Abstract
Few reports have investigated prognosis of canine gastrointestinal stromal tumor (GIST) cases treated by surgical resection alone. In the present study, we investigated the overall survival (OS) and prognostic factors for dogs with GIST treated by surgical complete resection alone. Fifty-three dogs were included, and the median OS was 18 months. Multivariate analysis showed that primary tumors in small intestine (P=0.04) is significantly associated with shorter OS, and median OS of the cases with cecum lesion and those with small intestine lesion was 22 and 6 months, respectively. The present study suggested primary tumor site was a novel prognostic factor for dogs with GIST treated by surgical complete resection alone.
Keywords: canine, gastrointestinal stromal tumor, overall survival, prognosis, surgery
Gastrointestinal stromal tumor (GIST) is submucosal neoplasm that is thought to be of Cajal cell origin [15, 16]. Common clinical signs in dogs with GIST include lethargy, anorexia and diarrhea, and abdominal distension due to tumors can be also observed [2, 15]. Common primary sites of GIST are cecum and small intestine, and it also occurs in stomach [2, 15]. Although GIST had been rarely diagnosed in dogs, 66–85% of leiomyomas and leiomyosarcomas were later reclassified as GIST through the immunohistological examinations for mesenchymal tumors occurred in the gastrointestinal tract [11, 16]. Accordingly, the actual incidence of GIST may be higher than previously reported in both human and veterinary medicine [15, 16], and GIST has become to be diagnosed based on the immunohistological examination of KIT, which is receptor tyrosine kinases (RTKs), and discovered-on-GIST 1 (DOG1) has been also used as a marker of GIST in human medicine [13]. It is also the characteristics of canine GIST that mutations in c-kit gene are frequently observed [17], which is similar to human GIST [4, 8], and the effectiveness of chemotherapeutic agents that target this RTK has been reported in canine GIST [2, 5, 6, 8, 10].
Treatment guidelines for human GIST recommend surgical resection as first-line therapy unless patients have metastasized and/or non-resectable tumors, and a similar approach is also recommended for canine GISTs [7, 12]. Previous studies in human medicine indicated that independent prognostic factors for GIST include the mitotic index, tumor size and tumor location [12]. In veterinary medicine, a study reported that the median survival time was 11.6 months in 17 dogs with GIST treated by surgery and 37.4 months in cases that survived the perioperative period [16]. This study also showed that GIST occurs in various locations throughout gastrointestinal tract and prognosis of dogs with GIST treated by surgery was various among cases (range: 0–96 months). However, there has been no study that examined prognostic factors for canine GIST cases treated by surgical resections alone.
Based on these backgrounds, the purpose of the present study was set to investigate the survival time and prognostic factors for dogs with GISTs that were completely resected by surgery and not receiving any chemotherapeutic drugs before or after surgery.
The records in a diagnostic laboratory that conducts histopathological examinations of animal samples (North Lab) were retrospectively reviewed and those of dogs with GIST that were completely resected between January 2008 and December 2015 were obtained. Then, immunohistological examination of KIT was performed using the paraffin-embedded tissue blocks in storage as described in Supplementary Materials and Methods, and 80 cases were confirmed to be positive for KIT expression. Among these cases, 3 cases were excluded because they received chemotherapy after surgery, and 24 cases were also excluded because the information on prognosis of these cases could not be obtained. Thus, the remaining 53 dogs were finally included in the present study. The tumor samples of these cases were sent from 45 veterinary hospitals located throughout Japan.
Information extracted from medical record included signalment, primary tumor site, tumor size, and prognosis of the cases. The clinical examinations that were conducted to identify the primary tumors included radiographic evaluation, ultrasonography, and computed tomography (CT), and the examinations performed were various among the facilities. The findings in these examinations confirmed that metastases could not be detected before surgery.
The size of each tumor was recorded by measuring the longest diameter. The tissues were fixed in formalin and embedded in paraffin, and histopathological and immunohistochemical staining for KIT and Ki-67 were conducted as described in Supplementary Materials and Methods and mitotic index was calculated, because Ki-67 expression and mitotic index in histopathological examinations have been reported as prognostic factors in human GIST [12]. The number of positive nuclear reactions for Ki-67 per 500 nuclei was counted in each case, and Ki-67 score was calculated as the mean number of positive nuclei per 100 nuclei. The mitotic index was calculated as the number of cells undergoing mitosis per 50 high-power (400×) fields under a light microscope. The mitotic index was categorized into >10 or ≤10, and the Ki-67 score was categorized into >2 or ≤2 according to a previous study [2]. The mitotic index, and cells positive for KIT or Ki-67 were evaluated by a veterinary pathologist who is accredited by the Japanese College of Veterinary Pathologists and was blinded to the prognosis of each case.
The median follow-up period was 18 months (range: 1–86 months). Whether the causes of death were associated with GIST (GIST group) or not (non-GIST group) was determined based on the existence of mass of GIST identified by radiographic evaluation, ultrasonography, or CT examination at the time of death. Overall survival (OS) was defined as the duration from surgery to death. Dogs were censored if they were alive at the time of the data analysis.
The associations of various factors with OS were evaluated using log-rank test as univariate analyses, and Cox proportional hazards test was also performed as multivariate analysis. As for the primary tumor site, since GIST occurs most frequently in cecum, we decided to compare OS between dogs with GIST in cecum and those in other sites (non-cecum). The parameters used in univariate analysis included the following covariates: age (>10 years or ≤10 years), sex, body weight (>10 kg or ≤10 kg), primary tumor site (cecum or non-cecum), longest diameter of tumor (>5 cm or ≤5 cm and 10 cm or ≤10 cm), mitotic index (>10 or ≤10), Ki-67 score (>2 or ≤2), and cause of death (GIST or non-GIST). In addition, longest diameter of tumor, mitotic index, and Ki-67 index were compared between cases with tumors in cecum and those in small intestine using Mann-Whitney U test. P values of <0.05 were considered as statistically significant. All statistical analyses were conducted using JMP version 15 software (SAS Institute, Inc., Cary, NC, USA).
Breeds of the 53 cases included mixed (16 dogs, 30.2%), Miniature Dachshunds (9 dogs, 17.0%), Golden Retrievers (8 dogs, 15.1%) and other breeds. There were 26 males (13 were intact) and 27 females (9 were intact). The median age was 11 (7–16) years, and 42 cases (79.2%) were older than 10 years. The median body weight was 10.9 (1.3–38) kg, and those of 30 cases (56.6%) were ≥10 kg.
The primary tumor site was the cecum in 35 cases (66.0%) and the small intestine in 18 cases (34.0%). The longest diameter of tumors ranged from 1 cm to 24 cm, and those of 11 cases (20.8%) were ≥10 cm. Mitotic index ranged from 0 to 100; <10 in 31 cases (58.5%) and ≥10 in 22 cases (41.5%). Ki-67 expression was detected in 29 cases. Ki-67 score ranged from 0.2 to 20 in these cases and it was ≥2 in 16 of 29 cases (55.2%).
At the time of data analysis, 43 cases (81.1%) died from any causes, and the causes of death were associated with GIST in 18 cases and not in 16 cases. The causes of death were unclear in remaining 9 cases. The median OS was 20 (0.1–86) months (Fig. 1a). Univariate analysis showed a significant difference in OS between cases with the primary tumor site of cecum and those with the primary tumor site of small intestine (median OS was 22 and 6 months, respectively, P=0.004, Fig. 1b). There was also a significant difference in OS between cases with the longest tumor diameter of <10 cm and those with the longest tumor diameter of ≥10 cm (median OS was 20 and 7 months, respectively, P=0.009, Fig. 1c), although there was no significant difference in OS between cases with the longest tumor diameter of <5 cm and those with the longest tumor diameter of ≥5 cm (median OS was 18 months and 20 months, P=0.90). No significant association with OS was observed in the other parameters in univariate analysis.
Fig. 1.
(a) Kaplan-Meier curve showing the overall survival (OS) of all cases with gastrointestinal stromal tumor (GIST) included in the present study. (b) Kaplan-Meier curves showing the significant difference in OS between GIST cases with the primary tumor site of cecum and those with the primary tumor site of small intestine. The median OS of the cases with cecum lesion and those with small intestine lesion was 22 and 6 months, respectively (P=0.004 in univariate analysis), and the hazard ratio in multivariate analysis was 13.3 (95% confidence interval: 1.9–108.7, P=0.03). (c) Kaplan-Meier curves showing the significant difference in OS between GIST cases with the longest tumor diameter of <10 cm and those with the longest tumor diameter of ≥10 cm. The median OS of the cases with the longest tumor diameter of <10 cm and those with the longest tumor diameter of ≥10 cm was 20 and 7 months, respectively (P=0.009 in univariate analysis), and the hazard ratio in multivariate analysis was 2.3 (95% confidence interval: 0.1–32.3, P=0.48).
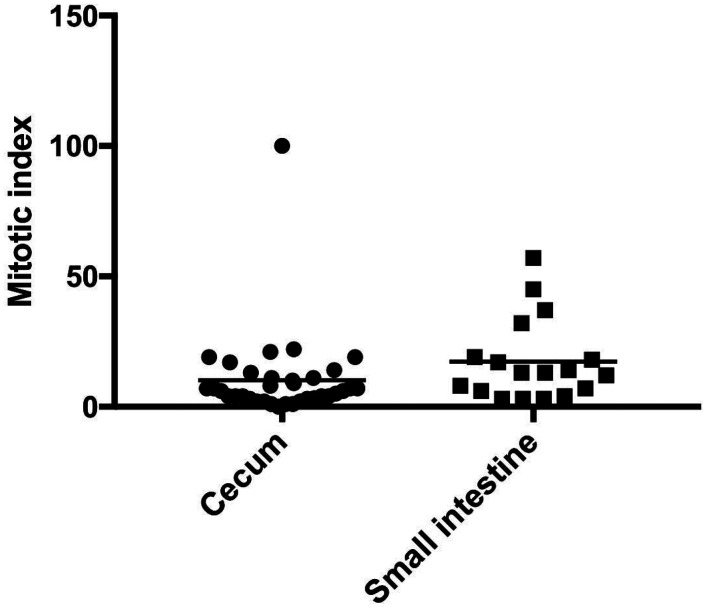
Based on these results, we performed multivariate Cox proportional hazards analysis with the parameters of primary tumor sites and tumor sizes, and it was revealed that primary tumor site was significantly associated with OS (P=0.01). Thus, we compared longest diameter of tumor, mitotic index, and Ki-67 index between cases with tumors in cecum and those in small intestine. There was not significant difference in the longest tumor diameter (P=0.18) or Ki-67 index (P=0.52) between cases with tumors in cecum and those in small intestine. Meanwhile, it was found that mitotic index of tumors in small intestine was significantly higher than those in cecum (median value was 13 and 6, respectively, Fig. 2).
Fig. 2.
Comparison of mitotic index between gastrointestinal stromal tumor cases with the primary tumor site of cecum and those with the primary tumor site of small intestine.
In this retrospective cohort study, we investigated the prognosis and the factors associated with OS in dogs with GISTs treated by surgical complete resection. In the current study, DOG-1 was not used to diagnose GIST. Both humans and dogs have been reported to be KIT-negative and to be diagnosed with GIST by DOG-1 positivity. In the future, it was considered that the dyeing of DOG-1 also had to be considered [3, 13]. In the present study, the median OS of all cases included in this study was 20 months. A previous study reported a shorter median survival time (MST) of 11.6 months in dogs with GIST treated by surgery [2], and it appeared to be relatively shorter than that in the present study. However, it was also reported in the previous study that MST in cases that survived the perioperative period was 37.4 months. Therefore, it is possible that there might be any factors that determined the prognosis of the cases and differences in these factors might be the causes of the differences in the prognosis of the cases between the studies.
In the investigations of prognostic factors, the univariate analyses showed that prognosis was poorer in cases with primary tumors in small intestine and those with the longest diameter of ≥10 cm, and multivariate analysis identified primary tumor sites as a significant prognostic factor. It was shown that mitotic index was significantly higher in tumors in small intestine compared with those in cecum, although mitotic index was not an independent prognostic factor in the present study.
Recently, it was reported that there were significant prognostic differences between mitotic index of 9 or less or 9 or more as prognostic factors for GIST 32 cases [1]. In our report, there were no prognostic differences in mitotic index as a whole, but differences were observed in the small intestine. Interestingly, in this report, we showed that immunostaining for KIT is strong and has a significantly longer prognostic value [1]. These needs to be investigated with a further increase in the number of cases.
Therefore, it was possible that GISTs occurred in small intestine were more aggressive compared with those in cecum and this higher malignancy was associated with the poorer prognosis of the cases with GIST in small intestine. Further studies are needed to investigate how the primary tumor sites affect the prognosis of canine GIST patients.
The distribution of location of GIST lesion in the present study was different from that in a previous study [14], which showed that GIST lesion existed in cecum, duodenum, jejunum, colon, and stomach in 59, 15, 11, 8 and 7% of the cases, respectively. This difference might be derived from the difference in inclusion criteria, because the cases treated by surgical complete resection were included in the present study.
As chemotherapy for GIST, successful uses of imatinib mesylate [7, 9] and toceranib phosphate [6, 11] have been reported in canine GIST cases with metastasized and/or non-resectable lesions. A recent study examined the effects of toceranib phosphate in 27 dogs with GIST, and it was reported that median progression-free interval in cases with gross disease was 110 weeks and that in cases with microscopic disease was 67 weeks [2]. Since the results of the present study indicated that the prognosis of dogs with GIST in small intestine was poorer than those with GIST in cecum, further studies should be conducted to investigate which treatment strategy including chemotherapy should be made for cases with GIST in small intestine.
The present study suggested that Ki-67 expression and mitotic index were not associated with OS in canine GIST cases treated by complete surgical resection alone, although it was indicated that mitotic index was significantly higher in tumors in small intestine compared with those in cecum as described above. In human GIST, Ki-67 expression and mitotic index in histopathological examinations have been reported as prognostic factors [12], and mitotic index was reported to be the most important parameter to estimate the risk of recurrence [9]. It is unclear the causes of the difference in prognostic factors between human and dogs, and it is needed to investigate the clinical significances of these parameters using a larger number of canine GIST cases.
In the present study, the cases where surgical complete resection could not be performed were excluded, because the aim of study was to investigate the prognosis of GIST cases treated by surgical complete resection alone. Further studies are needed to investigate the prognosis or effectiveness of additional therapy for the cases where incomplete resection was performed.
There are some limitations in the present study. At first, the retrospective nature might affect the case selection in the present study, and the important clinical information such as progression-free intervals (PFI) could not be obtained. Further prospective studies using a large number of cases are needed to investigate the prognostic factors associated with PFI in canine GIST cases. The examinations conducted before surgery were not standardized among the facilities and accuracy of metastasis detection might be various among cases. The longest diameter of tumor was used as a parameter to indicate the tumor size, but the shapes of tumors might be various among cases and it might have been needed to calculate total tumor volumes.
In conclusion, we investigated the prognosis of dogs with surgically resected GISTs and it was revealed that primary tumor site was significantly associated with OS. Further studies are needed to investigate how the primary tumor sites affect the prognosis of canine GIST patients.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Supplementary
Acknowledgments
We would like to thank the animal hospitals for their cooperation in the present study.
RFFERENCES
- 1.Alcazar C. M. D., Mahoney J. A., Dittrich K., Stefanovski D., Church M. E.2021. Outcome, prognostic factors and histological chracterization of canine gastrointestinal sarcomas. Vet. Comp. Oncol. 27:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Berger E. P., Johannes C. M., Jergens A. E., Allenspach K., Powers B. E., Du Y., Mochel J. P., Fox L. E., Musser M. L.2018. Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of gastrointestinal stromal tumors of dogs. J. Vet. Intern. Med. 32: 2045–2053. doi: 10.1111/jvim.15335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dailey D. D., Ehrhart E. J., Duval D. L., Bass T., Powers B. E.2015. DOG1 is a sensitive and specific immunohistochemical marker for diagnosis of canine gastrointestinal stromal tumors. J. Vet. Diagn. Invest. 27: 268–277. doi: 10.1177/1040638715578878 [DOI] [PubMed] [Google Scholar]
- 4.Demetri G. D., van Oosterom A. T., Garrett C. R., Blackstein M. E., Shah M. H., Verweij J., McArthur G., Judson I. R., Heinrich M. C., Morgan J. A., Desai J., Fletcher C. D., George S., Bello C. L., Huang X., Baum C. M., Casali P. G.2006. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368: 1329–1338. doi: 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 5.Elliott J. W., Swinbourne F., Parry A., Baines L.2017. Successful treatment of a metastatic, gastrointestinal stromal tumour in a dog with toceranib phosphate (Palladia). J. Small Anim. Pract. 58: 416–418. doi: 10.1111/jsap.12657 [DOI] [PubMed] [Google Scholar]
- 6.Irie M., Takeuchi Y., Ohtake Y., Suzuki H., Nagata N., Miyoshi T., Kagawa Y., Yamagami T.2015. Imatinib mesylate treatment in a dog with gastrointestinal stromal tumors with a c-kit mutation. J. Vet. Med. Sci. 77: 1535–1539. doi: 10.1292/jvms.15-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joensuu H.2008. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 39: 1411–1419. doi: 10.1016/j.humpath.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M., Kuroki S., Ito K., Yasuda A., Sawada H., Ono K., Washizu T., Bonkobara M.2013. Imatinib-associated tumour response in a dog with a non-resectable gastrointestinal stromal tumour harbouring a c-kit exon 11 deletion mutation. Vet. J. 198: 271–274. doi: 10.1016/j.tvjl.2013.05.035 [DOI] [PubMed] [Google Scholar]
- 9.Landi B., Blay J. Y., Bonvalot S., Brasseur M., Coindre J. M., Emile J. F., Hautefeuille V., Honore C., Lartigau E., Mantion G., Pracht M., Le Cesne A., Ducreux M., Bouche O., Thésaurus National de Cancérologie Digestive (TNCD), Fédération Francophone de Cancérologie Digestive (FFCD), Fédération Nationale de Centres de Lutte Contre les Cancers (UNICANCER), Groupe Coopérateur Multidisciplinaire en Oncologie (GERCOR), Société Française de Chirurgie Digestive (SFCD), Société Française de Radiothérapie Oncologique (SFRO), Société Française d’Endoscopie Digestive (SFED), Société Nationale Française de Gastroentérologie (SNFGE). 2019. Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 51: 1223–1231. doi: 10.1016/j.dld.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 10.London C. A., Hannah A. L., Zadovoskaya R., Chien M. B., Kollias-Baker C., Rosenberg M., Downing S., Post G., Boucher J., Shenoy N., Mendel D. B., McMahon G., Cherrington J. M.2003. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin. Cancer Res. 9: 2755–2768. [PubMed] [Google Scholar]
- 11.Maas C. P., ter Haar G., van der Gaag I., Kirpensteijn J.2007. Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histologic, and immunohistochemical evaluation. Vet. Surg. 36: 302–313. doi: 10.1111/j.1532-950X.2007.00271.x [DOI] [PubMed] [Google Scholar]
- 12.Nishida T., Blay J. Y., Hirota S., Kitagawa Y., Kang Y. K.2016. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 19: 3–14. doi: 10.1007/s10120-015-0526-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novelli M., Rossi S., Rodriguez-Justo M., Taniere P., Seddon B., Toffolatti L., Sartor C., Hogendoorn P. C., Sciot R., Van Glabbeke M., Verweij J., Blay J. Y., Hohenberger P., Flanagan A., Dei Tos A. P.2010. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 57: 259–270. doi: 10.1111/j.1365-2559.2010.03624.x [DOI] [PubMed] [Google Scholar]
- 14.Russell K. N., Mehler S. J., Skorupski K. A., Baez J. L., Shofer F. S., Goldschmidt M. H.2007. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990–2003). J. Am. Vet. Med. Assoc. 230: 1329–1333. doi: 10.2460/javma.230.9.1329 [DOI] [PubMed] [Google Scholar]
- 15.Selting K. A.2013. Intestinal tumors. pp. 412–423. In: Small Animal Clinical Oncology, 5th ed. (Withrow, S. J., Vail, D. M. and Page, R. L. eds.), Elsevier, Amsterdam. [Google Scholar]
- 16.Streutker C. J., Huizinga J. D., Driman D. K., Riddell R. H.2007. Interstitial cells of Cajal in health and disease. Part II: ICC and gastrointestinal stromal tumours. Histopathology 50: 190–202. doi: 10.1111/j.1365-2559.2006.02497.x [DOI] [PubMed] [Google Scholar]
- 17.Takanosu M., Amano S., Kagawa Y.2016. Analysis of c-KIT exon 11 mutations in canine gastrointestinal stromal tumours. Vet. J. 207: 118–123. doi: 10.1016/j.tvjl.2015.10.051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.