Key Points
Question
What is the nodal recurrence rate in patients with clinically node-positive (cN1) breast cancer treated with sentinel lymph node biopsy (SLNB) alone after neoadjuvant chemotherapy (NAC)?
Findings
In this cohort study of 610 patients with cN1 breast cancer, among 234 consecutive patients whose cancer was rendered cN0 with NAC treated with SLNB alone with 3 or more negative SLNs retrieved, rates of axillary failure at a median follow-up of 40 months were low (0.4%), without routine nodal clipping.
Meaning
These data support potential omission of axillary lymph node dissection in patients with cN1 breast cancer who achieve nodal pathologic complete response with NAC and are treated with SLNB alone.
This cohort study examines nodal recurrence rates in patients with clinically node-positive cancer treated with sentinel lymph node biopsy alone after neoadjuvant chemotherapy.
Abstract
Importance
Prospective trials have demonstrated sentinel lymph node (SLN) false-negative rates of less than 10% when 3 or more SLNs are retrieved in patients with clinically node-positive breast cancer rendered clinically node-negative with neoadjuvant chemotherapy (NAC). However, rates of nodal recurrence in such patients treated with SLN biopsy (SLNB) alone are unknown because axillary lymph node dissection (ALND) was performed in all patients, limiting adoption of this approach.
Objective
To evaluate nodal recurrence rates in a consecutive cohort of patients with clinically node-positive (cN1) breast cancer receiving NAC, followed by a negative SLNB using a standardized technique, and no further axillary surgery.
Design, Setting, and Participants
From November 2013 to February 2019, a cohort of consecutively identified patients with cT1 to cT3 biopsy-proven N1 breast cancer rendered cN0 by NAC underwent SLNB with dual tracer mapping and omission of ALND if 3 or more SLNs were identified and all were pathologically negative. Metastatic nodes were not routinely clipped, and localization of clipped nodes was not performed. The study was performed in a single tertiary cancer center.
Intervention
Omission of ALND in patients with cN1 breast cancer after NAC if 3 or more SLNs were pathologically negative.
Main Outcome and Measures
The primary outcome was the rate of nodal recurrence among patients with cN1 breast cancer treated with SLNB alone after NAC.
Results
Of 610 patients with cN1 breast cancer treated with NAC, 555 (91%) converted to cN0 and underwent SLNB; 234 (42%) had 3 or more negative SLNs and had SLNB alone. The median (IQR) age of these 234 patients was 49 (40-58) years; median tumor size was 3 cm; 144 (62%) were ERBB2 (formerly HER2)-positive, and 43 (18%) were triple negative. Most (212 [91%]) received doxorubicin-based NAC; 205 (88%) received adjuvant radiotherapy (RT), and 164 (70%) also received nodal RT. At a median follow-up of 40 months, there was 1 axillary nodal recurrence synchronous with local recurrence in a patient who refused RT. Among patients who received RT (n = 205), there were no nodal recurrences.
Conclusions and Relevance
This cohort study found that in patients with cN1 disease rendered cN0 with NAC, with 3 or more negative SLNs with SLNB alone, nodal recurrence rates were low, without routine nodal clipping. These findings potentially support omitting ALND in such patients.
Introduction
In patients with clinically node-positive (cN1) breast cancer, neoadjuvant chemotherapy (NAC) can eradicate disease in axillary lymph nodes, with nodal pathologic complete response (pCR) rates exceeding 40%,1 reducing the need for axillary lymph node dissection (ALND).2 Four prospective multi-institutional trials demonstrated that patients with cN1 breast cancer whose disease converted to clinically node-negative (cN0) after treatment with NAC can be reliably staged with sentinel lymph node biopsy (SLNB), with false-negative rates (FNRs) of less than 10% when 3 or more SLNs are retrieved.1,3,4,5 Because the patients enrolled in these trials underwent ALND to establish the FNR of SLNB, they do not provide data on rates of axillary recurrence in this population, which remain uncertain.
The aim of this study was to evaluate nodal recurrence rates in a consecutive cohort of patients with cN1 breast cancer treated with NAC, followed by a negative SLNB result using a standardized SLN technique.
Methods
Beginning in November 2013 it became standard practice at Memorial Sloan Kettering Cancer Center (MSKCC) to offer NAC to patients with biopsy-proven cN1 breast cancer to downstage the axilla and avoid ALND. Consecutive patients with clinical T1 to T3 biopsy-proven N1 breast cancer who received NAC and converted to cN0 by physical examination were eligible for SLNB; ALND was omitted in all instances when 3 or more SLNs were identified and all were pathologically negative. This retrospective study was approved by the MSKCC institutional review board, with a waiver for informed consent. Our prospectively maintained HIPAA-compliant database was queried to identify patients treated from November 2013 to February 2019.
Sentinel lymph node biopsy was performed after NAC using dual tracer mapping with technetium-99m sulfur colloid and isosulfan blue dye. Radioactive, blue, or palpably abnormal nodes were considered SLNs. In patients presenting with clips, localization of the clipped node was not performed and surgeons were unaware if the clipped node was retrieved during the SLNB.
Nodal recurrence was defined as a recurrence in the ipsilateral axillary, supraclavicular, or internal mammary nodal basins. Local recurrence was defined as an ipsilateral breast tumor recurrence; distant failure included any distant metastases. Time to recurrence was calculated from date of surgery. Patients were censored at time of recurrence or death, or at last known follow-up.
Statistical Analysis
Clinical and treatment characteristics were reported as median and interquartile range for continuous variables and frequency (No., %) for categorical variables. Locoregional recurrence was reported as a crude rate owing to the small number of events. Kaplan-Meier curves were constructed to estimate cumulative distant recurrence rates and overall survival (OS). Standard errors of survival estimates were estimated using the Greenwood formula to generate 95% CIs. All statistical analyses were conducted using R statistical software (version 3.5.3, R Foundation).
Results
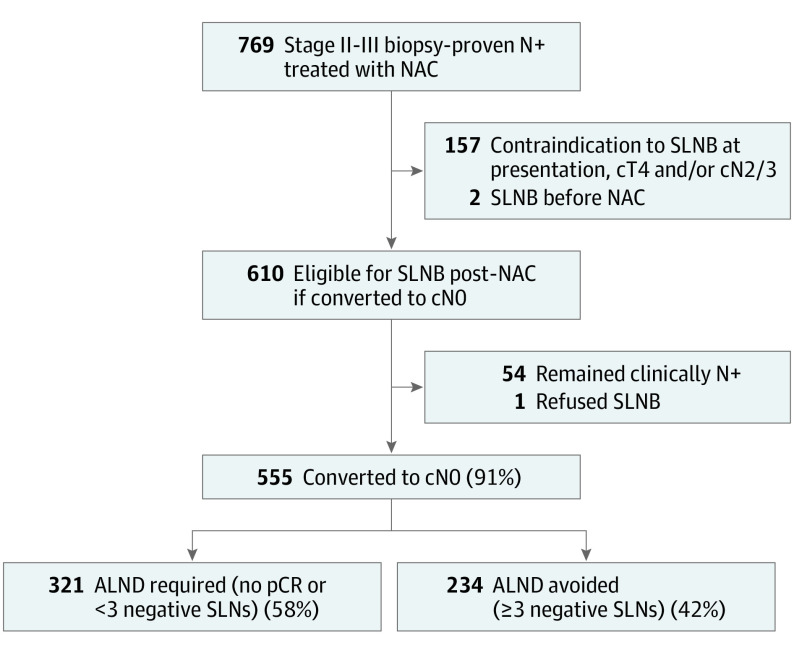
Between November 2013 and February 2019, 769 consecutive patients with stage II to stageIII biopsy-proven node-positive breast cancer received NAC followed by axillary surgery. Overall, 610 had cN1 disease and were eligible for SLNB after NAC; reasons for ineligibility are detailed in the Figure. Of patients with cN1 disease, 555 (91%) converted to cN0 by physical examination and received SLNB, and 513 (92%) had 3 or more SLNs retrieved. Overall, ALND was avoided in 234 patients with 3 or more pathologically negative (ypN0) SLNs (Figure).
Figure. Study Flowchart.
ALND indicates axillary lymph node dissection; cN, clinical nodal stage; cT, clinical tumor stage; NAC, neoadjuvant chemotherapy; N+, node positive; pCR, pathologic complete response; SLN, sentinel lymph node; and SLNB, sentinel lymph node biopsy.
Clinical and Treatment Characteristics and Follow-up Time
Clinical and treatment characteristics of the 234 patients with ypN0 SLNs are described in Table 1 and Table 2. Median follow-up was 40 months (range, 2.3-76 months), with 772 patient-years of follow-up. Of 234 patients, 224 had at least 1 year of follow-up; 24 patients (10%) were lost to follow-up.
Table 1. Clinicopathologic Characteristics of Study Cohorta.
| Clinicopathologic features | Overall (n = 234) |
|---|---|
| Age, median (IQR), y | 49 (40-58) |
| Menopausal status | |
| Premenopausal | 126 (54) |
| Postmenopausal | 108 (46) |
| Race and ethnicity | |
| Asian | 35 (15) |
| Black | 29 (12) |
| Hispanic | 16 (7) |
| White | 126 (54) |
| Other/unknown | 28 (12) |
| Tumor size at presentation, median (IQR), cm | 3.0 (2.2-5.0) |
| SLNs retrieved, median (IQR) | 4 (3-5) |
| Histologic findings | |
| Ductal | 211 (90) |
| Lobular and mixed | 7 (3) |
| Micropapillary and mixed | 10 (4) |
| Other | 3 (1) |
| Occult | 3 (1) |
| Differentiation | |
| Well | 1 (0.5) |
| Moderate | 36 (15) |
| Poor | 196 (84) |
| Unknown | 1 (0.5) |
| Receptor status | |
| HR+/ERBB2− | 47 (20) |
| HR+/ERBB2+ | 80 (34) |
| HR−/ERBB2+ | 64 (28) |
| HR−/ERBB2− | 43 (18) |
| Breast surgery | |
| Breast-conserving surgery | 118 (50) |
| Mastectomy | 116 (50) |
| Breast pCR (ypT0/Tis) | 161 (69) |
Abbreviations; ERBB2, Erb-B2 receptor tyrosine kinase 2 (formerly HER2, human epidermal growth factor receptor 2); HR, hormone receptor; pCR, pathologic complete response; and SLNs, sentinel lymph nodes.
Frequency (No., %) reported for categorical variables and median (IQR) reported for continuous variables.
Table 2. Treatment Characteristics.
| Treatment characteristics | No. | No. (%) |
|---|---|---|
| NAC Regimen | 234 | |
| AC-T | 197 (84) | |
| AC-T + Carbo | 15 (6.4) | |
| TC | 8 (3.4) | |
| Other | 14 (6) | |
| Neoadjuvant anti-ERBB2 therapy | 144 | |
| HP | 144 (100) | |
| Adjuvant RT | 234 | |
| Yes | 205 (88) | |
| No | 29 (12) | |
| Nodal RTa | 234 | |
| Yes | 164 (70) | |
| No | 70 (30) | |
| Adjuvant endocrine therapy | 127 | |
| Yes | 109 (86) | |
| No | 18 (14) | |
| Endocrine therapy regimen | 107 | |
| Tamoxifenb | 42 (39) | |
| Aromatase inhibitorb | 66 (61) | |
| Toremifene | 1 (<1) | |
| Adjuvant anti-ERBB2 therapy | 144 | |
| Yes | 132 (92) | |
| No | 12 (8) |
Abbreviations: AC-T, adriamycin and cyclophosphamide followed by taxol; Carbo, carboplatin; ERBB2, Erb-B2 receptor tyrosine kinase 2 (formerly HER2, human epidermal growth factor receptor 2); HP, trastuzumab (H) and pertuzumab (P); NAC, neoadjuvant chemotherapy; RT, radiotherapy; TC, taxotere and cyclophosphamide.
Includes the ipsilateral level I and II axillary nodes, supraclavicular and infraclavicular fossa, and the internal mammary nodal chain in the first 3 intercostal spaces.
Ovarian suppression plus tamoxifen (n = 5); ovarian suppression plus aromatase inhibitor (n = 28).
Nodal Recurrence
There was 1 nodal recurrence—an axillary nodal recurrence, synchronous with a local recurrence, occurring 5.4 months after surgery in a patient who refused adjuvant RT. Among patients who received RT (n = 205), there were no nodal recurrences.
Local Recurrence, Distant Recurrence, and OS
One patient experienced an isolated local recurrence 6 years after surgery. Overall, 13 patients developed a distant recurrence, all isolated distant failures. The 5-year distant recurrence-free survival was 92.7% (95% CI, 86.7%-96.1%). Ten patients died during the study period. The 5-year OS was 94.2% (95% CI, 89.0%-97.0%).
Discussion
Among 234 patients with cN1 disease treated with NAC followed by SLNB with dual tracer mapping and retrieval of 3 or more pathologically negative SLNs without nodal clipping, there was 1 nodal recurrence (0.4%), in a patient who declined adjuvant RT. Among patients who received RT (n = 205), there were no nodal failures. These findings add to the existing literature demonstrating the oncologic safety of SLNB alone after NAC in patients with cN1 breast cancer, and confirm rates of axillary recurrence comparable to those observed in other retrospective studies (0%-1.6%).6,7,8 Unique to our study is the standardized approach to SLNB, with all patients receiving dual tracer mapping and retrieval of 3 or more negative SLNs required for omission of ALND. Kahler-Ribeiro-Fontana et al6 published their 10-year follow-up of 222 patients with cN1/N2 disease treated with NAC, of whom 123 had a negative SLN and were treated with SLNB alone; nodal clipping was not employed, and there were no requirements for number of SLNs retrieved (74% had ≤2 SLNs removed). At a median follow-up of 9.2 years, 2 (1.6%) of 123 ypN0 patients developed an axillary recurrence,6 similar to the rate in our study, taking into account differences in follow-up time. These studies provide preliminary evidence that patients with cN1 disease who achieve nodal pCR with NAC can be treated with SLNB alone, without nodal clipping, with low rates of nodal failure, although longer follow-up is needed in this study to confirm these findings.
This SLN protocol at MSKCC was developed based on the results of multi-institutional prospective trials demonstrating a strong relationship between the number of SLNs removed and the FNR in patients with cN1 breast cancer who downstage after treatment with NAC.1,3,4,5 This finding was confirmed in a meta-analysis in which retrieval of 3 or more SLNs resulted in an FNR of 4% (95% CI, 0%-9%).9 In contrast, the evidence supporting the need for localization and retrieval of clipped nodes is less robust and is based largely on retrospective studies, primarily in patients with suboptimal mapping techniques and retrieval of less than 3 SLNs.10,11Although the FNR of retrieval of the clipped nodes and the SLNs is comparable to that seen with retrieval of 3 or more SLNs, failure to retrieve the clipped node occurs in up to 30% of cases,12,13 resulting in a dilemma of whether ALND is then required. Although we have had success with retrieval of 3 or more SLNs after NAC, critics of this approach note that retrieval of 3 or more SLNs occurred infrequently in other studies (34%-67%),1,5,14 although most were mapped with a single tracer. In institutions where retrieval of 3 or more SLNs occurs infrequently, retrieving the clipped node and the SLNs is a reasonable alternative to stage the axilla after NAC, although long-term nodal recurrence rates with this approach are needed.
Limitations and Strengths
Our study is limited by its performance at a single institution and short median follow-up. However, based on known patterns of axillary failure, with most axillary recurrences occurring within 5 years,15 longer follow-up will unlikely result in a substantial increase in nodal recurrence rates. In addition, although our rate of identifying 3 or more SLNs with the use of dual tracer mapping exceeds 90%, this has not been replicated in other studies, which may limit the generalizability of this approach. Strengths of our study include the standardized approach to SLNB allowing for an assessment of recurrence in a homogeneously treated population.
Conclusions
Given that the prospective studies that established the accuracy of SLNB in this patient population all required ALND, studies like ours provide important data regarding the oncologic safety of surgical deescalation in patients with cN1 disease after treatment with NAC.
References
- 1.Boughey JC, Suman VJ, Mittendorf EA, et al. ; Alliance for Clinical Trials in Oncology . Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455-1461. doi: 10.1001/jama.2013.278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol. 2020;27(11):4515-4522. doi: 10.1245/s10434-020-08650-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258-264. doi: 10.1200/JCO.2014.55.7827 [DOI] [PubMed] [Google Scholar]
- 4.Classe JM, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019;173(2):343-352. doi: 10.1007/s10549-018-5004-7 [DOI] [PubMed] [Google Scholar]
- 5.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609-618. doi: 10.1016/S1470-2045(13)70166-9 [DOI] [PubMed] [Google Scholar]
- 6.Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47(4):804-812. doi: 10.1016/j.ejso.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 7.Martelli G, Barretta F, Miceli R, et al. Sentinel node biopsy alone or with axillary dissection in breast cancer patients after primary chemotherapy: long-term results of a prospective interventional study. Ann Surg. 2020. doi: 10.1097/SLA.0000000000004562 [DOI] [PubMed] [Google Scholar]
- 8.Piltin MA, Hoskin TL, Day CN, Davis J Jr, Boughey JC. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27(12):4795-4801. doi: 10.1245/s10434-020-08900-0 [DOI] [PubMed] [Google Scholar]
- 9.Tee SR, Devane LA, Evoy D, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105(12):1541-1552. doi: 10.1002/bjs.10986 [DOI] [PubMed] [Google Scholar]
- 10.Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072-1078. doi: 10.1200/JCO.2015.64.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diego EJ, McAuliffe PF, Soran A, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol. 2016;23(5):1549-1553. doi: 10.1245/s10434-015-5052-8 [DOI] [PubMed] [Google Scholar]
- 12.Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018;44(9):1307-1311. doi: 10.1016/j.ejso.2018.05.035 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges. Ann Surg Oncol. 2017;24(10):3011-3016. doi: 10.1245/s10434-017-6023-z [DOI] [PubMed] [Google Scholar]
- 14.Laws A, Hughes ME, Hu J, et al. Impact of residual nodal disease burden on technical outcomes of sentinel lymph node biopsy for node-positive (cN1) breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(12):3846-3855. doi: 10.1245/s10434-019-07515-4 [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AE, Ballman K, McCall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg. 2016;264(3):413-420. doi: 10.1097/SLA.0000000000001863 [DOI] [PMC free article] [PubMed] [Google Scholar]