This cohort study examines the associations between precancer mental health and pain and postcancer receipt of mental health, substance use disorder, or palliative care services with risk of suicidal self-directed violence.
Key Points
Question
Among survivors of head and neck cancer (HNC), which precancer characteristics and postcancer health services are associated with suicidal self-directed violence (SSDV) after cancer diagnosis?
Findings
Among 7803 US veteran survivors of HNC, precancer chronic pain or mood disorders and postcancer mental health and substance use disorder treatment were associated with increased risk of SSDV. Use of postcancer palliative care was associated with decreased risk of SSDV.
Meaning
Additional suicide prevention efforts should be directed toward survivors of HNC with precancer and postcancer risk factors for SSDV; palliative care may be an important component of supportive cancer care.
Abstract
Importance
Head and neck cancer (HNC) survivors are about twice as likely to die by suicide compared with other cancer survivors.
Objective
To examine the associations between precancer mental health and pain and postcancer receipt of mental health, substance use disorder (SUD), or palliative care services with risk of suicidal self-directed violence (SSDV).
Design, Setting, and Participants
This retrospective cohort study used the Veterans Health Administration data of 7803 veterans with a diagnosis of HNC (stage I-IVB) who received cancer treatment between January 1, 2012, and January 1, 2018. Data were analyzed between May 2020 and July 2021.
Exposures
Presence of precancer chronic pain and SUD diagnoses, and postcancer SUD, mental health, or palliative care treatment. Exposures were defined using International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in Veterans Health Administration administrative data.
Main Outcomes and Measures
Documented SSDV event, including suicide attempt or death by suicide, after HNC diagnosis.
Results
Among the cohort of 7803 veterans (7685 [98.4%] male; mean [SD] age, 65 [10.7] years), 72 (0.9%) had at least 1 documented SSDV event following their cancer diagnosis, and 51 (0.7%) died by suicide. Four adjusted modified Poisson regression analyses identified that precancer chronic pain (incidence rate ratio [IRR], 2.58; 95% CI 1.54-4.32) or mood disorder diagnoses (IRR, 1.95; 95% CI, 1.17-3.24) were associated with higher risk of postcancer SSDV. Those who had at least 1 documented mental health (IRR, 2.73; 95% CI, 1.24-6.03) or SUD (IRR, 3.92; 95% CI, 2.46-6.24) treatment encounter in the 90 days following HNC diagnosis were at higher risk for SSDV. A palliative care encounter within 90 days of postcancer diagnosis was associated with decreased risk of SSVD (IRR, 0.49; 95% CI, 0.31-0.78).
Conclusions and Relevance
In this cohort study, a high proportion of HNC survivors with an SSVD event died from their injuries. Identification of risk factors for SSDV among HNC survivors may help direct additional resources to those who are at high risk. Referral to palliative care appears to be an important component of supportive oncologic care to reduce the risk of SSDV.
Introduction
Suicide is the 10th leading cause of death among US adults,1 and cancer survivors have twice the risk of death by suicide compared with those without cancer.2 Results from the Surveillance, Epidemiology, and End Results database found that survivors of head and neck cancer (HNC) are more than twice as likely to die by suicide compared with survivors of other cancer types (63.4 vs 23.6 suicides per 100 000 person-years) and 3 times as likely compared with the general population.3 Despite recent advances in cancer care that have improved overall survival, the risk of death by suicide among survivors of HNC has increased 27% in recent years.3 Individuals within 5 years of their HNC diagnosis are at the highest risk of death by suicide.4
Survivors of HNC have high rates of pain during and after cancer treatment,5,6 high substance use before and after treatment,7 and mental health (MH) comorbidities.8 In addition, survivors of HNC have a high degree of disease and treatment burden such as difficulty swallowing, eating, and speaking, which causes functional impairment and psychological distress.9 The combination of these clinical characteristics places individuals with HNC at higher risk for death by suicide.10,11 Other nonmodifiable individual-level clinical and sociodemographic factors have been associated with incidence of suicide among survivors of HNC, including late-stage disease, being single or divorced, White race, and pharyngeal involvement.12,13
Little is known about modifiable risk factors, including how health services use modifies suicide risk in survivors of HNC. Research in veterans with late-stage lung cancer showed engagement in palliative care services decreased the risk of death by suicide.14 Data are needed to understand how use of services such as MH treatment, substance use disorder (SUD) treatment, or palliative care may be associated with a reduction in suicide risk among survivors of HNC.
In this cohort study, we examined the associations between precancer clinical risk factors as well as postcancer use of palliative care and MH or SUD treatment with risk of suicidal self-directed violence (SSDV) events (including suicide attempt or death by suicide) among a large national cohort of US veteran survivors of HNC. We hypothesized that chronic pain and comorbid MH and/or SUD diagnoses before cancer would independently increase risk of SSDV. We also hypothesized that use of palliative care and MH or SUD treatment would be associated with a reduction in the likelihood of having an SSDV event.
Methods
Cohort
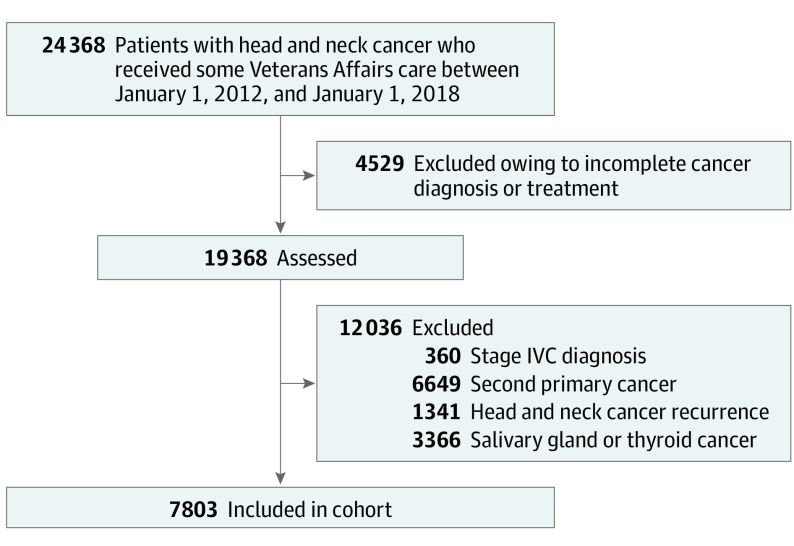
Using US Department of Veterans Affairs (VA) administrative data extrapolated from the Corporate Data Warehouse (CDW), we identified individuals who were diagnosed with a cancer in the head and neck region (ie, nasal cavity, nasal sinus, nasopharynx, oropharynx, oral cavity, hypopharynx, larynx) and had documented episodes of cancer care in the Veterans Health Administration (VHA) between January 01, 2012, and January 01, 2018. To create a cohort of patients who would be expected to have a longer survivorship, we excluded individuals diagnosed with stage IVC disease, who had a documented second primary cancer, or who had HNC recurrence in the 2 years following their index HNC diagnosis date (eTable 1 in the Supplement). We excluded those with salivary or thyroid primary site because these cancers are associated with different risk factors and treatment pathways. An overview of the sample selection is provided in Figure 1. This study was approved by the joint VA Portland Health Care System–Oregon Health & Science University institutional review board. A waiver of informed consent documentation and process was obtained for the following reasons: (1) this was determined to be a low-risk study; (2) it is not possible to obtain consent from those who have died, which comprised an essential population of the study; and (3) the potential risk of mailing consent (which contains personal identifiable information and protected health information, including social security numbers) to and from potential participants poses the risk of a breach of confidentiality. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed.
Figure 1. Cohort Flowchart.
Data Sources and Elements
Corporate Data Warehouse
The VA’s CDW is a comprehensive, relational database that aggregates electronic health record data from VA clinics and hospitals nationally. Data about cancer diagnosis and treatment were extracted from the CDW’s oncology raw domain,15 and International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes were used to identify MH and SUD diagnoses and treatment use (defined as at least 1 completed treatment encounter using a clinic stop code; eTable 1 in the Supplement). We identified palliative care use using the requested service name in the CDW consults table. We extracted pain scores, which are documented in CDW using the Numeric Rating Scale and ranging from 0 to 10, with 0 indicating no pain and 10 indicating the worst pain imaginable.16 Chronic pain was defined as having a Numeric Rating Scale score of 4 or more documented in 3 separate months within a single year.16,17,18,19 We obtained opioid prescription data for any opioid-based medication, except tramadol, including prescription names, doses, dates, and number of fills from CDW pharmacy data tables.20 We extracted all aforementioned data for the 1 year prior to the HNC diagnosis (precancer) and the 2 years following the HNC diagnosis (postcancer).
Suicide Prevention Applications Network
Suicide Prevention Applications Network is a validated database that VA maintains for comprehensive records on suicide attempts, suicide behavior reports, suicide risk assessments, and other suicide-related clinical documentation submitted by suicide prevention coordinators.21 We obtained dates, means, and outcome (eg, death, hospitalization, outpatient MH treatment) of every SSDV attempt. All suicide-related behaviors are categorized using Centers for Disease Control and Prevention guidelines.22 We included veterans who had a suicide death or a self-directed violence event with suicidal intent as SSDV. Any documented SSDV event, at any time precancer or postcancer, in VA records was included in the data. Any SSDV events preceding the HNC diagnosis were considered pre-HNC SSDV events, and those following the HNC diagnosis were considered post-HNC SSDV events. Date of final Suicide Prevention Applications Network extraction was May 22, 2019.
Suicide Data Repository
We obtained cause of death data, primarily death by suicide, from the US Department of Defense’s Suicide Data Repository, which is populated by the National Death Index.21 The Suicide Data Repository has been used in health services research as a validated source for death by suicide.14
Centers for Medicare & Medicaid Services
Veteran Affairs maintains curated Centers for Medicare & Medicaid Services data for veterans 65 years and older who are also covered by Medicare. From these data we obtained Medicare Part D (outpatient pharmacy claims) to capture opioid medications obtained outside of VHA. Combined with the VA CDW pharmacy data, we calculated the total daily morphine milligram equivalent for opioid medications filled in VHA and outside of VA using standardized conversions.23
Data Aggregation and Quality Assessment
Data from these sources were linked using scrambled social security numbers. We followed data quality assessment guidelines.24 Plausibility and correctness were assessed using examination of agreement between data elements and consultation with clinical and research experts in VA oncology electronic medical record data and Centers for Medicare & Medicaid Services or VA opioid prescription data. Data concordance and completeness were assessed by a trained data analyst (R.H.) with consultation from the principal investigator (S.M.N). We sought consultation from the VA Portland Health Care System cancer registrar, which oversees data input for the VA’s CDW oncology raw domain variables, following the Commission on Cancer’s 2016 Facility Oncology Registry Data Standards manual.25
Statistical Analysis
We examined frequencies and measures of central tendency for sociodemographic and clinical variables of interest 1 year prior and 2 years after HNC diagnosis. We conducted unadjusted bivariate comparisons to examine whether clinical variables of interest differed between those who did not have a SSDV event and those who did. We conducted modified Poisson regression analyses (a Poisson regression with robust error parameter estimates) to calculate incidence rate ratios (IRRs) for the dichotomous outcome.26 We ran adjusted models that controlled for age, marital status, and sex, with each precancer risk factor: chronic pain (dichotomous), a documented alcohol use disorder (dichotomous), a documented mood disorder (dichotomous), and the presence of a precancer SSDV (dichotomous). The fully adjusted model included all precancer risk factors, as well as age, marital status, and sex to assess for independence. For these analyses, SPSS statistical software, version 26 (IBM Corp), was used. Covariates were chosen a priori based on literature and use of directed analytic graphs. All available data for the 2-year observation period were used in the models.
We ran 3 adjusted modified Poisson regression models examining the association between postcancer diagnosis supportive services use (ie, palliative care, MH and SUD treatment) and SSDV event. Modified Poisson models are useful for analyzing rare events when patients are followed for a variable length of time. Each model controlled for cancer stage, age, marital status, sex, and documented precancer use of palliative care, MH treatment, or SUD treatment, respectively. We dichotomized use of these services (any documented use vs no documented use) from a count variable, minimizing immortal time bias, because those with longer survival would be more likely to have higher service use counts. To further reduce immortal time bias, we only examined treatment encounters in the 3 months after HNC diagnosis. We only included treatment encounters occurring prior to the first SSDV event after the cancer diagnosis. We conducted a sensitivity analysis excluding those who were given immunotherapy. For all modified Poisson regression models, we calculated the average marginal effects using Stata, version 15 (StataCorp).
Results
A total of 7803 survivors of HNC were included in the cohort. The majority were male (n = 7685; 98.4%) and identified as non-Hispanic White (n = 6179; 79.2%), and the mean (SD) age was 65 (10.7) years (Table 127). Regarding cancer stage of survivors, 2016 (25.8%) were diagnosed with stage I, 941 (12.1%) with stage II, 1189 (15.2%) with stage III, and 3657 (46.9%) with stage IVA or IVB. Radiation was the most common mode of treatment (n = 5166; 66.2%), followed by chemotherapy (n = 3284; 42.1%) and surgery (n = 2906; 37.2%). Treatments are not mutually exclusive. Squamous cell carcinoma was the most common tumor histologic finding (n = 7500; 96.1%); the most common primary tumor sites were pharyngeal (n = 3439; 44.0%) and laryngeal (n = 2365; 30.3%).
Table 1. Sociodemographic and Clinical Characteristics of Survivors of Head and Neck Cancer With and Without Suicidal Self-directed Violence (SSDV) Events.
| Sociodemographic and clinical characteristics | No. (%) | ||
|---|---|---|---|
| Total cohort (n = 7803) | Without SSDV (n = 7731) | With SSDV (n = 72) | |
| Age at diagnosis, mean (SD) | 64.6 (10.7) | 66.2 (9.1) | 61.0 (9.1) |
| Sex | |||
| Male | 7685 (98.4) | 7616 (98.5) | 69 (95.8) |
| Female | 118 (1.5) | 115 (1.5) | 3 (4.2) |
| Racea | |||
| American Indian | 68 (0.9) | 68 (0.9) | 0 |
| Asian | 11 (0.1) | 11 (0.1) | 0 |
| Black | 1098 (14.1) | 1087 (14.1) | 11 (15.3) |
| Pacific Islander | 61 (0.8) | 61 (0.8) | 0 |
| White | 6179 (79.2) | 6120 (79.2) | 59 (81.9) |
| Marital status | |||
| Divorced/separated | 3253 (41.7) | 3223 (41.7) | 30 (41.7) |
| Married/domestic partnership | 3116 (39.9) | 3091 (40.0) | 25 (34.7) |
| Single (never married) | 849 (10.9) | 835 (10.8) | 14 (19.4) |
| Unknown/multiple statuses | 69 (0.9) | 69 (0.9) | 0 |
| Widowed | 516 (6.6) | 513 (6.6) | 3 (4.2) |
| Mode of treatmentb | |||
| Surgery | 2906 (37.2) | 2884 (37.3) | 22 (30.6) |
| Radiation | 5166 (66.2) | 5116 (66.2) | 50 (69.4) |
| Chemotherapy | 3284 (42.1) | 3254 (42.1) | 30 (41.7) |
| Immunotherapy | 270 (3.5) | 263 (3.4) | 7 (9.7) |
| Histology | |||
| Squamous cell carcinoma | 7500 (96.1) | 7431 (96.1) | 69 (95.8) |
| Other | 304 (3.9) | 301 (3.9) | 3 (4.2) |
| Cancer sitec | |||
| Pharynx | 3436 (44.0) | 3436 (44.4) | 34 (47.2) |
| Larynx | 2365 (30.3) | 2365 (30.6) | 21 (29.2) |
| Lip and oral cavity | 1856 (23.8) | 1856 (24.0) | 17 (23.6) |
| Nasal cavity and paranasal sinuses | 146 (1.9) | 146 (1.9) | 0 (0) |
| Stage of cancer | |||
| I | 2016 (25.8) | 1999 (25.9) | 17 (23.6) |
| II | 941 (12.1) | 934 (12.1) | 7 (9.7) |
| III | 1189 (15.2) | 1177 (15.2) | 12 (16.7) |
| IVA/IVB | 3657 (46.9) | 3621 (46.8) | 36 (50.0) |
Unknown category for race accounts for percentages not equal to 100.
Treatments are not mutually exclusive, so total percentage may exceed 100.
Categorization of cancer sites according to the American Joint Committee on Cancer staging system.27
Of the total cohort, 5040 (64.6%) survivors had a documented MH or SUD diagnosis after their cancer diagnosis (Table 2). Mood disorders, including major depressive disorder, bipolar disorder, and dysthymia, were the most common MH diagnoses before (n = 1560; 20.0%) and after (n = 2046; 26.2%) HNC diagnosis. Nicotine use disorder was the most commonly diagnosed SUD before (n = 2355; 23.7%) and after (n = 2445; 24.6%) HNC diagnosis. The proportion of those who had any documented MH or SUD diagnosis increased for all diagnoses from the precancer period to the postcancer period (2198 [28.2%] vs 3401 [43.6%]). Of the total cohort, 2747 (35.2%) survivors experienced chronic pain in the 2 years following their cancer diagnosis compared with 1572 (19.6%) precancer.
Table 2. Mental Health (MH) and Substance Use Disorder (SUD) Characteristics of Survivors of Head and Neck Cancer (HNC) Before and After Diagnosis With and Without Suicidal Self-directed Violence (SSDV) Events.
| MH And SUD diagnoses and treatment encounters | No. (%) | |||||
|---|---|---|---|---|---|---|
| Total cohort (n = 7803) | Without SSDV (n = 7731) | With SSDV (n = 72) | ||||
| Before HNC diagnosis | After HNC diagnosis | Before HNC diagnosis | After HNC diagnosis | Before HNC diagnosis | After HNC diagnosis | |
| MH and SUD diagnosesa | ||||||
| PTSD | 926 (11.9) | 1072 (13.7) | 910 (11.8) | 1053 (13.6) | 16 (22.2) | 19 (26.4) |
| Anxiety disorder | 620 (8.0) | 1055 (13.5) | 608 (7.9) | 1028 (13.3) | 12 (16.7) | 27 (37.5) |
| Mood disorder | 1560 (20.0) | 2046 (26.2) | 1530 (19.8) | 1995 (25.8) | 30 (41.7) | 51 (70.8) |
| Any MH diagnosis | 1391 (17.8) | 1907 (24.4) | 1366 (17.7) | 1867 (24.1) | 25 (34.7) | 40 (55.6) |
| Nicotine SUD | 2355 (23.7) | 2445 (24.6) | 2326 (30.1) | 2412 (31.2) | 29 (40.3) | 33 (45.8) |
| Alcohol SUD | 1245 (12.5) | 1356 (13.6) | 1229 (15.9) | 1330 (17.2) | 16 (22.2) | 26 (36.1) |
| Cannabis SUD | 203 (2.0) | 258 (2.6) | 198 (2.6) | 244 (3.2) | 5 (6.9) | 14 (19.4) |
| Opioid SUD | 128 (1.3) | 129 (1.3) | 123 (1.6) | 115 (1.5) | 5 (6.9) | 14 (19.4) |
| Any SUD diagnosis | 2958 (37.9) | 3133 (40.2) | 2918 (37.7) | 3084 (40.0) | 40 (55.6) | 49 (68.0) |
| MH and SUD treatment use | ||||||
| Any MH visits | 2198 (28.2) | 3401 (43.6) | 2157 (27.9) | 3340 (43.2) | 41 (56.9) | 61 (84.7) |
| MH Treatment encounters,b median (IQR) | 4.0 (1-10) | 5.0 (1-12) | 4.0 (1-10) | 23.0 (4-62) | 13.0 (3-30) | 5.0 (1-11) |
| Time to first MH treatment encounter, mean (SD), d | NA | 210.1 (220.4) | NA | 208.7 (219.6) | NA | 233.0 (237.4) |
| Any SUD treatment visits | 399 (5.1) | 435 (5.6) | 388 (5.0) | 411 (5.3) | 11 (15.3) | 24 (33.3) |
| Time to first SUD treatment encounter, mean (SD), d | NA | 136.5 (167.2) | NA | 137.3 (167.4) | NA | 92.4 (151.4) |
| SUD Treatment encounters,b median (IQR) | 7.0 (2-34) | 4.0 (2-23) | 7.0 (4-34) | 4.0 (2-20) | 10.0 (5-94) | 16.5 (8-89) |
| Time to first palliative care encounter, mean (SD), d | NA | 151.0 (173.7) | NA | 149.2 (172.0) | NA | 275.6 (242.4) |
| Pain variables | ||||||
| Chronic pain | 1572 (19.6) | 2747 (35.2) | 1495 (19.3) | 2696 (34.9) | 32 (44.4) | 51 (70.1) |
| Prescription opioid MME,c mean (SD) (n = 6880) | 50.4 (96.7) | 88.8 (133.8) | 50.5 (97.3) | 77.9 (128.8) | 42.6 (35.4) | 121.6 (130.6) |
Abbreviations: MME, morphine milligram equivalent; NA, not applicable; PTSD, posttraumatic stress disorder.
Diagnoses are not mutually exclusive, so total percentage may exceed 100.
The MH and SUD treatment encounter medians and IQRs were calculated among those who had at least 1 encounter.
Total MME was only calculated for those who were currently prescribed opioids.
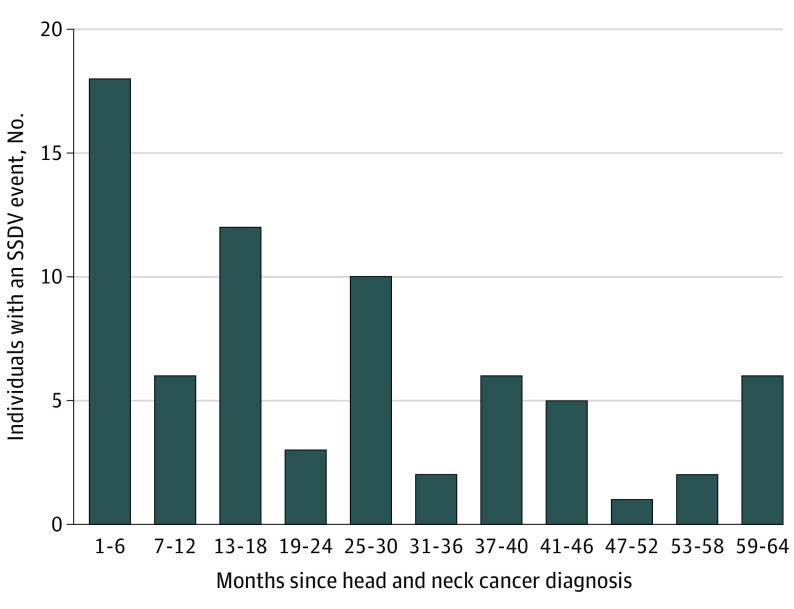
Seventy-two individuals (0.9%) had at least 1 documented SSDV event following their cancer diagnosis; 51 of whom died by suicide. Patients were followed for a mean (SD) of 3.2 (2.1) years for a total of 25 015 person-years, yielding an incidence of 203.9 deaths and 287.8 SSDV events per 100 000 person-years. The most common means of SSDV were intentional self-poisoning with various substances (n = 28, 14 of whom died) or firearm discharge (n = 33, all of whom died). Almost half (n = 32; 44.4%) of SSDV events were more than 2 years after the diagnosis, though 18 (25.0%) individuals had an event within 6 months of their cancer diagnosis (Figure 2). For 67 (93.1%) individuals with a postcancer SSDV event, it was the first documented SSDV event in their medical history.
Figure 2. Time Between Head and Neck Cancer Diagnosis and First Documented Suicidal Self-directed Violence (SSDV) Event.
Survivors of HNC with an SSDV event had higher rates of precancer MH and SUD diagnoses (except alcohol and nicotine use disorder; Table 2). Among those who had a documented palliative care consult (n = 2074), MH treatment encounter (n = 3401), or SUD treatment encounter (n = 435), average days to first use of each of these services were examined (Table 2). Those with documented SUD treatment prior to their SSDV event (n = 24) had shorter time to SUD treatment (mean [SD], 92 [151] days) compared with those without documented SSDV (n = 411; mean [SD], 137 [167] days). Those who received palliative care and had documented SSDV (n = 29) had a longer time to first palliative care treatment (mean [SD], 276 [242] days) compared with those who did not have an SSDV event (n = 2045; mean [SD], 149 [172] days).
Several modified Poisson regression models were conducted to examine associations between precancer risk factors and a postcancer SSDV event (Table 3). In separate adjusted models, all risk factors showed an increase in risk of a SSDV event. In the fully adjusted model, the presence of a precancer mood disorder (IRR, 1.95; 95% CI, 1.17-3.24), precancer chronic pain (IRR, 2.58; 95% CI, 1.54-4.32), and precancer SSDV (IRR, 3.56; 95% CI, 1.15-11.02) all were independently associated with increased risk of a postcancer SSDV event. Marginal effects were calculated to assess the difference in predicted probability of the outcome associated with each of these risk factors (Table 3). For example, the probability of an SSDV event among veterans with an SSDV before their cancer diagnosis was 0.032 (32 veterans per 1000) and 0.009 (9 veterans per 1000) for those with no SSDV event before their cancer diagnosis.
Table 3. Association of Precancer Risk Factors and Postcancer Use of Mental Health, Substance Use Disorder, or Palliative Care Treatment With Postcancer Suicidal Self-directed Violence (SSDV) Events in Survivors of Head and Neck Cancera.
| Precancer risk factor | Adjusted IRR (95% CI) | Predicted probability of postcancer SSDV average marginal effects (95% CI) | ||
|---|---|---|---|---|
| Risk factor | No risk factor | Difference | ||
| Alcohol substance use disorder | 0.89 (0.50 to 1.58) | 0.008 (0.004 to 0.013) | 0.095 (0.007 to 0.012) | –0.001 (–0.006 to 0.004) |
| Mood disorder diagnosis | 1.94 (1.17 to 3.24) | 0.014 (0.009 to 0.020) | 0.007 (0.005 to 0.010) | 0.007 (0.001 to 0.013) |
| Chronic pain | 2.58 (1.54 to 4.32) | 0.017 (0.011 to 0.024) | 0.007 (0.005 to 0.009) | 0.011 (0.004 to 0.018) |
| Precancer SSDV | 3.56 (1.15 to 11.02) | 0.032 (–0.003 to 0.067) | 0.009 (0.007 to 0.011) | 0.023 (–0.012 to 0.058) |
| Service use within 90 d after cancer diagnosis | ||||
| Mental health treatment | 5.04 (2.60 to 9.76) | 0.041 (0.016 to 0.067) | 0.008 (0.006 to 0.010) | 0.033 (0.008 to 0.059) |
| Palliative care | 0.49 (0.31 to 0.78) | 0.005 (0.003 to 0.007) | 0.010 (0.008 to 0.120) | –0.005 (–0.008 to –0.002) |
| Substance use disorder treatment | 3.92 (2.46 to 6.24) | 0.020 (0.014 to 0.026) | 0.005 (0.003 to 0.007) | 0.015 (0.009 to 0.021) |
Abbreviation: IRR, incidence rate ratio.
The precancer risk factor model controlled for age, marital status, and sex. Service use models included cancer stage and precancer use of that service, in addition to age, sex, and marital status.
Separate analyses examined the association between any MH treatment, palliative care, or SUD treatment encounter (preceding the SSDV event and occurring within 90 days of the HNC diagnosis) and a SSDV event (Table 3). Postcancer use of MH treatment (IRR, 2.73; 95% CI, 1.24-6.03) and SUD treatment (IRR, 3.92; 95% CI, 2.46-6.24) were associated with a higher risk of a subsequent SSDV event. Use of palliative care was associated with decreased risk of SSDV (IRR, 0.49; 95% CI, 0.31-0.78). Results of sensitivity analyses, which excluded all who received immunotherapy, did not differ appreciably from primary analyses, with the exception of the palliative care model, which was no longer associated (eTable 2 in the Supplement).
Discussion
Identifying precancer and postcancer risk factors for SSDV is essential to direct prevention efforts in a population that experiences increasing rates of SSDV. The present cohort had an SSDV incidence of 203.9 deaths by suicide and 287.8 SSDV events per 100 000 person-years. This is about 4 times the incidence of suicide compared with veterans with any diagnosed substance use or mental health disorders28 and 3 times higher than nonveteran HNC survivors.3 The presence of chronic pain, a mood disorder, or a self-directed violence event pre-HNC diagnosis were all independently associated with an increased risk of an SSDV event following HNC diagnosis. In addition, we found use of SUD and MH treatment within 90 days of the HNC diagnosis were associated with increased risk of a subsequent SSDV event. Use of palliative care in the 90 days following cancer diagnosis decreased the risk of a subsequent SSDV event.
Survivors of HNC have high symptom burden such as disfigurement and pain during speaking, swallowing, and eating, secondary to their disease and cancer treatment,9 all contributing to distress and poor quality of life. This study’s findings are consistent with the high pain and psychological symptom burden following HNC treatment found in other studies.29 The present findings also portray a unique picture of relatively high prevalence of precancer pain, SUD diagnoses, and MH comorbidities. We know from observational studies of those with HNC that high pain during survivorship and premorbid MH diagnoses place individuals with HNC at higher risk of death by suicide30 compared with those with other types of cancer and the general population.4,5,7,30 The present findings corroborate the increased risk among those with pain and MH comorbidities31 but add to the literature in that we also examined a history of a precancer SSDV event.
There are several clinical implications of these findings. Palliative care was associated with lower risk of a subsequent SSDV event. This is similar to a recent study of veterans that found reduced risk of death by suicide among those with advanced stage lung cancer who received palliative care.14 Taken together, these findings suggest that there may be important and unique components of palliative care, such as the combination of pain management, mental health and spiritual support, social work, and nutrition that offer the range of wraparound services needed to support those survivors of HNC who have severe symptom and emotional burden.32 It is important to acknowledge that heterogeneity exists as to how palliative care teams receive referrals within and outside of VA, which may enhance generalizability of the present findings to other health care systems. Also, other supportive services such as cancer rehabilitation may have some overlapping services provided to palliative care. Relatedly, multidisciplinary care models have been found to enhance survival33 and quality of life,29,34 and these care models, in collaboration with palliative care, may work synergistically to lengthen survival.
Patients who are engaged in MH or SUD treatment appear to be more likely to have a subsequent SSDV. Those with more severe MH and SUD challenges may be more likely to engage in treatment and also may be at increased risk for SSDV. Thus, use of these services might be an opportunity for identification of high-risk individuals. While distress screening is recommended by several national guidelines,35,36 screening related to suicide is less commonly a part of cancer care. The Suicidal Behaviors Questionnaire-Revised37 is an appropriate screening tool for suicidality among survivors of HNC and could be incorporated into oncology clinics and used earlier in the cancer treatment trajectory.32 Prior work has identified a screening and follow-up workflow protocol, including assessment and treatment, for those with HNC who have positive screening results.38 Robust evidence suggests that increasing screening does not increase likelihood of acting on suicidal thoughts,39 though screening must occur in the context of a clinical infrastructure to support follow-up MH care.
Determining the best timing to screen for suicidal ideation is important. Prior work found that those with HNC were at highest risk for suicidal ideation between 6 months and 5 years postdiagnosis.40 Similarly, results from this study suggest that the majority of the cohort had their first SSDV event within 2 years of their cancer diagnosis (with a peak at 6 months). For the majority of those with SSDV, the event following the cancer diagnosis was the first event in their medical record, suggesting that the HNC diagnosis and/or treatment played a role in precipitating the SSDV event. Prior work indicates that the combination of stressful life events, perceived burdensomeness of adverse effects, and chemical changes caused by the combination of steroids, chemotherapy, and other medications may exacerbate MH crises34 and increase acquired capability for suicide.35 Importantly, SSDV in the present cohort often resulted in death because individuals used highly lethal means such as firearms and medication overdose. Because SSDV (and death by suicide) can occur sometime after acute phase treatment of HNC, facilitating long-term follow-up MH support and regularly screening may help mitigate suicide risk over time.
Limitations
We note several limitations to this study. First, the cohort comprised veterans who did not have an HNC recurrence or second primary cancer in the 2 years following their index HNC diagnosis; this may limit generalizability of the findings to those with more severe, recurrent, or second primary cancer. In addition, the composition of the cohort may limit generalizability to nonveterans, female patients, or those not receiving health care in VA. Second, these data are based on documented events in VA administrative data, so it is possible that service use, especially treatment obtained outside of VA, would not be documented and would artificially lower estimated use rates. It is possible that SSDV events may be underreported and death by suicide may be misclassified as another cause of death. Finally, there are limitations to the use of International Classification of Diseases codes as a proxy for the presence of an MH or SUD diagnosis, which may affect the accuracy of the prevalence estimate.
Conclusions
Results of this cohort study demonstrate that survivors of HNC are at high risk for self-directed violence and death by suicide. The findings offer insights into precancer MH, SUD, and pain clinical characteristics as well as how use of postcancer services are associated with SSDV. Identification of precancer and postcancer risk factors can enhance SSDV screening and prevention efforts, potentially improving HNC survival. Palliative care service use is associated with a decrease in postcancer SSDV and should be considered an important component of comprehensive cancer care. Additional research might examine the relationship between palliative care and decreased risk of SSDV to elucidate what components of palliative care may reduce SSDV risk.
eTable 1. Study population ICD-9-CM/ICD-10-CM diagnosis codes
eTable 2. Sensitivity analyses excluding those on immunotherapy (n=7532)
References
- 1.Crosby AE, Ortega L, Stevens MR; Centers for Disease Control and Prevention (CDC) . Suicides—United States, 2005-2009. MMWR Suppl. 2013;62(3):179-183. [PubMed] [Google Scholar]
- 2.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738. doi: 10.1200/JCO.2007.13.8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osazuwa-Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124(20):4072-4079. doi: 10.1002/cncr.31675 [DOI] [PubMed] [Google Scholar]
- 4.Kam D, Salib A, Gorgy G, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1075-1081. doi: 10.1001/jamaoto.2015.2480 [DOI] [PubMed] [Google Scholar]
- 5.Macfarlane TV, Wirth T, Ranasinghe S, Ah-See KW, Renny N, Hurman D. Head and neck cancer pain: systematic review of prevalence and associated factors. J Oral Maxillofac Res. 2012;3(1):e1. doi: 10.5037/jomr.2012.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer JD, Johnson JT, Nilsen ML. Pain in head and neck cancer survivors: prevalence, predictors, and quality-of-life impact. Otolaryngol Head Neck Surg. 2018;159(5):853-858. doi: 10.1177/0194599818783964 [DOI] [PubMed] [Google Scholar]
- 7.Duffy SA, Terrell JE, Valenstein M, Ronis DL, Copeland LA, Connors M. Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. Gen Hosp Psychiatry. 2002;24(3):140-147. doi: 10.1016/S0163-8343(02)00180-9 [DOI] [PubMed] [Google Scholar]
- 8.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27(12):1969-1975. doi: 10.1200/JCO.2008.18.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alias A, Henry M. Psychosocial effects of head and neck cancer. Oral Maxillofac Surg Clin North Am. 2018;30(4):499-512. doi: 10.1016/j.coms.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 10.Anguiano L, Mayer DK, Piven ML, Rosenstein D. A literature review of suicide in cancer patients. Cancer Nurs. 2012;35(4):E14-E26. doi: 10.1097/NCC.0b013e31822fc76c [DOI] [PubMed] [Google Scholar]
- 11.Cracchiolo JR, Klassen AF, Young-Afat DA, et al. Leveraging patient-reported outcomes data to inform oncology clinical decision making: introducing the FACE-Q Head and Neck Cancer Module. Cancer. 2019;125(6):863-872. doi: 10.1002/cncr.31900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendal WS. Suicide and cancer: a gender-comparative study. Ann Oncol. 2007;18(2):381-387. doi: 10.1093/annonc/mdl385 [DOI] [PubMed] [Google Scholar]
- 13.Yu GP, Mehta V, Branovan D, Huang Q, Schantz SP. Non-cancer-related deaths from suicide, cardiovascular disease, and pneumonia in patients with oral cavity and oropharyngeal squamous carcinoma. Arch Otolaryngol Head Neck Surg. 2012;138(1):25-32. doi: 10.1001/archoto.2011.236 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan DR, Forsberg CW, Golden SE, Ganzini L, Dobscha SK, Slatore CG. Incidence of suicide and association with palliative care among patients with advanced lung cancer. Ann Am Thorac Soc. 2018;15(11):1357-1359. doi: 10.1513/AnnalsATS.201805-299RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zullig LL, Ramos K, Berkowitz C, et al. Assessing key stakeholders’ knowledge, needs, and preferences for head and neck cancer survivorship care plans. J Cancer Educ. 2019;34(3):584-591. doi: 10.1007/s13187-018-1345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abel EA, Brandt CA, Czlapinski R, Goulet JL. Pain research using Veterans Health Administration electronic and administrative data sources. J Rehabil Res Dev. 2016;53(1):1-12. doi: 10.1682/JRRD.2014.10.0246 [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3)(suppl):S3-S14. doi: 10.4065/mcp.2009.0649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi: 10.1097/j.pain.0000000000000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011;26(9):965-971. doi: 10.1007/s11606-011-1734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846-857. doi: 10.1097/00005650-199909000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Surveillance of all veteran suicides. US Department of Veterans Affairs. Accessed September 2, 2021. https://www.mirecc.va.gov/suicideprevention/Data/data_index.asp
- 22.Crosby AE, Ortega L, Melanson C. Self-directed violence surveillance: uniform definitions and recommended data elements. National Center for Injury Prevention and Control. February 2011. Accessed August 29, 2021. https://www.cdc.gov/suicide/pdf/Self-Directed-Violence-a.pdf
- 23.Opioid oral morphine milligram equivalent (MME) conversion factors. Utah Department of Health Medicaid. Accessed September 2, 2021. https://medicaid.utah.gov/Documents/files/Opioid-Morphine-EQ-Conversion-Factors.pdf
- 24.Weiskopf NG, Bakken S, Hripcsak G, Weng C. A data quality assessment guideline for electronic health record data reuse. EGEMS (Wash DC). 2017;5(1):14. doi: 10.5334/egems.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Commission on Cancer . Facility Oncology Registry Data Standards. American College of Surgeons. January 1, 2016. Accessed August 29, 2021. https://www.facs.org/quality-programs/cancer/ncdb/call-for-data/fordsmanual
- 26.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 27.American Joint Committee on Cancer . Cancer staging system. American College of Surgeons. Accessed September 2, 2021. https://www.facs.org/quality-programs/cancer/ajcc/cancer-staging
- 28.National veteran suicide prevention annual report. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention . 2020. Accessed August 29, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
- 29.Lin LA, Bohnert ASB, Blow FC, et al. Polysubstance use and association with opioid use disorder treatment in the US Veterans Health Administration. Addiction. 2021;116(1):96-104. doi: 10.1111/add.15116 [DOI] [PubMed] [Google Scholar]
- 30.Henry M, Rosberger Z, Bertrand L, et al. Prevalence and risk factors of suicidal ideation among patients with head and neck cancer: longitudinal study. Otolaryngol Head Neck Surg. 2018;159(5):843-852. doi: 10.1177/0194599818776873 [DOI] [PubMed] [Google Scholar]
- 31.Robson A, Scrutton F, Wilkinson L, MacLeod F. The risk of suicide in cancer patients: a review of the literature. Psychooncology. 2010;19(12):1250-1258. doi: 10.1002/pon.1717 [DOI] [PubMed] [Google Scholar]
- 32.National Consensus Project. Clinical Practice Guidelines for Quality Palliative Care, 4th edition. National Coalition for Hospice and Palliative Care. https://www.nationalcoalitionhpc.org/ncp. 2018. Accessed August 29, 2021.
- 33.Ahmad FI, Clayburgh DR. Venous thromboembolism in head and neck cancer surgery. Cancers Head Neck. 2016;1:13. doi: 10.1186/s41199-016-0014-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun. 2019;10(1):207. doi: 10.1038/s41467-018-08170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shreders A, Niazi S, Hodge D, et al. Universal screening for depression in cancer patients and its impact on management patterns. J Clin Oncol. 2016;34(suppl 26):232. doi: 10.1200/jco.2016.34.26_suppl.232 [DOI] [Google Scholar]
- 36.Guidelines for patients. National Comprehensive Cancer Network. Accessed September 2, 2021. https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients
- 37.Osman A, Bagge CL, Gutierrez PM, Konick LC, Kopper BA, Barrios FX. The Suicidal Behaviors Questionnaire-Revised (SBQ-R): validation with clinical and nonclinical samples. Assessment. 2001;8(4):443-454. doi: 10.1177/107319110100800409 [DOI] [PubMed] [Google Scholar]
- 38.Anderson EM, Luu M, Balzer BL, et al. Variations in the association of grade with survival across the head and neck cancer landscape. Head Neck. 2021;43(4):1105-1115. doi: 10.1002/hed.26566 [DOI] [PubMed] [Google Scholar]
- 39.Blades CA, Stritzke WGK, Page AC, Brown JD. The benefits and risks of asking research participants about suicide: a meta-analysis of the impact of exposure to suicide-related content. Clin Psychol Rev. 2018;64:1-12. doi: 10.1016/j.cpr.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 40.Anusa AM, Thavarajah R. Risk of cognition alteration and emotional frailty via circulating transcriptome in treatment naïve head and neck squamous cell cancer patients. J Oral Biol Craniofac Res. 2019;9(2):143-150. doi: 10.1016/j.jobcr.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Study population ICD-9-CM/ICD-10-CM diagnosis codes
eTable 2. Sensitivity analyses excluding those on immunotherapy (n=7532)