Abstract
Three eastern bongos (Tragelaphus eurycerus isaaci) presented acutely with hemorrhagic diarrhea at the Singapore Zoo, thought to be caused by a mouldy batch of hay. Repeated fecal tests were negative of parasites and common gastrointestinal bacteria including salmonella and campylobacter. The diarrhea resolved for all individuals after a week of leaf-only diet. However, 2 individuals developed signs of colic. Both animals were anesthetized for examination including blood tests and imaging studies. The findings were consistent of gastrointestinal ileus and a possible impaction. With intensive treatment involving repeated sedations for fluid therapy administration and treatments for gastrointestinal impaction, one individual eventually made a full recovery, but the other individual died due to septic peritonitis secondary to a rupture in the spiral colon. Persistent supportive therapy may be vital in treating severe gastrointestinal disease in this species.
Keywords: bongo, gastroenteritis, ileus, impaction, Tragelaphus eurycerus
The eastern bongo (Tragelaphus eurycerus isaaci) is a critically endangered animal antelope species found in the mountainous regions of Kenya. The animal is classified by the IUCN Antelope Specialist Group as critically endangered, with more individuals in captivity than in the wild. The Singapore Zoo has held populations of the eastern bongo for many years. Experientially, the species has been described to have a sensitive gastrointestinal system, commonly presenting with diarrhea especially when there are variations in the diet.
Diarrhea is a common clinical sign seen in enteritis and can be the result of either increased secretion or decreased absorption of the intestines [10]. In adult domestic ruminants, acute onset hemorrhagic diarrhea is commonly attributed to infectious causes including parasitic diseases like coccidiosis as well as bacterial diseases like salmonellosis and clostridiosis [2, 15, 17]. There are also non-infectious causes including mycotoxin exposure in moldy feed and iatrogenic reasons like drugs reactions [20]. Fluid and electrolyte losses are common with diarrhea, therefore fluid therapy is an important part of the management of severe enteritis. Intravenous therapy is more effective than oral rehydration because maldigestion and malabsorption are dominant underlying pathophysiological processes in enteritis. If losses exceed intake, systemic effects will be observed on clinical examination. A reduction in effective circulating volume leads to hypovolemia, hypotension and shock. Worsening dehydration and acidosis can lead to weakness, recumbency and eventually death [12].
Three female bongos at the Singapore Zoo presented acutely with hemorrhagic diarrhea in April 2020. There were no recent changes to the husbandry management of the animals prior to the incident, although an exceptionally dusty batch of alfalfa hay was reported. The animals were housed in three separate adjacent concrete dens overnight, with access to a common large terraced grass yard during the day only. Access to the exhibit had not been given for a week prior to initial presentation. All animals were fed in a common area with a mixture of leafy alfafa hay, Boskos Browser and Hi-Pro pellets (WESenterprises Pty Ltd., Thabazimbi, South Africa), an assortment of locally available leaves with branches (Acacia auriculiformis, Adenanthera pavonina, Artocarpus spp, Averrhoa carambola, Ficus spp, Leucaena leucocephala, Muntingia calabura, Nephelium lappaceum and Pennisetum purpureum) and token feedings of carrot and cabbage. The fecal consistency improved in all individuals five days after removing all but the leaves in the diet and the diarrhea was resolved in all individuals by day 9. All animals were reportedly bright and alert from when the diarrhea first started to when the diarrhea resolved, with good appetite throughout. Their body condition scores remained stable with a score of 5 out of 9 [3]. Two consecutive fecal checks showed no signs of gastrointestinal parasites and fecal PCR tests (Genesig q16 PCR Easy Kit Salmonella and Campylobacter, Primerdesign Ltd., Camberley, UK) for salmonella and campylobacter were negative. Despite a similar initial presenting sign, the disease progression was different for all three animals with treatment (Table 1). Case 1 achieved clinical resolution without intensive medical intervention. The other two cases developed signs of gastrointestinal impaction including gut stasis and progressively worsening signs of colic (Fig. 1). Case 2 was treated successfully with intensive medical management despite a poor prognosis. Case 3 died despite treatment and was found to have a ruptured spiral colon on post mortem examination.
Table 1. Summary of treatments for three cases.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| 8 year old female, 200 kg | 11 year 8 month old female, 180 kg | 7 year 2 month old female, 180 kg | |
| Clinical presentation | Hemorrhagic diarrhea | Hemorrhagic diarrhea followed by gastrointestinal ileus | Hemorrhagic diarrhea followed by gastrointestinal ileus |
| Number of days to clinical resolution | 9 | 21 | 17 |
| Outcome | Alive | Alive | Dead |
| Total number of sedations | 0 | 6 | 4 |
| Intravenous fluid therapy | None | · Lactated ringers solution (LRS; PT. Otsuka, Malang, Indonesia; 7 l) | · Lactated ringers solution (LRS; 7 l) |
| · Multivitamin solution (Amino B Plex, Jaapharm Canada Inc., Woodbridge, Ontario, Canada; 500 ml) | · Multivitamin solution (Amino B Plex; 500 ml) | ||
| Enteral fluid therapy | Water ad lib | · Water ad lib | · Water ad lib |
| · Oral liquid paraffin (TRM, Kildare, Ireland; 2 l) mixed with 2 l of water | · Oral liquid paraffin (TRM; 2 l) mixed with 2l of water | ||
| · Rectal water (5 l) and liquid paraffin (3 l) | · Rectal water (5 l) and liquid paraffin (3 l) | ||
| Electrolyte therapy | None | · Calcium gluconate (Calcium Gluconate, B Braun Melsungen AG, Melsungen, Germany; 0.5 mg/kg slow IV) | · Calcium gluconate (Calcium Gluconate; 0.5 mg/kg slow IV) |
| · Potassium chloride (7.45% Potassium Chloride, B Braun Medical Industries, Penang, Malaysia; 0.11 mEq/kg slow IV) | · Potassium chloride (7.45% Potassium Chloride; 0.11 mEq/kg slow IV) | ||
| Antibiotic therapy | Marbofloxacin (Marbocyl P 80 mg, Vetoquinol UK Limited, Northamptonshire, UK; 2 mg/kg PO, once daily for 5 days) | · Marbofloxacin (Marbocyl, Vetoquinol UK Limited, Northamptonshire, UK; 2 mg/kg IV or IM, once daily for 5 days) | · Marbofloxacin (Marbocyl, 2 mg/kg IM, once daily for 9 days) |
| · Ceftiofur crystalline free acid (Excede, ZoetisUS, Parsippany, NJ, USA; 5 mg/ml IM Q3d for 3 doses) | · Ceftiofur crystalline free acid (Excede; 5 mg/ml IM Q3d for 3 doses) | ||
| Anti-inflammatory | None | · Dexamethasone (Ilium Dexason, Troy Laboratories Australia Pty Ltd., Glendenning, NSW, Australia; 0.12 mg/kg IV or IM, once daily for 4 days) | · Dexamethason (Ilium Dexason; 0.12 mg/kg IM) |
| Gut motility agents | None | · Metoclopramide (Metomide, Ceva Animal Health Pty Ltd., Glenorie, NSW, Australia; 0.4 mg/kg IV) | · Metoclopramide (Metomide; 0.4 mg/kg IV) |
Fig. 1.
Case 2 (pictured on the right) and Case 3 (pictured on the left) showing mild signs of abdominal discomfort, including rolling to the side and staring at the flanks.
The two individuals presenting with signs of gastrointestinal impaction animal were observed in the morning to be in sternal recumbency and weak. Both cases had sunken eyes during physical examination, suggesting dehydration. Thoracic auscultation was within normal limits but abdominal auscultation revealed no borborygmi, suggesting ileus. A mucoid hemorrhagic plug was found at the anus (Fig. 2) of Case 2, which was confirmed to be intestinal slough on histopathology. Anesthesia on both animals was carried out with medetomidine (Ilium Medetomidine, Troy Laboratories Australia Pty Ltd., Glendenning, NSW, Australia; 0.03 mg/kg IM), azaperone (Stresnil, Elanco Australasia Pty Ltd., Macquarie Park, NSW, Australia; 0.2 mg/kg IM) and butorphanol (Butomidor, Ausrichter Pty Ltd., Camperdown, NSW, Australia; 0.1 mg/kg IM), topped up with ketamine (Ilium Ketamil, Troy Laboratories Australia Pty Ltd., Glendenning, NSW, Australia; 0.5 mg/kg IV) as required and reversed with naltrexone (Trexonil, Wildlife Pharmaceuticals Inc., Windsor, CO, USA; 0.2 mg/kg IM) and atipamezole (Atipam, East Tamaki, Auckland, New Zealand; 0.15 mg/kg IM). Repeated sedations were achieved with just butorphanol (Butomidor; 0.5 mg/kg IM) alone. This was sufficient for most procedures to be carried out, including imaging studies, oral and rectal fluid administration and blood collection.
Fig. 2.

Mucoid hemorrhagic plug retrieved from the anus after the animal was seen straining.
The response of Case 2 to treatment was minimal and its condition progressively worsened over the following 9 days. The signs of colic worsened, which included staring and nipping at the flanks, laying down in sternal and right lateral recumbency, bruxism and tenesmus. The ventral abdomen was also distended and felt doughy on palpation. There was minimal response to the treatment over the 9 days, with the animal still showing signs of severe colic. Arterial blood samples were taken whenever possible from the auricular artery for blood gas analyses (refer to Table 2) [8]. The animal was monitored closely and coax fed with leaves and syringe fed with oral electrolytes at intervals throughout the day and night, but showed only minimal interest in browse in the evening. Faint borborygmi could be auscultated only after 6 days of treatment, which was when a cursory abdominal ultrasound scan was conducted. There were no signs of free fluid in the abdomen but a markedly hyperechoic serosal layer of the small intestines. The fecal consistency gradually improved and the animal was back to normal 21 days after the first presenting sign of hemorrhagic diarrhea.
Table 2. Case 2 arterial blood gas analysis (i-STAT Corp., Princeton, NJ, USA).
| Parameter (Cattle RI)8 | Day 8 | Day 14 | Day 15 | Day 16 | Day 16 |
|---|---|---|---|---|---|
| pH (7.35–7.50)8 | 7.39 | 7.48 | - | 7.56 | 7.67 |
| paCO2 / mmHg (35–44)8 | 46.30 | 40.10 | - | 38.50 | 30.40 |
| paO2 / mmHg (92)8 | 39.00 | 53.00 | - | 47.00 | 68.00 |
| Base Excess mmol/l | 3.00 | 6.00 | - | 13.00 | 15.00 |
| HCO3 mmol/l (20–30)8 | 28.10 | 30.00 | - | 34.80 | 35.10 |
| Na mmol/l | 133.00 | 129.00 | 129.00 | 133.00 | 134.00 |
| K mmol/l | 3.20 | 2.50 | 3.10 | 2.50 | 2.80 |
| Ionised Calcium mmol/l | 0.99 | 0.92 | - | 0.90 | 1.00 |
| Glucose mg/dl | 8.00 | 3.60 | - | 51.00 | 102.00 |
Unlike the severe signs of colic displayed by Case 2, Case 3 showed only mild signs including lethargy, inappetence and a wide based stance. The animal was still keen to eat although the food intake progressively reduced till the day 11 when the animal was completely anorectic. From day 15, the animal would display signs of abdominal discomfort and would kneel on its carpal joints for a short while before lying down in lateral recumbency, presumably to alleviate abdominal pain. Similar to Case 2, blood was sampled for blood gas analysis and similar treatment was administered. Ultrasonographic assessment of the animal on day 14 showed no signs of free fluid at the ventral abdominal wall but multiple hypoechoic nodules were observed within the submucosa of the small intestine with a markedly hyperechoic serosal layer. The animal’s condition worsened and was found dead on the morning of day 17.
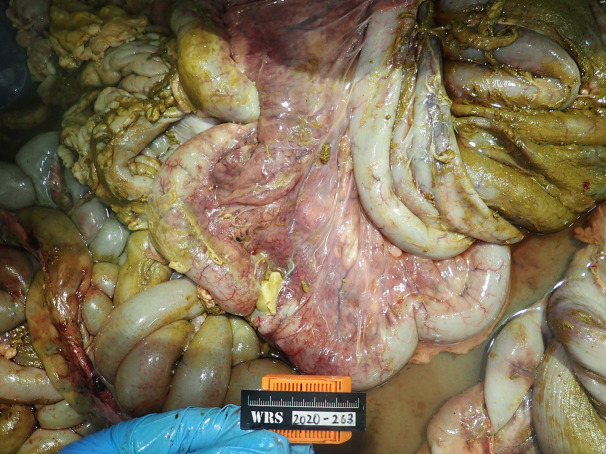
Post-mortem examination showed a large amount of digesta throughout the peritoneum with large amounts of adhesions between the intestines and mesentery. There was a large amount of moist roughage in the forestomach and large focal areas of the abomasal mucosa were discolored red. The spiral colon had multiple segments discolored grey with red streaks. The mesentery attached was congested (Fig. 3). An approximately 10 cm segment of the spiral colon was discolored purple with an 8 mm perforation of the intestinal wall on the antimesenteric border (Fig. 4). A 4 cm phytobezoar was found lodged firmly in the transverse colon, 1.5 m from the rectum. The mucosa proximal to the phytobezoar appeared dull grey with multifocal erosions and the intestinal lumen was distended (Fig. 5). There was no digesta or feces in the alimentary tract distal to the phytobezoar.
Fig. 3.
The spiral colon showing multiple segments discolored grey with red streaks. The mesentery attached also shows signs of congestion.
Fig. 4.

A perforation on the intestinal wall on the antimesenteric border seen in the spiral colon.
Fig. 5.
A phytobezoar found lodged in the transverse colon.
Representative samples from all major organs were taken for histopathology examination and H&E staining. The main findings from histopathology examination showed that all serosal surfaces of the intestines were diffusely effaced with large amounts of necrotic cellular material, degenerate neutrophils and large numbers of bacterial colonies, confirming a bacterial fibrinonecrotizing peritonitis. Destroyed intestinal crypts were present along the entire mucosa of the spiral colon. These were replaced with large numbers of mixed inflammatory cells, congestion, edema and fibrin, confirming necrotizing colitis. The inflammation and necrosis extended to the serosa in some colon sections.
The cause of the hemorrhagic enteritis in the three animals is likely to be a nutritional problem. Episodes of diarrhea in ungulates at the Singapore Zoo are usually diet related especially when leaves of particular varieties like the miracle plant (Leucaena leucocephala) are offered in too large an amount. The common approach to cases of diarrhea in the ruminants in the zoo is to withhold all high sugar food items and offer only select browse and hay. Most cases of diarrhea are self-limiting within a few days from the change in diet. All three animals developed clinical symptoms at the same time, which makes it likely that they were concurrently exposed to the same etiological agent. The animal care staff reported an inferior batch of alfafa hay, described to be dusty and easily crumbled. The hay is stored centrally in an air-conditioned container but loose hay from bags that are already in use is stored in a covered tub under shelter, adjacent to the dens of the eastern bongos. The ambient humidity is likely to be high as the zoo was experiencing torrential tropical monsoon rains during the period of the incident. The combination of the high environmental moisture content and heat could favor the growth of mold, which resulted in mycotoxicosis and a hemorrhagic bowel in the three animals. Unfortunately, mycotoxin residues are not easily detected in the animal and the symptoms are often non-specific [20]. Feed analyses of mycotoxins would also be problematic as the suspicion was only for a particular batch of hay that had already been given to the animal and the lack of testing facilities within the country.
As all animals had excellent demeanor in the first few days from when the diarrhea first began, debilitating infectious diseases are less likely. The body temperatures of the animals examined were normal and the complete blood counts (Procyte, IDEXX Laboratories Inc., Westbrook, ME, USA) were also not indicative of any systemic inflammation based on the reference range established for the speices (Zims Species360, Bloomington, MN, USA) which would be expected during an infection. In-house PCR testing capabilities could rule out the involvement of only Salmonella or Campylobacter, although further testing to rule out other infectious organisms including Clostridium would have been beneficial for more targeted treatment. Routine fecalysis of the individuals have been negative for parasites.
The clinical signs observed in Cases 2 and 3 were fitting of a gastrointestinal obstruction or impaction. Both cases had reduced borborygmi, ventral abdominal distension, dehydration, anorexia, absent fecal production and colic symptoms. Fatal abomasal and omasal impactions have been reported in captive bongos with identical clinical signs [11]. The clinicopathological findings were also fitting of abomasal impaction, with both animals presenting with progressively worsening metabolic alkalosis, hypochloremia and hypokalemia [18]. These findings are however not specific to abomasal impactions but also with obstructive intestinal disease occurring near the duodenum or pylorus [7]. Definitive diagnosis of abomasal impaction requires exploratory laparotomy, which was not done for both cases [9]. Necropsy findings of abomasal mucosal erosions in Case 3 could be suggestive of the involvement of the abomasum in the disease process, although interestingly the digesta in the forestomach was described to be consistently moist and not impacted. The latter could however be an effect of the oral liquid paraffin and fluids administered. It is unlikely that pathology causing the erythema in the abomasum is the directly contributing factor for the febrile state of Case 3 as these animals are often described to have mild or no clinical signs only [6].
Abomasal impactions in cattle occur commonly in winter and is thought to be associated with the consumption of poor-quality, coarse roughage in the face of restricted water intake [19]. For cases 2 and 3, the consumption of the inferior batch of hay could have been exacerbated by the fluid losses and dehydration from the diarrhea, resulting in mechanical impaction. Compromised gastric innervation due to ileus, vagus indigestion or hypocalcemia could have also contributed to abomasal atony and as a result, functional impaction.
The prognosis for bongos with abomasal impaction is poor to grave, with no treatments reported to be successful. In a report of the eleven bongos with abomasal impaction, all animals died from the disease. Two individuals were euthanized, one on the basis that the condition carries a grave prognosis and the other after four days of intensive treatment without signs of recovery [11, 18, 19]. The persistence of supportive therapy is thought to have contributed to the clinical resolution of Case 2. Whilst the outcome for Case 2 was successful, there were no markers of an improving condition right until diarrheic feces were observed in the den overnight on the eighth day after medical interventions under anesthesia started. Quite the contrary, the signs of colic and depressed demeanor were increasingly worrying such that an exploratory laparotomy was planned to obtain a diagnosis and potentially decide on euthanasia for the animal. It is thought that as a species, the eastern bongo may have a lower pain threshold compared to other ungulates and therefore appear more drastically unwell even though their general condition may be improving. Treatments were only continued for both Cases 2 and 3 despite the poor prognosis because despite having a drastically reduced appetite, both animals were still observed to be chewing on fodder. The slight interest in food was thought to be a positive prognostic indicator for both animals, although this is erroneous as even Case 3 was observed to be chewing on fodder hours before death.
The initial treatments administered were focused on the correction of fluid and electrolyte losses from the diarrhea in the animals. Fluid therapy in ruminant medicine is often better described for smaller ruminants as several factors limit the frequency of use and effectiveness of fluid therapy in the larger animal. These factors include the size of the patient and the volume of fluids required for the animal and proper restraint for long term intravenous administration [13]. The key limiting factor for the sedated medical interventions was to keep the total time under an hour, which was set with due consideration to the potential complications associated with long procedures in ruminant anesthesia, including bloat and regurgitation [16]. This placed constraints on the total volume of fluids that could be safely administered to the animals at a reasonable rate. This volume was 7 l in the anesthetized animal with two venous accesses and 3 l in a lightly sedated animal with one venous access. Although lactated ringers solution was used, 0.9% sodium chloride solution would have been a better base solution especially with the developing metabolic alkalosis. It is also generally reported that alkalosis is more common than acidosis in adult cattle [13]. Supplementary potassium chloride was added based on the severity of the hypokalemia, ensuring that the total rate of administration did not exceed 0.5 mEq/kg/hr. The provision of a high-potassium, chloride-rich solution provides extracellular anions in relative excess to cations, which is the treatment of choice for metabolic alkalosis [13]. Glucose was added to correct hypoglycemic states but in concentrations not greater than 2.5%. Calcium gluconate was also added to correct the low ionized calcium levels detected that in turn could improve smooth and skeletal muscle function. The rate of calcium administration did not exceed 0.5 mEq/kg/hr with constant auscultation of the heart for the development of bradyarrhythmias. Fluids were administered orally and rectally to compensate for the remaining fluid deficit after intravenous fluid therapy. The ideal scenario would be to leave the fluid line in the patient and administer fluid therapy gradually over a longer period of time. This was however assessed to be unfavorable unless the animals were placed on a sedative as access to the fluid line and the animal would have been unsafe.
The other treatments administered were treatments that have been attempted in other reported cases of abomasal impaction in bongos [11]. Prokinetic agents and oral paraffin oil were administered for the purpose of relieving the presumed mechanical obstruction. Broad spectrum intravenous and long acting intramuscular antibiotics were mainly prophylactic in the moribund animal. The choice of ceftiofur however was targeted to the possibility that the etiological agent could have been Clostridium. The choice of dexamethasone over non-steroidal anti-inflammatory drugs (NSAIDs) was for stronger anti-inflammatory effects. Potent NSAIDs are also associated with gastrointestinal side effects due to non-selective COX inhibition, which is undesirable especially when there may already be gastrointestinal ulcerations in the bongos with hemorrhagic diarrhea [5].
The post mortem findings in Case 3 were unexpected, insofar as an intestinal rupture and peritonitis were not suspected. The only finding that could have suggested a peritonitis was the hyperechoic serosal layer of the intestines noted on ultrasound examination. The rupture in the spiral colon was very likely due to the phytobezoar causing a complete intraluminal obstruction. The administration of promotility agents and oral paraffin oil could have softened any impaction, eventually dislodging the phytobezoar that moved distally into the transverse colon. The accumulation of digesta proximal to the site of impaction stretched the already diseased intestinal wall, resulting in a transmural perforation of the spiral colon.
Phytobezoar obstructions in the spiral colon have been reported in cattle, horse, camels and giraffes [1]. In most cases of phytobezoar occurrence, these structures remain in the rumen but occasionally can dislodge and move distally through the intestinal tract, with a risk of obstructing the intestinal lumen. There have been reports in cattle however, describing obstructions in the spiral colon due to adhesions causing extraluminal constriction, rather than intraluminal obstructions [14]. Peritonitis, which should be discerned from an intra-abdominal bacterial infection, can ensue from the peritoneal reaction to intestinal obstructions, compromised intestinal vascularization and gastrointestinal ulceration [4].
Hemorrhagic enteritis in the eastern bongo, although possibly self-resolving, can have dire consequences and further gastrointestinal effects. When gastrointestinal impaction or stasis is suspected, veterinary intervention should be prompt and aggressive. Despite there being few clinical markers to gauge the prognosis or recovery of the animals, persisting with treatment, which can be prolonged, can still achieve favorable outcomes.
CONFLICT OF INTEREST
The authors declare no conflicts of interest in the carrying out of this work.
Supplementary
Acknowledgments
The authors would like to thank the veterinarians, veterinary nurses and laboratory technicians for their medical support for these cases, as well as the animal care staff who have toiled around the clock for the animals they love and care for.
REFERENCES
- 1.Davis M. R., Langan J. N., Mylniczenko N. D., Benson K., Lamberski N., Ramer J.2009. Colonic obstruction in three captive reticulated giraffe (Giraffa camelopardalis reticulata). J. Zoo Wildl. Med. 40: 181–188. doi: 10.1638/2008-0102.1 [DOI] [PubMed] [Google Scholar]
- 2.Dennison A. C., VanMetre D. C., Callan R. J., Dinsmore P., Mason G. L., Ellis R. P.2002. Hemorrhagic bowel syndrome in dairy cattle: 22 cases (1997–2000). J. Am. Vet. Med. Assoc. 221: 686–689. doi: 10.2460/javma.2002.221.686 [DOI] [PubMed] [Google Scholar]
- 3.Disney Animal Programs.2005. Bongo body condition scores, Disney animal programs. Lake Buena Vista, FL: Disney’s Animal Kingdom. [Google Scholar]
- 4.Fecteau G.2005. Management of peritonitis in cattle. Vet. Clin. North Am. Food Anim. Pract. 21: 155–171. doi: 10.1016/j.cvfa.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 5.Francisco J. B., Guitian R., Moreno J., Francisco J., Galdo F.1999. Effect of anti-inflammatory drugs on COX-1 and COX−2 activity in human articular chondrocytes. J. Rheumatol. 26: 1366–1373. [PubMed] [Google Scholar]
- 6.Francoz D., Guard C. L.2009. Abomasal ulcers. pp. 861–863. In: Large Animal Internal Medicine, 4th ed. (Smith, B. P. ed), Elsevier, St. Louis. [Google Scholar]
- 7.Francoz D., Guard C. L.2009. Obstructive intestinal diseases. pp. 861–863. In: Large Animal Internal Medicine, 4th ed. (Smith, B. P. ed), Elsevier, St. Louis. [Google Scholar]
- 8.George J. W.1994. Water electrolytes and acid base, pp. 94–111. In: Veterinary Laboratory Medicine Clinical Pathology, 3rd ed. (Duncan, R. J., Prasse, K. W. and Mahaffey, E. A. eds), Iowa State University Press, Ames. [Google Scholar]
- 9.Guard C. L., Francoz D.2009. Abomasal impaction. pp. 864–866. In: Large Animal Internal Medicine, 4th ed. (Smith, B. P. ed), Elsevier, St. Louis. [Google Scholar]
- 10.Gunn A. A., Naylor J. A., House J. K.2009. Diarrhea. pp. 340–363. In: Large Animal Internal Medicine, 4th ed. (Smith, B. P. ed), Elsevier, St. Louis. [Google Scholar]
- 11.Gyimesi Z. S., Burns R. B., Campbell M., Knightly F., Kramer L. W., Wack R. F., Zuba J. R., Rings D. M.2011. Abomasal impaction in captive bongo (Tragelaphus eurycerus). J. Zoo Wildl. Med. 42: 281–290. doi: 10.1638/2010-0116.1 [DOI] [PubMed] [Google Scholar]
- 12.Heller M. C., Chigerwe M.2018. Diagnosis and treatment of infectious enteritis in neonatal and juvenile ruminants. Vet. Clin. North Am. Food Anim. Pract. 34: 101–117. doi: 10.1016/j.cvfa.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roussel A. J., Jr.1999. Fluid therapy in mature cattle. Vet. Clin. North Am. Food Anim. Pract. 15: 545–557. doi: 10.1016/S0749-0720(15)30163-8 [DOI] [PubMed] [Google Scholar]
- 14.Smith D. F., Donawick W. J.1979. Obstruction of the ascending colon in cattle: I. Clinical presentation and surgical management. Vet. Surg. 8: 93–97. doi: 10.1111/j.1532-950X.1979.tb00617.x [DOI] [Google Scholar]
- 15.Songer J. G.1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9: 216–234. doi: 10.1128/CMR.9.2.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steffey E. P.1986. Some characteristics of ruminants and swine that complicate management of general anesthesia. Vet. Clin. North Am. Food Anim. Pract. 2: 507–516. doi: 10.1016/S0749-0720(15)31203-2 [DOI] [PubMed] [Google Scholar]
- 17.Sudhakara Reddy B., Sivajothi S., Rayulu V. C.2015. Clinical coccidiosis in adult cattle. J. Parasit. Dis. 39: 557–559. doi: 10.1007/s12639-013-0395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguchi K.1995. Relationship between degree of dehydration and serum electrolytes and acid-base status in cows with various abomasal disorders. J. Vet. Med. Sci. 57: 257–260. doi: 10.1292/jvms.57.257 [DOI] [PubMed] [Google Scholar]
- 19.Trent A. M.1990. Surgery of the bovine abomasum. Vet. Clin. North Am. Food Anim. Pract. 6: 399–448. doi: 10.1016/S0749-0720(15)30868-9 [DOI] [PubMed] [Google Scholar]
- 20.Whitlow L. W., Hagler W. M.2005. Mycotoxins in dairy cattle: Occurrence, toxicity, prevention and treatment. Proc. Southwest Nutrition Conference, 124–128.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.