Abstract
We determined a comprehensive immunohistochemistry of putative isoforms of enzymes for prostaglandin (PG) F2α and PGE2 biosynthesis and these PGs levels in placenta and fetal membrane of normal pregnant rats in vivo. Placenta and fetal membrane showed positive immunoreactions for phospholipase A2 group 4A, but not group 2A, and cyclooxygenase (COX)-1 rather than COX-2. They showed positive immunoreactions for at least one isoform of each of PGF synthase and PGE synthase with tissue-dependent variations. PGF2α and PGE2 levels in both tissues were highest on day 12 and declined and remained low thereafter. Obtained data would be the basic information on the primary PGs synthesis in rat placenta and fetal membrane in normal pregnancy.
Keywords: fetal membrane, placenta, prostaglandin, rat
Prostaglandins (PGs) are a class of substances mediating a variety of auto/para-crine signaling. In mammalian reproduction, ovulation and corpus luteum regression in the ovary and myometrial contraction and cervical ripening in the uterus are well established processes by which the primary PGs, PGF2α and PGE2, mediate through binding to their specific, cell surface receptors (FP and EP1~EP4, respectively) [27]. On the other hand, it remains less known whether these PGs are produced and play physiological and/or pathological roles in gestation-associated, newly formed tissues, placenta and fetal membranes [23, 26]. PGF2α and PGE2 are generated via three enzymatic reactions: 1) cleavage of arachidonic acid (AA) from membrane glycerophospholipid by phospholipase A2 (PLA2), 2) AA conversion to PGH2 by cyclooxygenase (COX), and 3) metabolization of PGH2 to PGF2α and PGE2 by respective PG terminal synthases, PGF2α synthase (PGFS) and PGE2 synthase (PGES) (Supplementary Fig. 1) [36]. Mammalian PLA2 has over 20 isoforms, among which groups 2A, 4A, and 6A PLA2s have been recognized to have a wide range of biological activities [16]. COXs have only two isoforms, a housekeeping type COX-1 and an inducible type COX-2. PGESs have three isoforms with cytosolic type (cPGES) and microsomal types 1 and 2 (mPGES-1 and -2). PGFSs that belong to aldo-keto reductase (AKR) superfamily and catalyze PGH2 or PGE2 have three isoforms, AKRIC7, AKR1C11, and AKR1C5.
A great number of studies have intensively focused on COX (-1 and -2) and/or one or two PLA2s (groups 2A and/or 4A) among three steps of PG biosynthesis in placenta and fetal membranes of human [5, 11, 16, 18, 24, 34, 37] and other species [4, 8, 10, 19, 28, 30, 31, 35, 41, 43, 44, 45]. There are clearly not a few potential discrepancies among the results on temporal and labor-associated changes in expression, tissue distribution and regulation of the enzymes. In contrast to COXs and PLA2s, current knowledge about their down-stream enzymes, PGFSs and PGESs, in rodent gestational tissues is very limited and partial. Studies accumulated so far [12, 28, 33, 38] have focused on only a few isoforms of PGFS or PGES, and therefore, the information on the thorough biosynthetic pathways and its relevance to tissue PGs contents are almost unclear. Here, we attempt to identify enzyme isoforms of PGF2α and PGE2 biosynthesis comprehensively and determine the tissue levels of PGF2α and PGE2 in rat placenta and fetal membranes during normal pregnancy.
Three to five months-old female rats of Wistar-Imamichi strain were used. All procedures employed in this study were carried out following the Guidelines of the Animal Care and Use Committee of Kitasato University School of Veterinary Medicine. The day of fertilization, when vaginal smear showed the estrous stage with sperm presence, was designated as day 1 of pregnancy (depicted as PRG1 in this paper). Rats were sacrificed by cervical dislocation under light anesthesia on PRG12, 15, 19, 21, and 22 (the day before normal parturition). Following uteri and ovaries were first excised out from the body, fetal membranes, fetuses and placentas were freed from gravid uteri and excluded. Placenta and fetal membrane tissues were stored at −20°C for PGs analysis. Based on previous studies investigating PG synthase expression in late pregnancy and before parturition in rodent models [2, 12, 33, 38], placenta from rats on PRG19 and fetal membranes form PRG21 were instantly fixed in Bouin’s solution for the immunohistochemical processing. PGF2α and PGE2 were extracted from the tissue and assayed with commercial enzyme immunoassay kits (Cayman Chemical, Ann Arbor, MI, USA) as described previously [14]. Immunohistochemistry was also the same as the previous studies [15, 32]. Antibodies against a total of 11 kinds of synthetic enzymes which had been well characterized for cross-reactivities were described in Supplementary Table 1. The relative intensity of the immunoreaction was evaluated semi-quantitatively using criteria of both intensity and area with a scale grading from negative (−), slightly positive (+), to moderately or intensely positive (++).
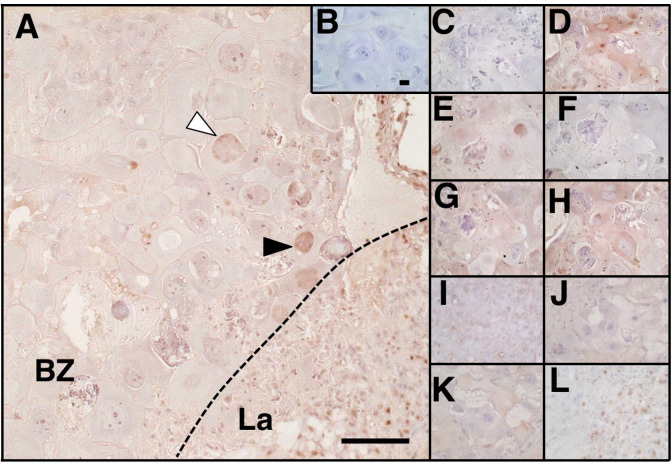
The immunoreactivities for each enzyme in each part of placenta (labyrinth zone, basal zone, decidua) and of fetal membranes (amnion and chorion) were summarized in Table 1. Typical photographic data for the placental labyrinth and basal zones and amnions are shown in Figs. 1 and 2, respectively. Placenta were slightly to intensely positive for group 4A PLA2 and clear immunoreactions were evident in giant cells, glycogen cells, and cytotrophoblasts (Fig. 1A). Other enzymes showing slightly to moderately positive reactions included group 6A PLA2 (Fig. 1D), COX-1 (Fig. 1E), AKR1C7 (Fig. 1G), AKR1C11 (Fig. 1H), AKR1C5 (Fig. 1I), cPGES (Fig. 1J), mPGES-1 (Fig. 1K) and mPGES-2 (Fig. 1L). Relatively intense reactions for group 6A PLA2, COX-1 and AKR1C11 were found in glycogen cells, and that for COX-1 and mPGES-2 in cytotrophoblasts. Placental immunoreactions for group 2A PLA2 (Fig. 1C) and COX-2 (Fig. 1F) were negative. These data of immunoreactive enzyme localization are, partly but not totally, consistent with that of human term placenta [20]. Likewise, decidua tissue was positive for at least one isoform in each step of enzymes, a part of which coincides with prior observations of group 4A PLA2 [15] and AKR1C5 [33]. Fetal membranes, especially amnion cells, were intensely immunoreactive for group 4A PLA2 (Fig. 2D), group 6A PLA2 (Fig. 2E), COX-1 (Fig. 2A), AKR1C11 (Fig. 2H), and mPGES-1 (Fig. 2K). They were faintly positive for group 2A PLA2 (Fig. 2C), COX-2 (Fig. 2F), AKR1C7 (Fig. 2G), and AKR1C5 (Fig. 2I), and were almost negative for cPGES (Fig. 2J) and mPGES-2 (Fig. 2L). These immunohistochemical data of PLA2s are consistent with others’ data of group 4A expression evaluated mostly by RT-PCR or Western blot analyses [5, 10, 11, 31, 34, 45] but inconsistent with that of group 2A PLA2 [5, 11, 16]. Frequent expression of group 6A PLA2 in gestational tissues is a novel finding, but it appears to be a less potential AA supplier than groups 4A and 2A due to biochemical characteristics [16]. Our data of COXs are almost consistent with others’ data of COX-1 expression evaluated by RT-PCR or Western blot analyses [8, 10, 11, 19, 44] but partly inconsistent with that of COX-2 [10, 19, 44]. In a murine model of normal parturition, COX-1 has a greater role than COX-2 [7, 8, 17, 29, 40], although the responsible gestational tissue(s) with COX-1-mediated PG generation are not clearly defined. In human fetal membranes cPGES and mPGES-1 are comparably present regardless of gestational age and labor occurrence [21], which is apparently distinct from our finding with a rat model. Factors causative of discrepancies between our current findings and others’ are supposed to include differential method and sensitivity of detection, animal species, time points examined, and others. Overall, our results indicate the positive immunoreaction of groups 4A and 6A PLA2s and COX-1, but not group 2A PLA2 and COX-2, and at least one isoform of each of PGFS or PGES in both tissues. These expressional pattern of enzyme isoforms tends to be similar to those in corpus luteum and uterus in rats [32]. The current qualitative observation of synthesizing enzymes is far from quantitative determination of expression and their relative contribution to PGs synthesis and is just on their distribution at the limited time point of intact pregnancy. As described below and contrast to our expectation, the active PG production occurred in the mid-pregnancy and may involve differential expression pattern of isoforms.
Table 1. Expression of each enzyme in placenta and fetal membrane.
| Enzyme |
Placenta |
Fetal membrane |
||||
|---|---|---|---|---|---|---|
| Group | Isoform | Labyrinth | Basal zone | Decidua | Amnion | Chorion |
| PLA2 | Group 2A | - | - | - | - | - |
| Group 4A | ++ | ++ | + | + | + | |
| Group 6A | + | + | + | + | + | |
| COX | -1 | + | + | + | ++ | + |
| -2 | - | - | + | - | - | |
| PGFS | AKR1C7 | + | + | - | - | + |
| AKR1C11 | + | + | + | + | + | |
| AKR1C5 | + | + | + | - | - | |
| PGES | cPGES | + | + | - | - | - |
| mPGES-1 | + | + | - | + | + | |
| mPGES-2 | - | - | + | - | - | |
Placenta of day 19 of pregnancy (PRG19) and fetal membrane of PRG21 were examined. Relative intensity of immunoreactivities in each part of the tissue were evaluated with negative (-), mild (+), and strong (++) reaction. Abbreviation PLA2, phospholipase A2; COX, cyclooxygenase; PGFS, prostaglandin F synthase; AKR, aldo-keto reductase; PGES, prostaglandin E synthase; cPGES, cytosolic type PGES; mPGES, microsomal type PGES.
Fig. 1.
Placental immunoreactivities for prostaglandin F2α and prostaglandin E2 biosynthetic enzymes. Placenta on day 19 of pregnancy was shown with immunostaining of negative control IgG (B), group 2A PLA2(C), group 4A PLA2(A), group 6A PLA2(D), COX-1 (E), COX-2 (F), AKR1C7 (G), AKR1C11 (H), AKR1C5 (I), cPGES (J), mPGES-1 (K) and mPGES-2 (L). White and black arrowheads depicted giant cell and glycogen cell, respectively. BZ, Basal zone; La, Labyrinth. Scale bars, 100 μm in A and 25 μm in B (applicable to C–L).
Fig. 2.
Fetal membrane immunoreactivities for prostaglandin F2α and prostaglandin E2 biosynthetic enzymes. Fetal membranes harvested on day 21 of pregnancy were shown with immunostaining of negative control IgG (B), group 2A phospholipase (PL) A2(C), group 4A PLA2(D), group 6A PLA2(E), cyclooxygenase (COX)-1 (A), COX-2 (F), aldo-keto reductase (AKR) 1C7 (G), AKR1C11 (H), AKR1C5 (I), cytosolic type prostaglandin E synthase (cPGES) (J), and microsomal type 1 PGES (mPGES-2) (K) and mPGES-2 (L). Scale bars, 25 μm in A and B (applicable to C–L).
To know the dynamics of PGs synthesis in placenta and fetal membranes, we directly determined PGF2α and PGE2 contents in vivo in the time-course from the tissues development to completion of gestational function. As shown in Table 2, placental PGF2α and PGE2 levels on PRG12 were 9.70 ± 0.63 and 9.98 ± 1.50 ng/mg protein, respectively, both of which were the maximum throughout the period investigated. PGF2α level gradually declined to 12.3% (vs. PRG12) on PRG19 and further through PRG22. PGE2 level declined more drastically to 2.1% (vs. PRG12) on PRG19 and remained low until PRG22. PGF2α and PGE2 levels in fetal membranes on PRG12 were 4.24 ± 0.35 and 3.37 ± 0.27 ng/mg protein, respectively. These values were both less than half of those in placenta and highest on the early period of tissue formation. PGF2α level declined to 23.3% (vs. PRG12) on PRG19 and showed subtle decrease through PRG22. PGE2 level in fetal membranes also declined to about 6% (vs. PRG12) on PRG19 and PRG21 and tended to increase again on PRG22. These data of PGs dynamics in fetal membranes throughout the gestation are presumably the most detailed one of normal pregnant animals.
Table 2. Temporal changes in prostaglandin F2α and prostaglandin E2 contents in placenta and fetal membrane.
| PRG | Placenta |
Fetal membrane |
||
|---|---|---|---|---|
| PGF2α | PGE2 | PGF2α | PGE2 | |
| 12 | 9.70 ± 0.63 a | 9.98 ± 1.50 a | 4.24 ± 0.35 a | 3.37 ± 0.27 a |
| 15 | 2.69 ± 0.46 b | 0.50 ± 0.08 b | 3.50 ± 0.73 a | 0.94 ± 0.24 b |
| 19 | 1.19 ± 0.25 c | 0.21 ± 0.04 c | 0.99 ± 0.24 b | 0.20 ± 0.11 c |
| 21 | 0.94 ± 0.26 c | 0.41 ± 0.11 b | 0.72 ± 0.14 b | 0.21 ± 0.06 c |
| 22 | 0.76 ± 0.28 c | 0.30 ± 0.04 bc | 0.62 ± 0.11 b | 0.97 ± 0.54 bc |
Tissues were sampled from day 12 of pregnancy (PRG12) through PRG22 and assayed for prostaglandin (PG) F2α and PGE2 concentrations by the respective enzyme immunoassay. Data were standardized by protein concentration (ng/mg protein) and were expressed as mean +/−SEM (n=3~6). Different alphabetical letters within the same columns mean significant difference (P<0.05, Tukey-Kramer test).
Both levels of PGF2α and PGE2 in placenta and fetal membranes were highest on PRG12 and declined thereafter. Placental contents of PGE2 and PGF2α was temporally similar to but different in relative concentration from those of previous reports with rat model [2, 3, 42] and human tissues [25]. Cook et al. observed a significant fluctuation in PGE2 and PGF2α contents in murine fetal membranes only for a short period in late pregnancy (from day 16 to 19.3) [4]. Our current data suggest that these primary PGs are likely implicated in the early phase of placental and fetal membrane development rather than their original function of fetuses support. It was demonstrated that 15-deoxy-Δ12,14-PGJ2 was produced in rat placenta and contributed to tissue development through acting on a nuclear receptor, peroxisome proliferator-activated receptor (PPAR) γ [1]. AA and/or some PG(s) other than PGE2 and PGF2α can interact with PPAR (α, γ, δ) as endogenous ligands [9]. Given our observation of frequent nuclear co-localization of group 4A PLA2 and COX-1 in distinct cell types, we may need to take non-primary PGs with direct genomic effect into consideration for future study.
Interestingly, the temporal dynamics of PGs in placenta and fetal membranes are in contrast to those in corpus luteum [13] and uterus and cervix [14] in the rat. These findings indicate that temporal regulation of PG synthesis differs among gestational tissues. No evidence is currently available to provide satisfactory explanation for it and we can only speculate that PG synthesis in several gestation-associated tissues is not affected similarly by endocrine hormonal milieu. PGF2α and PGE2 levels in association with PLA2 and COX activity in the corpus luteum, uterus and cervix rapidly increased toward the end of gestation [2, 13, 14, 42], causing multiple processes to successful parturition. Notably, all of EP1~EP4 and FP have been demonstrated to be expressed in human placenta and fetal membranes at term [39]. Low levels of PGF2α and PGE2 in placenta and fetal membrane in late pregnancy may be associated with uterine quiescence. It remains almost unclear whether endogenous PGs are locally involved in original functions of those gestational tissues such as supporting fetal growth and nutritional and signal transfer [6, 22]. Aberrant stimuli with inflammation or pathogenic infection before term cause abortion and pre-term labor [22, 23]. In this pathological condition, pro-inflammatory cytokines or lipopolysaccharide stimulation rapidly induces immediate early genes such as COX-2, group 4A PLA2, and mPGES-1, which should greatly enhance PG biosynthesis [22, 23]. Even in normal pregnancy and parturition, placenta [19] and fetal membranes [22] are thought to experience “inflammatory”-like status around peri-parturient period, but we here did not find significant rise in PGs content. Fetal membrane along with placenta are postulated to constitute an integral part of forming a positive feedback loop involving cortisol, PGs, surfactant protein A, and pro-inflammatory cytokines that trigger and execute normal or abnormal parturition cascade in several species [23]. Much further studies both in vivo and in vitro are needed to understand the physiological and pathological roles for PGs in these gestational tissues [26].
In conclusion, the current findings should be the basic information on PGF2α and PGE2 synthesis in placenta and fetal membranes in intact gestation and parturition in a rat model. A set of group 4A PLA2 and COX-1 is likely the general pathway for generating PGH2 and is associated with diverse isoforms of downstream PGFS and PGES. The tissue contents of these PGs are high in the developmental phase and remain low until the occurrence of parturition, unlike those in uterus and corpus luteum. The findings extend our knowledge of PGs synthetic mechanism in gestational tissues and serve as the basic information to next explore the possible implications of PGs in the physiology and pathophysiology of normal or aberrant maintenance and termination of gestation.
CONFLICT OF INTEREST
We declare no conflict of interest with respect to the publication of this paper.
Supplementary
Acknowledgments
We thank Drs I. Kudo and K. Watanabe for antibodies donation and M. Nakata for secretary assistance.
REFERENCES
- 1.Asami-Miyagishi R., Iseki S., Usui M., Uchida K., Kubo H., Morita I.2004. Expression and function of PPARgamma in rat placental development. Biochem. Biophys. Res. Commun. 315: 497–501. doi: 10.1016/j.bbrc.2004.01.074 [DOI] [PubMed] [Google Scholar]
- 2.Carminati P., Luzzani F., Soffientini A., Lerner L. J.1975. Influence of day of pregnancy on rat placental, uterine, and ovarian prostaglandin synthesis and metabolism. Endocrinology 97: 1071–1079. doi: 10.1210/endo-97-5-1071 [DOI] [PubMed] [Google Scholar]
- 3.Chan W. Y.1983. Uterine and placental prostaglandins and their modulation of oxytocin sensitivity and contractility in the parturient uterus. Biol. Reprod. 29: 680–688. doi: 10.1095/biolreprod29.3.680 [DOI] [PubMed] [Google Scholar]
- 4.Cook J. L., Shallow M. C., Zaragoza D. B., Anderson K. I., Olson D. M.2003. Mouse placental prostaglandins are associated with uterine activation and the timing of birth. Biol. Reprod. 68: 579–587. doi: 10.1095/biolreprod.102.008789 [DOI] [PubMed] [Google Scholar]
- 5.Freed K. A., Moses E. K., Brennecke S. P., Rice G. E.1997. Differential expression of type II, IV and cytosolic PLA2 messenger RNA in human intrauterine tissues at term. Mol. Hum. Reprod. 3: 493–499. doi: 10.1093/molehr/3.6.493 [DOI] [PubMed] [Google Scholar]
- 6.Furukawa S., Tsuji N., Sugiyama A.2019. Morphology and physiology of rat placenta for toxicological evaluation. J. Toxicol. Pathol. 32: 1–17. doi: 10.1293/tox.2018-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross G. A., Imamura T., Luedke C., Vogt S. K., Olson L. M., Nelson D. M., Sadovsky Y., Muglia L. J.1998. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc. Natl. Acad. Sci. USA 95: 11875–11879. doi: 10.1073/pnas.95.20.11875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta D. K., Sato T. A., Keelan J. A., Marvin K. W., Mitchell M. D.2001. Expression of prostaglandin H synthase-1 and -2 in murine intrauterine and gestational tissues from mid pregnancy until term. Prostaglandins Other Lipid Mediat. 66: 17–25. doi: 10.1016/S0090-6980(01)00122-8 [DOI] [PubMed] [Google Scholar]
- 9.Helliwell R. J. A., Berry E. B. E., O’Carroll S. J., Mitchell M. D.2004. Nuclear prostaglandin receptors: role in pregnancy and parturition? Prostaglandins Leukot. Essent. Fatty Acids 70: 149–165. doi: 10.1016/j.plefa.2003.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Jeng Y. J., Liebenthal D., Strakova Z., Ives K. L., Hellmich M. R., Soloff M. S.2000. Complementary mechanisms of enhanced oxytocin-stimulated prostaglandin E2 synthesis in rabbit amnion at the end of gestation. Endocrinology 141: 4136–4145. doi: 10.1210/endo.141.11.7761 [DOI] [PubMed] [Google Scholar]
- 11.Johansen B., Rakkestad K., Balboa M. A., Dennis E. A.2000. Expression of cytosolic and secreted forms of phospholipase A2 and cyclooxygenases in human placenta, fetal membranes, and chorionic cell lines. Prostaglandins Other Lipid Mediat. 60: 119–125. doi: 10.1016/S0090-6980(99)00057-X [DOI] [PubMed] [Google Scholar]
- 12.Kubota K., Kubota T., Kamei D., Murakami M., Kudo I., Aso T., Morita I.2005. Change in prostaglandin E synthases (PGESs) in microsomal PGES-1 knockout mice in a preterm delivery model. J. Endocrinol. 187: 339–345. doi: 10.1677/joe.1.06169 [DOI] [PubMed] [Google Scholar]
- 13.Kurusu S., Kaizo K., Ibashi M., Kawaminami M., Hashimoto I.1999. Luteal phospholipase A2 activity increases during functional and structural luteolysis in pregnant rats. FEBS Lett. 454: 225–228. doi: 10.1016/S0014-5793(99)00389-0 [DOI] [PubMed] [Google Scholar]
- 14.Kurusu S., Ishii S., Kawaminami M., Hashimoto I.2002. Enhanced activity in cytosolic phospholipase A2 in the rat uterus and cervix around parturition. J. Reprod. Dev. 48: 65–73. doi: 10.1262/jrd.48.65 [DOI] [Google Scholar]
- 15.Kurusu S., Endo M., Madarame H., Kawaminami M., Hashimoto I.1999. Cytosolic phospholipase A2 in rat decidual cells: evidence for its role in decidualization. FEBS Lett. 444: 235–238. doi: 10.1016/S0014-5793(99)00078-2 [DOI] [PubMed] [Google Scholar]
- 16.Lappas M., Rice G. E.2004. Phospholipase A2 isozymes in pregnancy and parturition. Prostaglandins Leukot. Essent. Fatty Acids 70: 87–100. doi: 10.1016/j.plefa.2003.04.001 [DOI] [PubMed] [Google Scholar]
- 17.Loftin C. D., Trivedi D. B., Langenbach R.2002. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J. Clin. Invest. 110: 549–557. doi: 10.1172/JCI0214924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López Bernal A., Newman G. E., Phizackerley P. J., Bryant-Greenwood G., Keeling J. W.1992. Human placental phospholipase A2 activity in term and preterm labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 43: 185–192. doi: 10.1016/0028-2243(92)90172-U [DOI] [PubMed] [Google Scholar]
- 19.Mark P. J., Lewis J. L., Jones M. L., Keelan J. A., Waddell B. J.2013. The inflammatory state of the rat placenta increases in late gestation and is further enhanced by glucocorticoids in the labyrinth zone. Placenta 34: 559–566. doi: 10.1016/j.placenta.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Meadows J. W., Eis A. L. W., Brockman D. E., Myatt L.2003. Expression and localization of prostaglandin E synthase isoforms in human fetal membranes in term and preterm labor. J. Clin. Endocrinol. Metab. 88: 433–439. doi: 10.1210/jc.2002-021061 [DOI] [PubMed] [Google Scholar]
- 21.Meadows J. W., Pitzer B., Brockman D. E., Myatt L.2004. Differential localization of prostaglandin E synthase isoforms in human placental cell types. Placenta 25: 259–265. doi: 10.1016/j.placenta.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 22.Menon R., Richardson L. S., Lappas M.2019. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 79: 40–45. doi: 10.1016/j.placenta.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesiano S., DeFranco E., Muglia L. J.2015. Parturition. pp. 1875–1925. In: Physiology of Reproduction, 4th ed. (Plant, T. M. and Zeleznik, A. J. eds.) (42th chapter), Academic Press, New York. [Google Scholar]
- 24.Mijovic J. E., Zakar T., Nairn T. K., Olson D. M.1997. Prostaglandin-endoperoxide H synthase-2 expression and activity increases with term labor in human chorion. Am. J. Physiol. 272: E832–E840. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell B. F., Rogers K., Wong S.1993. The dynamics of prostaglandin metabolism in human fetal membranes and decidua around the time of parturition. J. Clin. Endocrinol. Metab. 77: 759–764. [DOI] [PubMed] [Google Scholar]
- 26.Myatt L., Sun K.2010. Role of fetal membranes in signaling of fetal maturation and parturition. Int. J. Dev. Biol. 54: 545–553. doi: 10.1387/ijdb.082771lm [DOI] [PubMed] [Google Scholar]
- 27.Olson D. M., Ammann C.2007. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front. Biosci. 12: 1329–1343. doi: 10.2741/2151 [DOI] [PubMed] [Google Scholar]
- 28.Palliser H. K., Ooi G. T., Hirst J. J., Rice G., Dellios N. L., Escalona R. M., Young I. R.2004. Changes in the expression of prostaglandin E and F synthases at induced and spontaneous labour onset in the sheep. J. Endocrinol. 180: 469–477. doi: 10.1677/joe.0.1800469 [DOI] [PubMed] [Google Scholar]
- 29.Reese J., Paria B. C., Brown N., Zhao X., Morrow J. D., Dey S. K.2000. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc. Natl. Acad. Sci. USA 97: 9759–9764. doi: 10.1073/pnas.97.17.9759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal M. D., Albrecht E. D., Pepe G. J.1998. Developmental maturation of baboon placental trophoblast: expression of messenger ribonucleic acid and protein levels of cytosolic and secretory phospholipases A2. J. Clin. Endocrinol. Metab. 83: 2861–2867. [DOI] [PubMed] [Google Scholar]
- 31.Sato T. A., Gupta D. K., Keelan J. A., Marvin K. W., Mitchell M. D.2001. Cytosolic phospholipase A2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression in murine uterine and gestational tissues during late pregnancy. Prostaglandins Leukot. Essent. Fatty Acids 64: 247–251. doi: 10.1054/plef.2001.0267 [DOI] [PubMed] [Google Scholar]
- 32.Satoh H., Watanabe K., Kawaminami M., Kurusu S.2013. A comprehensive immunohistochemistry of prostaglandins F2α and E2 synthetic enzymes in rat ovary and uterus around parturition. Prostaglandins Other Lipid Mediat. 106: 23–28. doi: 10.1016/j.prostaglandins.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Shiota K., Seong H. H., Noda K., Hattori N., Ikeda A., Ogura A., Itagaki S., Takahashi M., Ogawa T.1993. 20 α-Hydroxysteroid dehydrogenase activity in rat placenta. Endocr. J. 40: 673–681. doi: 10.1507/endocrj.40.673 [DOI] [PubMed] [Google Scholar]
- 34.Skannal D. G., Brockman D. E., Eis A. L. W., Xue S., Siddiqi T. A., Myatt L.1997. Changes in activity of cytosolic phospholipase A2 in human amnion at parturition. Am. J. Obstet. Gynecol. 177: 179–184. doi: 10.1016/S0002-9378(97)70459-9 [DOI] [PubMed] [Google Scholar]
- 35.Stanfield K. M., Bell R. R., Lisowski A. R., English M. L., Saldeen S. S., Khan K. N. M.2003. Expression of cyclooxygenase-2 in embryonic and fetal tissues during organogenesis and late pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 67: 54–58 (Part A). doi: 10.1002/bdra.10032 [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto Y., Inazumi T., Tsuchiya S.2015. Roles of prostaglandin receptors in female reproduction. J. Biochem. 157: 73–80. doi: 10.1093/jb/mvu081 [DOI] [PubMed] [Google Scholar]
- 37.Teixeira F. J., Zakar T., Hirst J. J., Guo F., Sadowsky D. W., Machin G., Demianczuk N., Resch B., Olson D. M.1994. Prostaglandin endoperoxide-H synthase (PGHS) activity and immunoreactive PGHS-1 and PGHS-2 levels in human amnion throughout gestation, at term, and during labor. J. Clin. Endocrinol. Metab. 78: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 38.Unezaki S., Sugatani J., Masu Y., Watanabe K., Ito S.1996. Characterization of prostaglandin F2 α production in pregnant and cycling mice. Biol. Reprod. 55: 889–894. doi: 10.1095/biolreprod55.4.889 [DOI] [PubMed] [Google Scholar]
- 39.Unlugedik E., Alfaidy N., Holloway A., Lye S., Bocking A., Challis J., Gibb W.2010. Expression and regulation of prostaglandin receptors in the human placenta and fetal membranes at term and preterm. Reprod. Fertil. Dev. 22: 796–807. doi: 10.1071/RD09148 [DOI] [PubMed] [Google Scholar]
- 40.Welsh T., Mitchell C. M., Walters W. A., Mesiano S., Zakar T.2005. Prostaglandin H2 synthase-1 and -2 expression in guinea pig gestational tissues during late pregnancy and parturition. J. Physiol. 569: 903–912. doi: 10.1113/jphysiol.2005.098129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson L., Jr., Freinkel N.1982. Alterations in uterine and placental prostaglandin F and E with gestational age in the rat. Prostaglandins 24: 567–574. doi: 10.1016/0090-6980(82)90014-4 [DOI] [PubMed] [Google Scholar]
- 42.Winchester S. K., Imamura T., Gross G. A., Muglia L. M., Vogt S. K., Wright J., Watanabe K., Tai H. H., Muglia L. J.2002. Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology 143: 2593–2598. doi: 10.1210/endo.143.7.8926 [DOI] [PubMed] [Google Scholar]
- 43.Wu W. X., Ma X. H., Nathanielsz P. W.1999. Tissue-specific ontogenic expression of prostaglandin H synthase 2 in the ovine myometrium, endometrium, and placenta during late gestation and at spontaneous term labor. Am. J. Obstet. Gynecol. 181: 1512–1519. doi: 10.1016/S0002-9378(99)70398-4 [DOI] [PubMed] [Google Scholar]
- 44.Xu Y., Knipp G. T., Cook T. J.2005. Expression of cyclooxygenase isoforms in developing rat placenta, human term placenta, and BeWo human trophoblast model. Mol. Pharm. 2: 481–490. doi: 10.1021/mp0500519 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q., Wu W. X., Brenna J. T., Nathanielsz P. W.1996. The expression of cytosolic phospholipase A2 and prostaglandin endoperoxide synthase in ovine maternal uterine and fetal tissues during late gestation and labor. Endocrinology 137: 4010–4017. doi: 10.1210/endo.137.9.8756578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.