet et al., 2020; Wichmann et al., 2020).Low-molecular weight heparin was reported to reduce mortality rate in such COVID-19 associated obesity cases (Tang et al., 2020).
There is an increased risk of severity in COVID-19 patients with a BMI of >25 kg/m2 and fatty liver disease than in patients without obesity (Zheng et al., 2020).Obese patients due to physical inactivity, insulin resistance and gut dysbiosis, may result in inflammatory response to infection with COVID-19.Obese individuals suffer with chronic inflammation particularly elevated levels of IL-6 might be get beneficial results with the use of tocilizumab. It can reduce the fever and oxygen requirement, however it may not be beneficial with acute therapy (X. Xu et al., 2020). Horby et al. reported that dexamethasone use in obese patients with severe COVID-19 can reduce mortality rate by 8–26% (Horby et al., 2020). Limited information is available for the use of other treatment options like statins, nonsteroidal anti-inflammatory drugs and ARBs for obese patients with COVID-19 (Popkin et al., 2020).
3.4. Cardiovascular diseases
COVID-19 patients association with cardiovascular disease are also more prevailing and are at a higher risk of mortality (Babapoor-Farrokhran et al., 2020; B. Li et al., 2020). Kim et al. reported a COVID-19 patient associated with acute myocarditis (Kim et al., 2020). Buja et al. reported the cardiopulmonary pathology of coronavirus disease where an autopsy reported that 13 out of 23 patients shown cardiac manifestations associated with pulmonary issues (Buja et al., 2020). Li et al. has reported the influence of novel coronavirus on heart injury (J. J. W. Li et al., 2020). Atrial fibrillation and heart failure are also reported as major implications for death in covid affected patients (Gawałko et al., 2020; Sanyaolu et al., 2020).
Acute myocarditis has been found as a reverse stress-induced syndrome in COVID-19 patients (Sala et al., 2020). Possible mechanisms for cardiovascular complications might include reduced systemic oxygenation due to pneumonia with increased cardiac demand, immune dysregulation, electrolyte imbalance, or associated with adverse effects of drugs like azithromycin and hydroxychloroquine (Babapoor-Farrokhran et al., 2020).
For venous thromboembolic complications, anticoagulation therapy with low molecular weight heparin is found to reduce the mortality risk.COVID-19 patients with cardiovascular disease under treatment with antiplatelet agents and anticoagulants may show significant drug-drug interactions with antiviral therapies like remdesivir, lopinavir/ritonavir and antibody therapies like tocilizumab or sarilumab (Bikdeli et al., 2020).
Statins are known to be commonly prescribed for cardiovascular patients with COVID-19 for their anti-inflammatory effects which involves Myd88 and downstream nuclear factor kappa B signal pathways. They show beneficial effect on influenza infection and hyper-inflammatory ARDS (Calfee et al., 2018; Y. B. Su et al., 2020).
Chloroquine and hydroxychloroquine in combination with azithromycin can lead to QT prolongation in cardiovascular patients with COVID-19. They may also cause rare complications like cardiomyopathy, atrioventricular and bundle branch block (Chatre et al., 2018).
ACE inhibitors and ARBs are not known to affect the morbidity and mortality of COVID-19 patients with cardiovascular disease.
3.5. Others
High levels of c-reactive protein, ferritin, procalcitonin, neutrophil-to-lymphocyte ratio, inflammatory cytokines and chemokines are associated with both COVID-19 severity and mortality (Henry et al., 2020; Petrilli et al., 2020; Zhou et al., 2020). Severe inflammatory infiltration of lungs, hear, spleen, kidneys and lymph nodes is found with COVID-19 patients of severity (chen et al., 2020; Diao et al., 2020; Z. Xu et al., 2020; Jin jin Zhang et al., 2020). COVID-19 is also associated with increased coagulation activity (Tang et al., 2020). Pulmonary intravascular coagulation has been reported in COVID-19 patients (McGonagle et al., 2020).
4. Current status of COVID-19 vaccines development
Vaccines are said to the most successful approach in preventing several infectious diseases in view of the fact that they are more effective than the treatment and can trim down the morbidity and mortality rate devoid of long-lasting effects (André, 2001; C. Zhang et al., 2019). Since two decades, three corona viruses namely SARS-CoV, MERS-CoV, and SARS-CoV-2, were emerged throughout the world, creating significant threat to global health (Guarner, 2020). Scientific experts feel that the pandemic of SARS-CoV-2 might be curtailed only by vaccination. Historically, there have been several difficulties faced by researchers in developing the vaccine for coronavirus. Coronavirus vaccines are not proven as effective in preventing the acquisition of disease, besides they found to be immunogenic in animal models (Koirala et al., 2020). There is a concern that, vaccination may not induce lifelong immunity as with the natural infection and re-infection may be observed. Disappointedly, vaccines for SARS-CoV and MERS-CoV raised some safety concern related to Th2 mediated immunopathology in animal studies (Edridge et al., 2020; Roper and Rehm, 2009).
Coronaviruses consists of a helical nucleocapsid (N) covering single strand RNA genome and outer envelope having spike proteins (S), matrix proteins (M) and envelope protein (E) (Boopathi et al., 2020). The S protein contains receptor binding domain (RBD), which is responsible for binding of ACE2 receptor and entry into the cell. Among all structural proteins, S protein was the major target antigen in vaccine development, as it found to elicit neutralizing antibody (Buchholz et al., 2004; Walls et al., 2020).
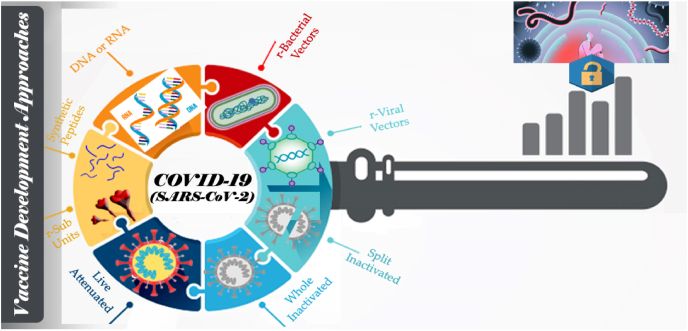
In current pandemic emergency of COVID-19, to contest this threatful condition, the world is truly required the potential vaccine. Vaccines approved for human use are generally live attenuated vaccines or inactivated viruses or protein polysaccharide conjugates or virus like particles etc. Multiple strategies as shown in Fig. 3 have been applied for SARS-CoV-2 vaccines to overcome the roadblocks that are associated with traditional vaccine development.
Fig. 3.
Strategies applied to develop vaccines against COVID-19.
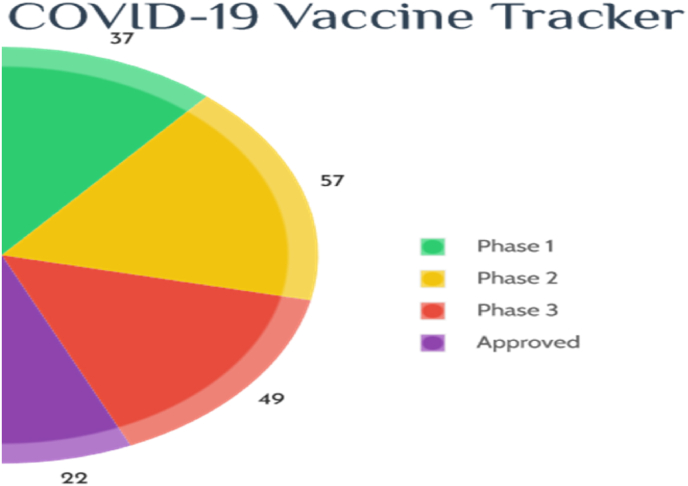
DNA and RNA composed vaccine, recombinant proteins and viral vectors are amongst them (X. X. Liu et al., 2020). As of October 01, 2021, more than 150 vaccines racing at various development stages in global COVID-19 vaccine research and development landscape and the progress was showed in Fig. 4. As per the existing data, 452 vaccine trails are in the various stages of development, their strategy and characteristics were given in Table 2 (approved) and Table 3(ongoing).
Fig. 4.
COVID Vaccine tracker.
Table 2.
List of approved COVID-19 vaccines.
| S. No | Name | Vaccine type | Primary developers | Country of origin |
|---|---|---|---|---|
| Moderna COVID-19 Vaccine (mRNA-1273); also called Spikevax | mRNA-based vaccine | Moderna, BARDA, NIAID | US | |
| Comirnaty (BNT162b2) | mRNA-based vaccine | Pfizer, BioNTech; Fosun Pharma | Multinational | |
| COVID-19 Vaccine AstraZeneca (AZD1222); also known as Vaxzevria and Covishield | Adenovirus vaccine | BARDA, OWS | UK | |
| Sputnik V | Recombinant adenovirus vaccine (rAd26 and rAd5) | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia | |
| Sputnik Light | Recombinant adenovirus vaccine (rAd26) | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Russia | |
| COVID-19 Vaccine Janssen (JNJ-78436735; Ad26.COV2.S) | Non-replicating viral vector | Janssen Vaccines (Johnson & Johnson) | The Netherlands, US | |
| CoronaVac | Inactivated vaccine (formalin with alum adjuvant) | Sinovac | China | |
| BBIBP-CorV | Inactivated vaccine | Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | China | |
| EpiVacCorona | Peptide vaccine | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology | Russia | |
| Convidicea (PakVac, Ad5-nCoV) | Recombinant vaccine (adenovirus type 5 vector) | CanSino Biologics | China |
Table 3.
Vaccines in the various stages of development, their characteristics.
| S. No | Candidate | Mechanism | Sponsor | Trial Phase | Institution |
|---|---|---|---|---|---|
| Unnamed vaccine candidate | Recombinant vaccine (Sf9 cells) | WestVac Biopharma Co., Ltd.; West China Hospital; Sichuan University; | Phase 3 | Jiangsu Province Centers for Disease Control and Prevention | |
| ARCoV | mRNA-based vaccine | Walvax Biotechnology Co., Ltd.; Abogen Biosciences Co. Ltd.; Yuxi Walvax Biotechnology Co., Ltd. | Phase 3 | Xiangfen CDC | |
| NVX-CoV2373 | Nanoparticle vaccine | Novavax | Phase 3 | Novavax | |
| Unnamed vaccine candidate | Plant-based adjuvant vaccine | Medicago; GSK; Dynavax | Phase 3 | Medicago | |
| VLA2001 | Inactivated vaccine | Valneva; UK National Institute for Health Research | Phase 3 | Multiple NIHR testing sites | |
| Corbevax | Adjuvanted protein subunit vaccine | Biological E, Baylor College of Medicine, Dynavax, CEPI | Phase 3 | Various | |
| Vidprevtyn | Recombinant protein vaccine | Sanofi; GlaxoSmithKline | Phase 3 | Various | |
| Nanocovax | Recombinant vaccine (Spike protein) | Nanogen Biopharmaceutical | Phase 3 | Military Medical Academy (Vietnam) | |
| V-01 | Recombinant protein vaccine | Guangdong Provincial Center for Disease Control and Prevention; Gaozhou Municipal Center for Disease Control and Prevention; Zhuhai Livzonumab Biotechnology Co., Ltd. | Phase 3 | Livzon Mabpharm Inc. | |
| Razi Cov Pars | Recombinant vaccine (Spike protein) | Razi Vaccine and Serum Research Institute | Phase 3 | Tehran Rasoul Akram Hospital; Karaj, Hesarak, Razi Vaccine and Serum Research Institute | |
| GBP510 | Nanoparticle vaccine | SK bioscience Co., Ltd.; GSK; University of Washington; CEPI | Phase 3 | Various | |
| CVnCoV | mRNA-based vaccine | CureVac; GSK | Phase 2b/3 | CureVac | |
| Bacillus Calmette-Guerin (BCG) vaccine | Live-attenuated vaccine | University of Melbourne and Murdoch Children's Research Institute; Radboud University Medical Center; Faustman Lab at Massachusetts General Hospital | Phase 2/3 | University of Melbourne and Murdoch Children's Research Institute; Radboud University Medical Center; Faustman Lab at Massachusetts General Hospital | |
| INO-4800 | DNA vaccine (plasmid) | Inovio Pharmaceuticals; Advaccine | Phase 2/3 | Center for Pharmaceutical Research, Kansas City. Mo.; University of Pennsylvania, Philadelphia | |
| No name announced | Adenovirus-based vaccine | ImmunityBio; NantKwest | Phase 2/3 | ||
| UB-612 | Multitope peptide-based vaccine | Vaxxinity | Phase 2/3 | United Biomedical Inc. (UBI) | |
| GRAd-COV2 | Adenovirus-based vaccine | ReiThera; Leukocare; Univercells | Phase 2/3 | Lazzaro Spallanzani National Institute for Infectious Diseases | |
| SCB-2019 | Protein subunit vaccine | GlaxoSmithKline, Sanofi, Clover Biopharmaceuticals, Dynavax and Xiamen Innovax; CEPI | Phase 2/3 | Linear Clinical Research (Australia) | |
| BBV154 | Intranasal vaccine | Bharat Biotech | Phase 2/3 | Various |
Just weeks after the identification of SARS-CoV-2 genetic sequence, Moderna Company together with NIAID, BARDA, made an announcement that mRNA-1273 (company's experimental COVID-19 vaccine) is all set for human trails (Zhai et al., 2020). This vaccine is one of the most hopeful and a notable fast vaccine development cycle was observed thereafter.
4.1. mRNA-1273
Moderna applied lipid nanocarrier technology for mRNA-1273 to deliver the mRNA into the host cell unlike the conventional vaccination approach i.e., using of weakened strain of the virus to obtain SARS-CoV-2 specific antibodies and effective immune responses. mRNA vaccine was proven to be superior in contrast to other conventional vaccines, as it possess high potency, safety and short production cycle (Ahn et al., 2020; Moderna, 2020). Dose dependent, open label Phase –I trail was conducted in 45 healthy volunteers to assess its safety with respect to effective dose. Few of the volunteers were identified with several adverse effects such as fatigue, fever, headache, pain at the site on administration and muscle ache. Phase III studies were started in July 2020 at 89 locations in the United States with almost 30,000 participants. An interventional, randomized and placebo-controlled study was designed to check for its immunogenicity and safety.
4.2. CoronaVac
CoronaVac, developed by SinoVac have the virions which are inactivated using radiation or chemicals or with/without adjuvant and antigenically similar to the live virus. Phase II studies among 600 volunteers, confirms the immunogenicity in showing 92% seroconversion even at lower doses. Pain at the site of injection and mild in severity were observed adverse effects. Phase III study was started in various location of China, Brazil, Bandung and Indonesia with a sample size of 8870 (Yan-Jun Zhang et al., 2020). Double blind, randomized design was employed for this study.
4.3. Sinopharm vaccine
Similar product of CoronaVac developed by Sinopharm in association with Wuhan Institute of Biological Products and China National Pharmaceutical Group (Chinese Clinical Trial Register (ChiCTR) - The world health organization international clinical trials registered organization registered platform, 2020). Neutralizing antibodies were produced after 14 days with 2 doses of administration. 21,000 healthy volunteers are participating in Phase III trials in United Arab Emirates and Bahrain.
4.4. Ad5-nCoV
Yet another approach on basis of Cansino's adenovirus type 5 as a vector was proposed by CanSino Biologics to load the SARS-CoV-2 gene fragments in order to express the S-protein. Preclinical data from the animal study demonstrated that Ad5-nCoV can show the strong immune response and encouraging safety profiles (F. C. N. Zhu et al., 2020). Dose escalation, open-label, non-randomized phase 1 trials were conducted in healthy adults aged between 18 and 60 years. Subjects were divided into three dose groups (5 × 1010, 1 × 1011, 1.5 × 1011 viral particles) to receive the test by intramuscular injection and safety was assessed over 28 days. The Ad5 vectored vaccine was concluded as tolerable and immunogenic and rapid specific T-cell responses were noted. Phase II studies conducted in 508 volunteers, showed the neutralizing antibody and T Cell responses.
Another phase 1/2 a studies were conducted on Ad26.COV2.S, a non-replicating adenovirus 26 based vector in 402 healthy volunteers. Ad26.COV2.S was administered at a dose level of 5 × 1010, 1 × 1011 viral particle per one dose of vaccine. The safety and immunogenicity after a single dose, supports for further clinical development (Sadoff et al., 2020).
4.5. AZD1222
This vaccine comes under the category of recombinant vaccine/viral vectors vaccine. It was developed by Oxford vaccine trial group together with AstraZeneca. Earlier phase studies concluded the formation of spike-specific antibodies and neutralizing antibodies on 28th and 56th day respectively. Additionally, paracetamol was administered for some participants to enhance the tolerability. Pedro M et al. group used adenovirus as a vector in this vaccine and this vector carries the gene that expresses the spike protein of SARS-CoV-2. This study was registered with clinicaltrials.gov and the registration number is NCT04324606.In this study they assessed the safety, tolerability, reactogenicity and immunogenicity of the vaccine.
In this study ChAdOx1 nCoV-19Vaccine formulation compared with control vaccine that is quadrivalent conjugate meningococcal vaccine (MenACWY). This study is single blind, randomized controlled study conducted in UK at 5 clinical sites. For this study they recruited 1077 healthy adults aged 18–55 years and with no history of laboratory confirmed COVID 19 or of COVID-19-like symptoms. All the participants were randomly assigned to receive either ChAdOx1 nCoV-19 or MenACWY vaccine at the dose of 5 × 101⁰ viral particles as a single intramuscular injection. After the vaccine dose, Anti spike IgG response peaked on day 28 and in more than 90% of participantsneutralising antibodies, humoral and cellular immunogenicity was generated.
Local and systemic adverse events such as fatigue, headache, and local tenderness occurred commonly in a group of volunteers who have received ChAdOx1 nCoV-19. However, all these symptoms were very mild and tolerable. This study results supported that this vaccine has good safety profile and able to induce both humoral and cell mediated immunity. This encouraging result supports the large-scale evaluation of this vaccine in phase 3 trial. Phase III interventional studies were conducting among 30,000 volunteers in 20 various locations of United Kingdom.Surprisingly, on 8thof September, AstraZeneca announced that, they voluntarily paused the randomized clinical trial of AZD1222 as the volunteers developed an unexpected illness and should allow sufficient time to review the safety data (Samudrala et al., 2020).
4.6. BNT162
Another mRNA approach was observed with BNT162 vaccine, a lipid nano formulation containing nucleoside-mRNA that encodes trimerized SARS-CoV-2 S-glycoprotein RBD developed by BioNTech. Strong RBD-binding of IgG and neutralizing antibodies were reached to maximum level after 7 days of booster dose administration. Mark J. Mulligan et al. reported the safety, immunogenicity and tolerability data from the placebo controlled, observer-blinded dose escalation studies in 45 healthy adults (18–55 years of age) (Mulligan et al., 2020). Subjects are received two doses of 10 μg, 30 μg or 100 μg separated by 21 days. Increased reactogenicity and increased immunogenicity was observed after a single dose compared to 30 μg of dose, accordingly 100 μg dose was not administered. These results support further evaluation of this mRNA vaccine candidate.
4.7. Sputnik V
On Aug 11, 2020, The Russian vaccine named Sputnik is the first approved vaccine against severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) by the Russian government. The vaccine, which is based on two adenovirus vectors, namely recombinant adenovirus type 26 (rAd26) vector and a recombinant adenovirus type 5 (rAd5) vector was developed by the Gamaleya National Center of Epidemiology and Microbiology, Russia. The phase 1/2 results have been published in The Lancet. This vaccine consisting of two components, rAd26 vector and a rAd5 vector, both these vectors are carrying the gene for SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S). Sputnik V was prepared in two formulations namely, frozen, and lyophilised forms. They have conducted phase 1 and phase 2 studies in 38 participants each and the design of the trial was open and non-randomized. In Phase 1 each formulation was received by 9 volunteers and in phase 2, 20 volunteers received the rAd26-S and rAd5-S (Logunov et al., 2020). The primary objective of the study was to determine the safety and antigen specific humoral immunity and secondary outcome was to measure the antigen-specific cellular immunity and change in neutralizing antibodies. As per their findings both the vaccine formulations were safe, well tolerated and shown only very common side effects. In phase 2, 85% of participants had detectable antibodies at 14 days after the priming dose, rising to 100% by day 21, with substantial titre rises after the boosting dose. All the participants who were treated with the formulations were produced both humoral and cell mediated immunity. Based on these findings Russian government and regulatory agencies has given approval for this vaccine to release into the Russian market for the common public.
However, scientific fraternity and regulatory agencies concerns that without the results of phase 3 study the approval is premature. Also, few scientists are stating that data on immune response alone would not generally be an adequate basis for approving a vaccine and “Immune response might not be directly proportional to the degree of protection.
Vaccine development is an organized, tedious, and long-run process, which usually takes around 10 years for the successful development. Nevertheless, researches and the developers are facing numerous challenges in diverse fronts, like regulatory constraints, time and worsening of disease, scale up as per the demand etc., that seems to be difficult to meet (Lv et al., 2020). A different line of trouble can be recognized, in producing large number of doses and their distribution among the worldwide population as it may require well-coordinated activities that should run in parallel (WHO Ebola Response Team, 2016). Impediment in vaccine distribution can results in several consequences as illustrated in 2013/14 Ebola epidemic in West Africa, which killed around 11,000 people along with economic burden over 53 billion dollars (Huber et al., 2018). The first vaccine was approved by FDA in 2019, after 43 years of Ebola viral outbreak. Tragically, the SARS 2003 epidemic was ended prior to complete vaccine development. All these challenges can result in accretion of invested resources, that finally elevates the financial risk. Consequently, several funding agencies diverted the allocated funds, leaving the manufacturers with huge financial loss. On September 4th, 2020, The WHO (World Health Organization) insist that it would never approve a vaccine that has not established its safety and effectiveness, amid concern in the hurry to develop the COVID-19 vaccine. Safety, efficacy, and potential adverse effects of currently used COVID-19 vaccines were summarized in Table 4.
Table 4.
Efficacy and potential adverse effects of COVID-19 vaccines.
| Name | Company/developer | Platform | Efficacy against symptomatic COVID-19 in clinical trials | Storage requirements | Common side effects | Rare adverse effects |
|---|---|---|---|---|---|---|
| BNT162b2 | Pfizer/BioNTech | mRNA | 95% (95% CI 90–98) after median of 2 months follow-up 91% (95% CI 89–93) after median of 6 months follow-up |
Ultracold freezer (−90 to −60 °C) then freezer (−25 to −15 °C) for up to 2 weeks cumulative time then refrigerated (2–8 °C) for up to 1 month | Local injection site reactions Systemic symptoms (fevers, chills, fatigue, myalgias, headache) |
Anaphylaxis (approximately 5 per million) Myocarditis/pericarditis (approximately 16 per million among 16–39 year olds) |
| mRNA-1273 | Moderna | mRNA | 94% (95% CI 89–97) after median of 2 months follow-up | Freezer (−25 to −15 °C) then refrigerated (2–8 °C) for up to 30 days | Local injection site reactions Systemic symptoms (fevers, chills, fatigue, myalgias, headache) |
Anaphylaxis (approximately 2.8 per million) Myocarditis/pericarditis (approximately 16 per million among 16–39 year olds) |
| Ad26.COV2.S | Janssen/Johnson & Johnson | Replication-incompetent adenovirus 26 vector | 67% (95% CI 59–73) against moderate to severe COVID-19 after median of 2 months follow-up | Refrigerated (2–8 °C) | Local injection site reactions Systemic symptoms (fevers, chills, fatigue, myalgias, headache) |
Thrombotic complications associated with thrombocytopenia: For females 30–39 years old: 12.4 cases/million For females 40–49 years old: 9.4 cases/million For females in other age ranges and males: 1.3 to 4.7 cases/million Guillain-Barre syndrome (approximately 8 cases/million) |
| ChAdOx1 nCoV-19/AZD1222 | AstraZeneca/University of Oxford/Serum Institute of India | Replication-incompetent chimpanzee adenovirus vector | 70% (95% CI 55–81) after median of 2 months follow-up | Refrigerated (2–8 °C) | Local injection site reactions Systemic symptoms (fevers, chills, fatigue, myalgias, headache) |
Very rare thrombotic complications associated with thrombocytopenia: Cerebral venous sinus thrombosis (169 of ≈34 million) Splanchnic vein thrombosis (54 of ≈34 million) Guillain-Barre syndrome (227 cases/51 million) |
5. Recent patents filed connected to COVID-19
5.1. Technological prospection
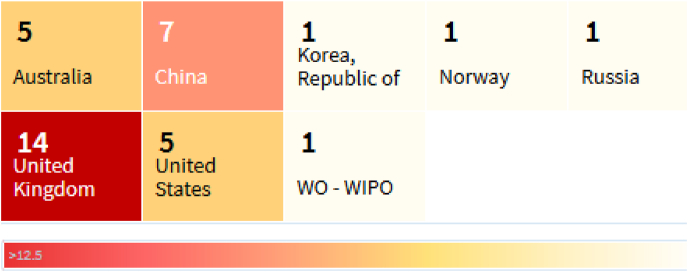
The present section confers on latest patents filed, connected with various applications in the management/preventing COVID-19. Excavation of patent portfolios was retrieved from The Lens (Free, open patent and scholarly search) portal. Database was searched with keywords a) [Title: COVID or Abstract: COVID or Claims: COVID]orb) [Title: Corona or Abstract: Corona or Claims: Corona] and Filters [Patent filed date (December 1, 2019–September 31, 2021)].A total of 202 patents were found in the database, but many of them are on basis of corona treatment, which is high frequency discharge that can augment the adhesion of plastic surfaces. Subsequently, we filtered these patents as they were out of the present scope of study and thus, selected 35 patents, which were having the application with respect to the COVID-19. Furthermore, we removed the patents which were not filed in English languages and duplicate patents. The short-listed patents are described in Table 5, (Patent analysis (COVID-19), 2020) which contains basic details like, Jurisdiction (country of protection), Classification, Publication number, Title, Applicants and Inventors.Of all these, majority of the patents (14) were filed from United Kingdom (UK) Jurisdiction. Country wise sharing of these patents were depicted in Fig. 5.
Table 5.
Patents filed on COVID-19/corona virus from December 2019 to till date.
| S. No | Juris-diction | Kind | Publication Number | Title | Applicants | Inventors |
|---|---|---|---|---|---|---|
| 1 | CN | A | CN 111041089 A | Application Of Covid-19 Infection Host Marker | Guangzhou Vision Gene Tech Co Ltd | Xu Teng |
| 2 | CN | A | CN 111081316 A | Method And Device For Screening Candidate Drugs For Covid-19 | Geneis Tech Beijing Co Ltd | Peng Lihong |
| 3 | US | A1 | US 2020/0214649 A1 | Detection of Covid-19 | Cogley Thomas Paul | Cogley Thomas Paul |
| 4 | CN | A | CN 111060691 A | Fluorescence Immunochromatography Device For Detecting Covid-19 And Using Method Thereof | Shenzhen Bioeasy Biotechnology Co Ltd | Zhou Hongwei |
| 5 | CN | A | CN 111024954 A | Colloidal Gold Immunochromatography Device For Combined Detection Of Covid-19 Antigen And Antibody And Use Method Thereof | Shenzhen Bioeasy Biotechnology Co Ltd | Chen Yiyao |
| 6 | CN | A | CN 111074008 A | Covid-19 (coronavirus Disease, 2019) Nucleic Acid Testing Method Capable Of Increasing Accurate Rate | Nanjing Synthgene Medical Tech Co Ltd | Tong Kun |
| 7 | CN | A | CN 111088283 A | Mvsv Virus Vector And Virus Vector Vaccine, And Covid-19 Vaccine Based On Mvsv Mediation | Suzhou Aoteming Pharmaceutical Tech Co Ltd | Qin Xiaofeng |
| 8 | AU | A4 | AU 2020/101189 A4 | Green Human Resource Management Practices Framework For Healthcare Organizations Under Stressful Covid-19 | A Velsamy Mr | |
| 9 | AU | A4 | AU 2020/100400 A4 | Proposed Therapy To Reduce Effects Of Viral Infections (may Help With Covid 19) 16 Mar. 2020 | Thompson Edgar | Thompson Edgar Geoffrey |
| 10 | GB | D0 | GB 202004993 D0 | Covid-19 Control | Enterobiotix Ltd | |
| 11 | GB | D0 | GB 202005556 D0 | Covid Face Mask | Aga Nanotech Ltd | |
| 12 | GB | D0 | GB 202007366 D0 | Covid-19 Washable Mask | Prabdial Yakeen | |
| 13 | GB | D0 | GB 202007652 D0 | Compounds For Treating Covid-19 | Katholieke Univ Leven | |
| 14 | US | A1 | US 2020/0179367 A1 | Method Of Treating Coronavirus | Mymd Pharmaceuticals Inc | Williams Jonnie R |
| 15 | GB | D0 | GB 202005097 D0 | Treatment And/or Prevention Of Covid-19 Infection | Mead Johnson Nutrition Co | |
| 16 | GB | D0 | GB 202003632 D0 | Sars-cov-2 (sars2, Covid-19) Antibodies | Harbour Antibodies Bv | |
| 17 | KR | A | KR 20200032050 A | Covid-19 Knockout Dna Covid-19 Suitable Triple Knockout Dnai Remedy | Kim Seung Chan | Kim Seung Chan |
| 18 | GB | D0 | GB 202003869 D0 | Method To Cure The Patients Of Covid-19 By Using Co2 In Special Rooms | Zaki George Abdel Messih | |
| 19 | GB | D0 | GB 202003870 D0 | Method To Cure The Patient Of Covid-19 By Using Ozone In Special Rooms | Zaki George Abdelmessih | |
| 20 | GB | D0 | GB 202008314 D0 | Chemical Compounds For Use In The Therapy Or Prohylaxis For The Medical Condition Covid-19 | Sheridan Richard John | |
| 21 | GB | D0 | GB 202005536 D0 | Space-time, 4-d Forces, 4-mechanics For Developing An Antibiotic For Covid-19 Corona Virus & Free Body Diagram | Moaqat Tarek Nadim Mahmood | |
| 22 | NO | A1 | NO 20200436 A1 | System And Method For The Removal Of Alveolar (thorax) Fluids In Patients With Infectious And/or Virus Diseases (Covid-19) | Modi Vivendi As | Myhr Gunnar |
| 23 | GB | D0 | GB 202007897 D0 | Essential Oil Formulation And Method For Delivery Through A Nebulizer For Use In Covid-19 And Other Upper Respiratory Tract Infections | Havercroft Nicholas Anthony | |
| 24 | WO | A2 | WO 2020/143892 A2 | Probiotic Which Cures Covid 19 Patients By Transfer Of Natural Passive Immunity From Immunised Cows | Lachlak Nassira | |
| 25 | AU | A4 | AU 2020/100564 A4 | Coronavirus Impact On The World Economy Problems Solving: I Invent The Equation For Solving The Forecast Of Number Of Covid-19 Cases In The Future So To Help A Country Can Re Open The Business As Early As Possible In The Minimizes Of Covid-19 | Phan Hung Thanh Mr | Phan Hung Thanh |
| 26 | GB | D0 | GB 202003829 D0 | Permanently Removing Nitrogen Dioxide, Carbon Particlulates And Covid 19 From The Air That We Breath Along With Other Viruses That Cause Respiratory Problems | Fordham Joseph Henry | |
| 27 | CN | A | CN 110960532 A | Anti-coronavirus Macleaya Cordata Benzylisoquinoline Alkaloid And Resveratrol Composition And Application Thereof | Jin Xiaofei | Jin Xiaofei |
| 28 | AU | A4 | AU 2020/100641 A4 | Quadruple Regime Using Azithromycin 500 mg Daily Plus Vitamin C Gram Twice Daily Plus Zinc 500 mg Daily Plus Low-dose Aspirin 100 mg Daily For 12 Weeks To Be Used As Prophylaxis To Prevent Covid-19 Virus/Corona Virus/Sars Covid 2 Virus Infection In Nursing Home/Aged Care Population In Illawarra Region Nsw Australia. | Balasubramaniam Vaidyanathan Dr | |
| 29 | AU | A4 | AU 2020/100453 A4 | Disinfectant Bomb - Disinfectant Dispersal System For Small, Medium And Large Areas. Disinfectant Slow Release Spray For All Modes Of Transport, Homes And Offices. | Giumelli Colin Boyd Mr | Giumelli Colin Boyd |
| 30 | GB | A | GB 2580006 A | Bubbles For Air Decontamination | Michael Anthony Holmes | Michael Anthony Holmes |
| 31 | GB | D0 | GB 202006266 D0 | Bubbles For Air Decontamination | Holmes Michael Anthony | |
| 32 | RU | C1 | RU 2727054 C1 | Method For Detecting Cdna Of Sars-cov-2 Coronavirus Using Synthetic Oligonucleotide Primers In Polymerase Chain Reaction | Drozd Sergej Feliksovich | Drozd Sergej Feliksovich |
| 33 | US | A1 | US 2020/0231128 A1 | Apparatus And Systems With Timer For Air-borne Cleaning Of Surfaces | Nuvinair Llc | Bailey Kyle |
| 34 | US | A1 | US 2020/0237689 A1 | Prevention And Treatment Of Coronavirus And Other Respiratory Infections Using Nanoemulsion Compositions | Bluewillow Biologics Inc | Peralta David |
| 35 | KR | A | KR 20200063275 A | Corona Virus Prevention And Treatment Drugs | Choi Gi Eun |
Fig. 5.
Heat map of country-wise patents filed on COVID.
5.2. Expert opinion
5.2.1. Classification of patent and concordance
Patent classification in the process of a grouping of patents as per their technical features. Earlier this was used only for the sorting of documents. The most accurate and finest way to search for patents is through their technical classification. There are numerous classifications used in many countries and this can be because of their own/independent patent laws. So, to consolidate patents, all the countries came together and formed a common code. These were mainly classified as CPC (Cooperative patent classification) and IPC (International patent classification). CPC came into action from Q4 of 2010 as an extension of IPC and managed by USPTO and EPO. CPC and IPC classifications were compared in Table 6.
Table 6.
Patent classifications along with entity.
| CPC Classification |
Entity | IPC Classification |
|---|---|---|
| Section | Section | |
| A | Human necessities | A |
| B | Performing operations transporting (notes & warnings) | B |
| C | Chemistry metallurgy (notes & warnings) | C |
| D | Textiles paper | D |
| E | Fixed constructions | E |
| F | Mechanical engineering lighting heating weapons blasting (notes & warnings) | F |
| G | Physics (notes & warnings) | G |
| H | Electricity (notes & warnings) | H |
| Y | Tagging of new technological developments | – |
Majority of the patents filed (15) were belongs to class A, contains patents related diagnosis, surgery, identification, analyzing, and biological material, etc. Subsection, A61P deals for the inventions for RNA viruses. Few subsections like A61K and A61L covers the preparations for medical, dental, or toilet purposes or pharmaceutical compounds or materials for deodorisation/disinfection/sterilisation of surgical materials or compositions thereof. Smoke or soap bubble toys used for producing smoke images were represented in the sub-class A63H. It was observed that, G01 N class occupies the subsequent place, which comprises measuring or testing process involving enzymes or microorganisms other than immunoassay and this was followed by G06 subclass, dealing with organisational models like scheduling, planning or allocating time or utilizing human of machine resources. Few patents were filed under C12Q class [synthesis of nucleic acids for analysis with precedence to polymerase chain reaction and colony counters etc] and G16H [covering healthcare informatics to handle or process the healthcare or medical data]. Fig. 6 shows the filed patents distribution among IPC classifications.
Fig. 6.
Word count representation of patents distribution among IPC classifications.
Since December 2019 to till date, numerous inventions were filed to receive intellectual protection on different strategies as shown in Fig. 7. No comprehensive literature has been published, in listing out all these recent patents filed in relation with COVID-19, that the present section intends to.
Fig. 7.
Different strategies in patents filed related to COVID-19.
5.2.2. Patents filed on diagnostic assays
Xu Teng, projected a patent on application of COVID-19 infection host marker. The gene expression difference between a COVID-19 positive patient and a COVID-19 negative patient is analyzed, an obtained gene with differential expression is used as the host marker. The most marker comprises any one of the following genes of RNR1, MFSD11, SYNE3 and SLC10A3 and this can be successfully used to prepare COVID-19 infection detection reagent or detection equipment. In another invention, device and method for screening of COVID-19 drugs were disclosed. Sequence and structure similarity information between viruses and pharmaceutical chemical structure was constructed using walkalgorithm to develop a screening model. This can be effectively used in reducing the development cost, enhancing the screening speed and accuracy. Cogley Thomas Paul, invented a device for the detection of Covid-19. The device consists of scintillator, which maximizes the light emissions positioned to a patient and produces light image of that patient on the screen with the help of a camera in operative proximity to the scintillator screen. Additionally, this was connected to a computer with specific software to capture and analyze the light images, followed by diagnosis for treatment of patient.
Patent CN 111060691 A, discloses a fluorescence immuno chromatography device for detecting a novel coronavirus COVID-19 and stated device is strong in specificity, high in sensitivity, strong specificity and short detection time (10–15 min). The invented device can be used in diverse places such as disease control centers, hospitals and airports etc. In another invention, COVID-19 nucleic acid testing method was applied, and the virus detection can be judged as per the obtained fluorescence signal data. This method can be helpful in monitoring all the stages of infection and capable of escalating the accurate rate. Drozd Sergej Feliksovich patented the method for detecting SARS-COV-2 coronavirus by polymerase chain reaction using synthetic oligonucleotide primers.
5.2.3. Patents filed on treatment strategies
Suzhou Aoteming Pharmaceutical Tech Co Ltd, invented mVSV virus vector vaccine, which comprises of a) Heterologous antigen which can fuses or embeds with the target virus between L and G genes of mVSV vector and b) antigen that can envelop or embeds the antigen that encoding the target virus.Another patent was filed on basis of proposed therapy in reducing the effects of viral infections. The inventors disclosed that combination of vitamin supplements and lifestyle changes etc., can lessen the impact of several virus infections. US 2020/0179367 A1 described that administering a pharmaceutical formualtion containing isomyosmine can reduce the oxidative stress, inhibit mitochondrial reactive oxygen species (mtROS) and thus can help in treating of coronavirus. Covid-19 Suitable Triple Knockout Dnai oligomer remedy was developed by Kim seung chan, which contains the single strand DNA coupled with three parts and transported by liposome to remove the virus genome. Lachlak Nassira developed a probiotic from immunised cows by transferring the natural passive immunity to treat COVID-19. Immunised cows produced colostrum, rich in anti-SARS-COV-2 IgG antibodies. Produced colostrum can be stored using simple cold lyophilisation technique to keep 99% of antibodies viable. Patients recovered by these probiotics are developed these antibodies, that can be evident from serological tests.Application of macleaya cordata benzylisoquinoline alkaloid and resveratrol composition was invented and filed the patent by Jin xiaofei. The foresaid composition has exceptional binding activity in coronavirus related protein target and expected to become a key ingredient in preparing anti-corona drugs. Balasubramaniam vaidyanathan invented that quadruple regimen consists azithromycin (500 mg Daily), vitamin c (1 Gram Twice Daily), zinc (500 mg Daily) and aspirin (100 mg Daily), for 12 weeks can acts as a prophylaxis in preventing COVID-19 infections.
5.2.4. Patents filed onscreening and disinfection procedures
Disinfectant Bomb, was invented to disinfectant commercial areas and also helps in slow release spray for homes, offices and all modes of transports. Similar invention was developed to decontaminate the air using soap bubbles, comprising a solvent, surfactant (sodium laury/laureth sulphate) and evaporation suppressant (glycerol/polyethylene glycol). Moreover, the process can be handled by connecting to wired or wireless devices to deliver or cease the production of soap bubbles.In another invention, apparatus and systems for cleaning of surfaces especially in an enclosable environment was described. The device or container spells out an air borne spray, spreads throughout the closed area and contact with the surfaces to be sterilized. The enclosableenvironment is maintained closed for an effective disinfection.
Majority of the researchers were focused not only on the treatment strategies and but also on diagnostic and disinfection devices, as evident from the above patent data. As the efficient diagnostic system helps for quick detection and isolation of COVID-19 effected patents, accordingly, mitigate the spread of the disease. Additionally, these tests can help to improve the understanding the virus spread and effectiveness of control measures. Health authorities throughout the world, are struggling to limit transmission of coronavirus. Disinfection is one of the suitable approaches. Disinfection will not reduce the spread of COVID-19, but also plays a significant role in flattening of the curve. Several inventors were filed the patents on all these strategies. Application of all these principles or methods can be effectively prevents/manage the current pandemic.
6. Potential influence of novel virus variants on the therapeutic approach
SARS-CoV-2, right from its onset, has shown multiple new variants of concern that made the therapeutic approach more complicated. Even the vaccine efficiency is encountered as a challenge with the changing variant of concerns. Due to the shared mutations in spike proteins mostly on S1 unit, there is a higher transmissibility rate and alter the viral virulence and clinical outcome. Even the mutations in non-structural proteins also became a challenge for researchers towards their vaccine development or therapeutic strategies. Several variants like alpha, beta, gamma, and delta are becoming complicated towards the management of patients. Hence, a combination of therapeutic strategies against viral cycle as well as immune host responses are in current trends (Khateeb et al., 2021).
7. Conclusion
COVID-19 cases are rising day by day due to release of lockdown and community spread. Cumulative death factor is increasing across the globe. Several scientists are working for development of diagnostics, vaccines, drugs and struggling to understand the disease conditions. Comorbid diseases are noted as more favourable conditions for easy spread and severity of coronavirus infection. Throughout the world, so many countries are involved in the drug discovery and development either as industries, academia, research institutes, or collaborations. Till date, no vaccine or drug has been discovered or developed. Drug repurposing is the only symptomatic remedy as of now with emergency use authorizations of some existing drugs. The present review helps researchers or individuals or health workers to understand and know about the severity of disease, vaccine development status, patents filing and clinical trials progress, and available inline treatment strategies.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
G.S.N. Koteswara Rao: Resources, Writing – original draft, Formal analysis. Buduru Gowthami: Resources, Writing – original draft. N. Raghavendra Naveen: Conceptualization, Resources, Writing – original draft, Formal analysis, Project administration. Pavan Kumar Samudrala: Conceptualization, Validation, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interestsor personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
N. Raghavendra Naveen, Email: raghavendra.naveen@gmail.com.
Pavan Kumar Samudrala, Email: pavanniper199@gmail.com.
References
- Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., Kim B.T., Kim S.J. vol. 30. Korean Society for Microbiology and Biotechnology; 2020. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) pp. 313–324. (Journal of Microbiology and Biotechnology). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy Maxmen. Medics check on people with COVID-19 in jinyintan hospital. Nature. 2020;578:347–348. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- André F.E. The future of vaccines, immunisation concepts and practice. Vaccine. 2001;19(17–19):2206–2209. doi: 10.1016/S0264-410X(00)00546-6. [DOI] [PubMed] [Google Scholar]
- Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Prato S. Del. Review COVID-19 in people with diabetes: understanding the reasons for worse outcomes. 2017. Www.Thelancet.Com/Diabetes-Endocrinology. 8. [DOI] [PMC free article] [PubMed]
- Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., Knighton P., Holman N., Khunti K., Sattar N., Wareham N.J., Young B., Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The Lancet Diabetes and Endocrinology. 2020;8(10):813–835. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C. Der, Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., et al. vol. 75. Elsevier USA; 2020. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review; pp. 2950–2973. (Journal of the American College of Cardiology). Issue 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathi S., Poma A.B., Kolandaivel P. Journal of Biomolecular Structure and Dynamics. Taylor and Francis Ltd; 2020. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R., Sacchi P., Maiocchi L., Patruno S., Filice G. vol. 38. Dig Liver Dis; 2006. Hepatotoxicity and antiretroviral therapy with protease inhibitors: a review; pp. 363–373. (Digestive and Liver Disease). Issue 6. [DOI] [PubMed] [Google Scholar]
- Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K., Collins P.L. Contributions of the structural proteins of severe respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. U.S.A. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja L.M., Wolf D., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., Aisenberg G.M., Madjid M., Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., et al. Engineering; 2020. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee C.S., Delucchi K.L., Sinha P., Matthay M.A., Hackett J., Shankar-Hari M., McDowell C., Laffey J.G., O'Kane C.M., McAuley D.F., Johnston A.J., Paikray A., Yates C., Polgarova P., Price E., McInerney A., Zamoscik K., Dempsey G., Seasman C., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. The Lancet Respiratory Medicine. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., Amadou C., Arnault G., Baudoux F., Bauduceau B., Borot S., Bourgeon-Ghittori M., Bourron O., Boutoille D., Cazenave-Roblot F., Chaumeil C., Cosson E., Coudol S., Darmon P., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celularity Announces Fda Clearance of Ind Application for Cynk-001 in Coronavirus, First in Cellular Therapy. (2020). https://www.prnewswire.com/news-releases/celularity-announces-fda-clearance-of-ind-application-for-cynk-001-in-coronavirus-first-in-cellular-therapy-301034141.html.
- Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. vol. 41. Springer International Publishing; 2018. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature; pp. 919–931. (Drug Safety). Issue 10. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu P., Gao H., Sun B., Chao D., Wang F., Zhu Y., Hedenstierna G., Wang C.G. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39(10):1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- chen y, Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Liu L., Wang C., Liu Y., Liu Y., Yuan Z., Ren L., Wu Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. MedRxiv. 2020 doi: 10.1101/2020.03.27.20045427. 2020.03.27.20045427. [DOI] [Google Scholar]
- Chen Y., Liu Q., Guo D. vol. 92. John Wiley and Sons Inc; 2020. Emerging coronaviruses: genome structure, replication, and pathogenesis; pp. 418–423. (Journal of Medical Virology). Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Clinical Trial Register . 2020. (ChiCTR) - the World Health Organization International Clinical Trials Registered Organization Registered Platform.http://www.chictr.org.cn/showprojen.aspx?proj=52227 [Google Scholar]
- Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid-19 related analytics, graphs, and charts . 2020. Corona Tracker.https://www.coronatracker.com/analytics/ [Google Scholar]
- Covid-19 Update . FDA; 2020. FDA Broadens Emergency Use Authorization for Veklury (Remdesivir) to Include All Hospitalized Patients for Treatment of COVID-19.https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized [Google Scholar]
- Cui J., Li F., Shi Z.L. vol. 17. Nature Publishing Group; 2019. Origin and evolution of pathogenic coronaviruses; pp. 181–192. (Nature Reviews Microbiology). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure E., Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes and Metabolic Syndrome: Clin. Res. Rev. 2020;14(4):349–350. doi: 10.1016/j.dsx.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M., Osborne V., Lane S., Roy D., Dhanda S., Evans A., Shakir S. Remdesivir in treatment of COVID-19: a systematic benefit–risk assessment. Drug Saf. 2020;43(7):645–656. doi: 10.1007/s40264-020-00952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., Laredo L., Laosa O., Centeno-Soto G.A., Ángeles Gálvez M., Puerro M., González-Rojano E., Pedraza L., de Pablo I., Abad-Santos F., Rodríguez-Mañas L., Gil M., Tobías A., Rodríguez-Miguel A., et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delang L., Abdelnabi R., Neyts J. vol. 153. Elsevier B.V; 2018. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses; pp. 85–94. (Antiviral Research). [DOI] [PubMed] [Google Scholar]
- Diao B., Wang C., Wang R., Feng Z., Tan Y., Wang H., Wang C., Liu L., Liu Y., Liu Y., Wang G., Yuan Z., Ren L., Wu Y., Chen Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020 doi: 10.1101/2020.03.04.20031120. 2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Patel A., Anderson C.S., Dong J., Ma C. vol. 73. Elsevier USA; 2019. Epidemiology of cardiovascular disease in China and opportunities for improvement: JACC international; pp. 3135–3147. (Journal of the American College of Cardiology). Issue 24. [DOI] [PubMed] [Google Scholar]
- Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., Prins M., Sastre P., Deijs M., Van Der Hoek L. Human coronavirus reinfection dynamics: lessons for SARS-CoV-2 Authors. 2020. [DOI]
- Fadini G.P., Morieri M.L., Longato E., Avogaro A. vol. 43. Springer; 2020. Prevalence and impact of diabetes among people infected with SARS-CoV-2; pp. 867–869. (Journal of Endocrinological Investigation). Issue 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. vol. 8. Lancet Publishing Group; 2020. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? p. e21. (The Lancet Respiratory Medicine). Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. vol. 14. International Advancement Center for Medicine and Health Research Co., Ltd; 2020. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. (BioScience Trends). Issue 1. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gawałko M., Kapłon-Cieślicka A., Hohl M., Dobrev D., Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. International Journal of Cardiology. Heart & Vasculature. 2020;30 doi: 10.1016/J.IJCHA.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Announces Results From Phase 3 Trial of Remdesivir in Patients With Moderate Covid-19. (2020). https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/gilead-announces-results-from-phase-3-trial-of-remdesivir-in-patients-with-moderate-covid-19.
- Government of India, and Ministry of Health and Family Welfare. (n.d.). Clinical Guidance on Diabetes Management at COVID-19 Patient Management Facility.
- Gozzo L., Viale P., Longo L., Vitale D.C., Drago F. vol. 11. Frontiers Media S.A; 2020. The potential role of heparin in patients with COVID-19: beyond the anticoagulant effect. A review; p. 1307. (Frontiers in Pharmacology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Ramos A.E. Is the ACE2 overexpression a risk factor for COVID-19 infection? Arch. Med. Res. 2020;51(4):345–346. doi: 10.1016/j.arcmed.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. vol. 12. MDPI AG; 2020. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. (Nutrients). Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group T.R.C. Effect of hydroxychloroquine in hospitalized patients with covid-19. 2020. Https://Doi.Org/10.1056/NEJMoa2022926. 2040,383,21,2030. [DOI]
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q., Li L., Chen P.Y., Sang L., Wang W., Li J.F., Li C.C., Ou L.M., Cheng B., et al. Comorbidity and its impact on 1,590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y., Liu X., Chen R., Tang C., Wang T., Ou C., Li L., Chen P., Sang L., Wang W., Li J., Li C., Ou L., Cheng B., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5):640. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am. J. Clin. Pathol. 2020;153(4):420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., De Oliveira M.H.S., Benoit S., Plebani M., Lippi G. vol. 58. De Gruyter; 2020. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis; pp. 1021–1028. (Clinical Chemistry and Laboratory Medicine). Issue 7. [DOI] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., et al. Dexamethasone for COVID-19-preliminary report effect of dexamethasone in hospitalized patients with COVID-19-preliminary report RECOVERY collaborative group. MedRxiv. 2020 doi: 10.1101/2020.06.22.20137273. 2020.06.22.20137273. [DOI] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. 2020. Www.Thelancet.Com. 395,497. [DOI] [PMC free article] [PubMed]
- Huber C., Finelli L., Stevens W. The economic and social burden of the 2014 Ebola outbreak in West Africa. JID (J. Infect. Dis.) 2018;218(Suppl. l_5):S698–S704. doi: 10.1093/infdis/jiy213. [DOI] [PubMed] [Google Scholar]
- Idf . ninth ed. 2019. Diabetes Atlas.https://www.diabetesatlas.org/en/ 2019. [Google Scholar]
- Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (Covid-19). (2020). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- Investigational Covid-19 Convalescent Plasma-Emergency INDs Frequently Asked Questions. (2020)..
- Keyaerts E., Vijgen L., Maes P., Neyts J., Ranst M. Van. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhan S.O., Fallahi H.R., Cheshmi B. Dysosmia and dysgeusia due to the 2019 Novel Coronavirus; a hypothesis that needs further investigation. Maxillofacial Plastic and Reconstructive Surgery. 2020;42(1):9. doi: 10.1186/s40902-020-00254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.M., Noor A., Madni A., Shafiq M. Emergence of novel coronavirus and progress toward treatment and vaccine. Rev. Med. Virol. 2020;30(4) doi: 10.1002/rmv.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateeb J., Li Y., Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care. 2021;25(1):1–8. doi: 10.1186/S13054-021-03662-X. 2021 25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I.C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020;41(19):1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala A., Joo Y.J., Khatami A., Chiu C., Britton P.N. vol. 35. W.B. Saunders Ltd; 2020. Vaccines for COVID-19: the current state of play; pp. 43–49. (Paediatric Respiratory Reviews). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., Khare S., Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes and Metabolic Syndrome: Clin. Res. Rev. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J.C., Weaver J., Kostka K., Duarte-Salles T., Abrahao M.T.F., Alghoul H., Alser O., Alshammari T.M., Biedermann P., Burn E., Casajust P., Conover M., Culhane A.C., Davydov A., DuVall S.L., Dymshyts D., Bertolín S.F., Fišter K., Hardin J., et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. MedRxiv. 2020 doi: 10.1101/2020.04.08.20054551. 2020.04.08.20054551. [DOI] [Google Scholar]
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Zhao R.C. Transplantation of ACE2- Mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging and Disease. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. vol. 19. NLM (Medline); 2020. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) pp. 149–150. (Nature Reviews. Drug Discovery). 3. [DOI] [PubMed] [Google Scholar]
- Li X.C., Zhang J., Zhuo J.L. vol. 125. Academic Press; 2017. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases; pp. 21–38. (Pharmacological Research). Issue Pt A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. vol. 109. Springer; 2020. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China; pp. 531–538. (Clinical Research in Cardiology). Issue 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.W., Han T.W., Woodward M., Anderson C.S., Zhou H., Chen Y.D., Neal B. Progress in Cardiovascular Diseases. W.B. Saunders; 2020. The impact of 2019 novel coronavirus on heart injury: a Systematic review and Meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. vol. 6. Springer Nature; 2020. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro; pp. 1–4. (Cell Discovery). Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu C., Liu G., Luo W., Xia N. vol. 10. Ivyspring International Publisher; 2020. COVID-19: progress in diagnostics, therapy and vaccination; pp. 7821–7835. (Theranostics). 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverpool Covid-19 Interactions. (2020). https://www.covid19-druginteractions.org/checker.
- Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Wu N.C., Mok C.K.P. COVID-19 vaccines: knowing the unknown. Eur. J. Immunol. 2020;50(7):939–943. doi: 10.1002/eji.202048663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythgoe M.P., Middleton P. vol. 41. Elsevier Ltd; 2020. Ongoing clinical trials for the management of the COVID-19 pandemic; pp. 363–382. (Trends in Pharmacological Sciences). Issue 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. vol. 2. Lancet Publishing Group; 2020. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia; pp. e437–e445. (The Lancet Rheumatology). 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. vol. 395. Lancet Publishing Group; 2020. COVID-19: consider cytokine storm syndromes and immunosuppression; pp. 1033–1034. (The Lancet). 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S., Pagotto A., Longo B., Vendramin I., Pecori D., Tascini C. Role of lopinavir/ritonavir in the treatment of covid-19: a review of current evidence, guideline recommendations, and perspectives. J. Clin. Med. 2020;9(7):2050. doi: 10.3390/jcm9072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo G. D. de, Lazarini F., Larrous F., Feige L., Kornobis E., Levallois S., Marchio A., Kergoat L., Hardy D., Cokelaer T., Pineau P., Lecuit M., Lledo P.-M., Changeux J.-P., Bourhy H. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol. Med. 2021;13(8):e14122. doi: 10.15252/EMMM.202114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. vol. 9. Taylor and Francis Ltd; 2020. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension; pp. 757–760. (Emerging Microbes and Infections). Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercorelli B., Palù G., Loregian A. vol. 26. Elsevier Ltd; 2018. Drug repurposing for viral infectious diseases: how far are we? pp. 865–876. (Trends in Microbiology). Issue 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderna . Moderna, Inc; 2020. Moderna Announces First Participant Dosed in NIH-Led Phase 1 Study of mRNA Vaccine (mRNA-1273) against Novel Coronavirus.https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participant-dosed-nih-led-phase-1-study [Google Scholar]
- Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Türeci Ö., Tompkins K.R., Walsh E.E., Jansen K.U. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;1–5 doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- Mycroft-West, Su D., Elli S., Guimond S.E., Miller G.J., Turnbull J.E., Yates E.A., Guerrini M., Fernig D.G., Lima M. A. de, Skidmore M.A. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv. 2020 doi: 10.1101/2020.02.29.971093. April, 2020.02.29.971093. [DOI] [Google Scholar]
- Paengsai N., Jourdain G., Salvadori N., Tantraworasin A., Mary J.Y., Cressey T.R., Chaiwarith R., Bowonwatanuwong C., Bhakeecheep S., Kosachunhanun N. Recommended first-line antiretroviral therapy regimens and risk of diabetes mellitus in HIV-infected adults in resource-limited settings. Open Forum Infectious Diseases. 2019;6(10) doi: 10.1093/ofid/ofz298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patent analysis (Covid-19) The Lens - free & open patent and scholarly search. 2020. https://www.lens.org/
- Paules C.I., Marston H.D., Fauci A.S. vol. 323. American Medical Association; 2020. Coronavirus infections-more than just the common cold; pp. 707–708. (JAMA - Journal of the American Medical Association). Issue 8. [DOI] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L.F., Chernyak Y., Tobin K., Cerfolio R.J., Francois F., Horwitz L.I. MedRxiv; 2020. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. 2020.04.08.20057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H., Alsukait R.F., Alluhidan M., Alazemi N., Shekar M. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obesity Reviews, obr. 2020;13128 doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., Hochman J.S. Renin–angiotensin–aldosterone system inhibitors and risk of covid-19. N. Engl. J. Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. vol. 395. Lancet Publishing Group; 2020. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease; pp. e30–e31. (The Lancet). 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roback J.D., Guarner J. vol. 323. American Medical Association; 2020. Convalescent plasma to treat COVID-19: possibilities and challenges; pp. 1561–1562. (JAMA - Journal of the American Medical Association). Issue 16. [DOI] [PubMed] [Google Scholar]
- Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper R.L., Rehm K.E. vol. 8. Expert Rev Vaccines; 2009. SARS vaccines: where are we? pp. 887–898. (Expert Review of Vaccines). Issue 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., Marit de Groot A., Stoop J., Tete S., Damme V., Leroux-Roels I., Berghmans P.-J., Kimmel M., Van Damme P., de Hoon J., Smith W., Stephenson E., Barouch D.H., De Rosa S.C., Cohen K.W., et al. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. MedRxiv. 2020 doi: 10.1101/2020.09.23.20199604. 2020.09.23.20199604. [DOI] [Google Scholar]
- Sala S., Peretto G., Gramegna M., Palmisano A., Villatore A., Vignale D., De Cobelli F., Tresoldi M., Cappelletti A.M., Basso C., Godino C., Esposito A. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., Criner G.J., Kaplan-Lewis E., Baden R., Pandit L., Cameron M.L., Garcia-Diaz J., Chávez V., Mekebeb-Reuter M., Menezes F. L. de, Shah R., González-Lara M.F., Assman B., Freedman J., Mohan S.V. Tocilizumab in patients hospitalized with covid-19 pneumonia. 2020. Https://Doi.Org/10.1056/NEJMoa2030340. 384,1,20,30. [DOI] [PMC free article] [PubMed]
- Samudrala P.K., Kumar P., Choudhary K., Thakur N., Wadekar G.S., Dayaramani R., Agrawal M., Alexander A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur. J. Pharmacol. 2020;883:173375. doi: 10.1016/j.ejphar.2020.173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. Sn Comprehensive Clinical Medicine. 2020;2(8):1. doi: 10.1007/S42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., D'Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., Maggi P., Coppola N., Paolisso G., Marfella R. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., McInnes I.B., McMurray J.J.V. vol. 142. Lippincott Williams and Wilkins; 2020. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms; pp. 4–6. (Circulation). 1. [DOI] [PubMed] [Google Scholar]
- Savarino A. Use of chloroquine in viral diseases. Lancet Infect. Dis. 2011;11(9):653–654. doi: 10.1016/S1473-3099(11)70092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. vol. 3. Lancet Publishing Group; 2003. Effects of chloroquine on viral infections: an old drug against today's diseases? pp. 722–727. (Lancet Infectious Diseases). Issue 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. vol. 6. Lancet Publishing Group; 2006. New insights into the antiviral effects of chloroquine; pp. 67–69. (Lancet Infectious Diseases). Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham H.L., Kempf D.J., Molla A., Marsh K.C., Kumar G.N., Chen C.M., Kati W., Stewart K., Lal R., Hsu A., Betebenner D., Korneyeva M., Vasavanonda S., McDonald E., Saldivar A., Wideburg N., Chen X., Niu P., Park C., et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1998;42(12):3218–3224. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., Feng J., Yan S., Guan Y., Xu D., He G., Chen C., Xiong X., Liu L., Li H., Tao J., Peng Z., Wang W. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- Shytaj I.L., Fares M., Lucic B., Gallucci L., Tolba M.M., Zimmermann L., Ayoub A.T., Cortese M., Neufeldt C.J., Laketa V., Chlanda P., Fackler O.T., Boulant S., Bartenschlager R., Stanifer M., Savarino A., Lusic M. The FDA-approved drug cobicistat synergizes with remdesivir to inhibit SARS-CoV-2 replication. BioRxiv. 2021 doi: 10.1101/2021.03.09.434219. 2021.03.09.434219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., Caizzo R., Caplan M., Cousin N., Duburcq T., Durand A., El kalioubie A., Favory R., Garcia B., Girardie P., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.B., Kuo M.J., Lin T.Y., Chien C.S., Yang Y.P., Chou S.J., Leu H.B. vol. 83. Wolters Kluwer Health; 2020. Cardiovascular manifestation and treatment in COVID-19; pp. 704–709. (Journal of the Chinese Medical Association). Issue 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18(5) doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünübol M., Ayhan M., Guney E. vol. 17. J Clin Rheumatol; 2011. Hypoglycemia induced by hydroxychloroquine in a patient treated for rheumatoid arthritis; pp. 46–47. (Journal of Clinical Rheumatology). Issue 1. [DOI] [PubMed] [Google Scholar]
- Usfda . 2020. (Veklury (Remdesivir) EUA Letter of Authorization). 10-01-2020. [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7):127–147. doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Chen Z., Zhang L., Wang X., Hao G., Zhang Z., Shao L., Tian Y., Dong Y., Zheng C., Wang J., Zhu M., Weintraub W.S., Gao R. Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. 2018;137(22):2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. vol. 30. Springer Nature; 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro; pp. 269–271. (Cell Research). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yeming, Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. vol. 81. Academic Press Inc; 2011. Coronavirus pathogenesis; pp. 85–164. (Advances in Virus Research). [DOI] [Google Scholar]
- Who Ebola Response Team After Ebola in West Africa — unpredictable risks, preventable epidemics. The New England Journal o f Medicine. 2016;375(6):587–596. doi: 10.1056/NEJMsr1513109. https://www.who.int/ebola/publications/nejm-after-ebola.pdf [DOI] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Zhang Y. vol. 395. Lancet Publishing Group; 2020. Hypertension, renin–angiotensin–aldosterone system inhibition, and COVID-19; pp. 1671–1673. (The Lancet). 10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongrakpanich S., Wongrakpanich A., Melhado K., Rangaswami J. vol. 9. International Society on Aging and Disease; 2018. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly; pp. 143–150. (Aging and Disease). Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. vol. 323. American Medical Association; 2020. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention; pp. 1239–1242. (JAMA - Journal of the American Medical Association). Issue 13. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Internal Medicine. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Wang Z. Can remdesivir and its parent nucleoside GS-441524 be potential oral drugs? An in vitro and in vivo DMPK assessment. Acta Pharm. Sin. B. 2021;11(6):1607–1616. doi: 10.1016/J.APSB.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Wang L., He J., Bi Y., Li M., Wang T., Wang L., Jiang Y., Dai M., Lu J., Xu M., Li Y., Hu N., Li J., Mi S., Chen C.S., Li G., Mu Y., Zhao J., et al. Prevalence and control of diabetes in Chinese adults. JAMA - Journal of the American Medical Association. 2013;310(9):948–958. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Yiwu, Zou Z., Sun Y., Li X., Xu K.F., Wei Y., Jin N., Jiang C. vol. 23. Nature Publishing Group; 2013. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model; pp. 300–302. (Cell Research). Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Yongli, Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Research and Care. 2020;8(1):1343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.W., Yang L., Luo R.G., Xu J.F. vol. 26. Elsevier B.V; 2020. Corticosteroid administration for viral pneumonia: COVID-19 and beyond; pp. 1171–1177. (Clinical Microbiology and Infection). Issue 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020;55(5):105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Maruggi G., Shan H., Li J. vol. 10. Frontiers Media S.A; 2019. Advances in mRNA vaccines for infectious diseases; p. 594. (Frontiers in Immunology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yan-Jun, Zeng Gang, Pan Hong-Xing, Li Chang-Gui, Kan Biao, Hu Ya-Ling, Mao Hai-Yan, Xin Qian-Qian, Chu Kai, Han Wei-Xiao, Chen Zhen, Tang Rong, Yin Wei-Dong, Chen Xin, Gong Xue-Jie, Qin Chuan, Hu Yuan-Sheng, Liu Xiao-Yong, Cui Guo-Liang, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy 2 adults aged 18-59 years: report of the randomized, double-blind, and 3 placebo-controlled phase 2 clinical trial. MedRxiv. 2020 doi: 10.1101/2020.07.31.20161216. 07.31.20161216,2020. [DOI] [Google Scholar]
- Zhang Jiancheng, Xie B., Hashimoto K. vol. 87. Academic Press Inc; 2020. Current status of potential therapeutic candidates for the COVID-19 crisis; pp. 59–73. (Brain, Behavior, and Immunity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Jin jin, Dong X., yuan Cao Y., Yuan Y. dong, Yang Y. bin, Yan Y. qin, Akdis C.A., Gao Y. dong. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy: European Journal of Allergy and Clinical Immunology. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Cui Y., Shen M., Zhang J., Liu B., Dai M., Chen L., Han D., Fan Y., Zeng Y., Li W., Lin F., Li S., Chen X., Pan P. Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res. Clin. Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., Ma H.L., Chen Y.P., Liu W.Y., George J., Zheng M.H. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metab. Clin. Exp. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris J.S.M., Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B. Sen, Wang Z., Wang L., Jia S.Y., Jiang H.D., Wang L., Jiang T., Hu Y., Gou J.B., Xu S.B., Xu J.J., Wang X.W., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., Li H., Zhang P., Song X., Chen X., Xiang M., Zhang C., Bai L., Xiang D., Chen M.M., Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;31(6):1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]