Key Points
Question
Which is the optimal endocrine partner (fulvestrant vs letrozole) for cyclin-dependent kinase 4 and 6 inhibitor palbociclib in previously untreated, endocrine-sensitive, hormone receptor–positive, ERBB2-negative advanced breast cancer?
Findings
In this randomized, open-label, phase 2 trial, 486 patients were assigned in equal numbers to receive palbociclib plus fulvestrant or letrozole. Median investigator-assessed progression-free survival was 27.9 months for fulvestrant-palbociclib vs 32.8 months for letrozole-palbociclib, a difference that was not statistically significant.
Meaning
Fulvestrant-palbociclib demonstrated no improvement in progression-free survival over letrozole-palbociclib, confirming letrozole as the preferred palbociclib partner in this patient population.
This randomized clinical trial investigates fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor–positive, ERBB2-negative breast cancer.
Abstract
Importance
The cyclin-dependent kinase 4 and 6 inhibitor palbociclib in combination with letrozole has become a standard first-line treatment for patients with endocrine-sensitive, hormone receptor–positive, ERBB2-negative advanced breast cancer. Meanwhile, the antiestrogen fulvestrant was shown to be superior to anastrozole in the absence of cyclin-dependent kinase 4 and 6 inhibition for this patient population.
Objective
To assess whether fulvestrant is superior to letrozole when combined with palbociclib in the first-line scenario.
Design, Setting, and Participants
In this international, randomized, open-label, phase 2 clinical study conducted from July 30, 2015, to January 8, 2018, patients with hormone receptor–positive, ERBB2-negative advanced breast cancer with no prior therapy in the metastatic setting and endocrine-sensitive criteria were recruited from 47 centers in 7 countries. Data were analyzed from February 11 to May 15, 2020.
Interventions
Patients were randomly assigned (1:1 ratio) to receive palbociclib with either fulvestrant or letrozole. Stratification factors were type of disease presentation (de novo vs recurrent) and the presence of visceral involvement (yes vs no).
Main Outcomes and Measures
The primary end point was investigator-assessed progression-free survival determined by Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
A total of 486 women (median age, 63 years [range, 25-90 years]; 3 Asian women [0.6%]; 4 Black women [0.8%]; 461 White women [94.9%]; 18 women of unknown race [3.7%]) were randomized (243 to fulvestrant-palbociclib and 243 to letrozole-palbociclib). Median investigator-assessed progression-free survival was 27.9 months (95% CI, 24.2-33.1 months) in the fulvestrant-palbociclib group vs 32.8 months (95% CI, 25.8-35.9 months) in the letrozole-palbociclib group (hazard ratio, 1.13; 95% CI, 0.89-1.45; P = .32). This result was consistent across the stratification factors. No significant differences were observed in objective response rate (46.5% vs 50.2%) and 3-year overall survival rate (79.4% vs 77.1%) for fulvestrant-palbociclib and letrozole-palbociclib, respectively. Grade 3-4 adverse events were comparable among treatment groups, and no new safety signals were identified. No treatment-related deaths were reported.
Conclusions and Relevance
Although fulvestrant-palbociclib demonstrated significant antitumor activity, this randomized clinical trial failed to identify an improvement in progression-free survival with this regimen over letrozole-palbociclib in patients with endocrine-sensitive, hormone receptor–positive, ERBB2-negative advanced breast cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT02491983
Introduction
Endocrine therapy is the mainstay of treatment for patients with hormone receptor–positive, ERBB2 (formerly HER2)-negative advanced breast cancer (ABC).1 Three phase 3 studies have demonstrated that addition of inhibitors of cyclin-dependent kinase 4 and 6 (CDK4/6) palbociclib, ribociclib, and abemaciclib to a nonsteroidal aromatase inhibitor (NSAI) in these patients improves median progression-free survival (PFS) from 14.5 to 16.0 months to 25.3 to 28.2 months, with hazard ratios (HRs) ranging between 0.54 and 0.58.2,3,4 Consequently, the combination of a CDK4/6 inhibitor and NSAI has become the standard front-line regimen for this patient population.
The selective estrogen-receptor downregulator fulvestrant is currently the most commonly used endocrine agent for patients progressing on prior endocrine therapy including an NSAI; therefore, it has been widely used for treatment of patients with endocrine-resistant, hormone receptor–positive, ERBB2-negative ABC.5 Three phase 3 trials have also confirmed the benefit of adding a CDK4/6 inhibitor to fulvestrant in NSAI-pretreated patients, increasing median PFS from 3.8 to 11.4 months for fulvestrant monotherapy to 9.2 to 18.8 months for the combination, with HRs ranging from 0.42 to 0.55.6,7,8 Two of these trials were associated with significant overall survival (OS) benefits.9,10 Simultaneously, the phase 3 Fulvestrant and Anastrozole Compared in Hormonal Therapy Naive Advanced Breast Cancer (FALCON) trial compared fulvestrant and NSAI in postmenopausal, endocrine-sensitive patients with hormone receptor–positive, ERBB2-negative ABC, and fulvestrant in the absence of CDK4/6 inhibition showed a significant PFS benefit over anastrozole (16.6 vs 13.8 months, respectively; HR, 0.80).11
Findings from previous studies raised the question of the optimal endocrine partner for CDK4/6 inhibitors in women with endocrine-sensitive, hormone receptor–positive, ERBB2-negative ABC. The PARSIFAL (for palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with hormone receptor–positive/ERBB2-negative metastatic breast cancer) trial assessed the superiority of fulvestrant plus palbociclib over letrozole plus palbociclib as initial therapy in this patient population.
Methods
Study Design and Patients
This international, randomized, open-label clinical trial with 2 parallel groups enrolled patients from July 30, 2015, to January 8, 2018, at 47 sites in 7 countries (eTables 1 and 7 in Supplement 1). It was designed to test the superiority of fulvestrant plus palbociclib compared with letrozole plus palbociclib first and then the noninferiority of fulvestrant plus palbociclib compared with letrozole plus palbociclib if the superiority objective was not achieved. The trial protocol and statistical analysis plan are available in Supplement 2 and Supplement 3, respectively.
Eligible women were aged 18 years or older with any menopausal status and locally confirmed hormone receptor–positive, ERBB2-negative, unresectable, locally advanced, or metastatic breast cancer not amenable to surgical resection or radiotherapy with curative intent. Patients had not received systemic therapy for advanced disease and had measurable or nonmeasurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Endocrine therapy in the neoadjuvant or adjuvant setting was permitted if the patient had a disease-free interval of more than 12 months from the completion of endocrine therapy. An Eastern Cooperative Oncology Group performance status score of 0 to 2 and adequate organ function were also required. Key exclusion criteria were visceral crisis and prior treatment with a CDK4/6 inhibitor. The other eligibility criteria are listed in eTable 2 in Supplement 1. Information on patient race but not ethnicity was collected. Patients self-identified their race, and some patients did not want to have this information identified. We did not consider race data to be relevant for the study.
This study was performed in agreement with the guidelines of the International Conference on Harmonization, the ethical principles in the Declaration of Helsinki, and all applicable regulations. All patients provided written informed consent before participation in any study-related activities. Approvals from regulatory authorities and ethics committees were appropriately obtained. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Masking
Patients were assigned in a 1:1 ratio to receive palbociclib plus either fulvestrant or letrozole. A central block randomization procedure with a block size of 4 was set up with the web-based software OpenClinica, version 3.14. Randomization was stratified according to type of disease (de novo metastatic or recurrent) and the presence or absence of visceral involvement (visceral or nonvisceral). An independent biometrical company (SAIL Biometría) developed the sequence generation and allocation concealment. The recruitment, selection, and treatment procedures were conducted by investigators and site staff (eTable 1 in Supplement 1). All study participants were aware of treatment assignment.
Procedures
Study treatment was initiated at randomization (day 0). Patients were orally administered 125 mg palbociclib per day (in cycles of 3 weeks of treatment followed by 1 week off) in combination with 500 mg fulvestrant on days 1, 15, 29, and once monthly thereafter, administered intramuscularly, or 2.5 mg letrozole per day, administered orally (continuous treatment). Premenopausal or perimenopausal women received a gonadotropin-releasing hormone agonist.
Treatment continued until disease progression, unacceptable toxicity, death, or patient withdrawal for any reason. Dosing interruptions and dose reduction were allowed for palbociclib as defined by prespecified protocol guidelines but were not applicable to fulvestrant and letrozole per label. Patients were permitted to discontinue palbociclib and continue with endocrine therapy alone.
Tumor assessments were carried out by computed tomography or magnetic resonance imaging according to RECIST, version 1.1 at baseline and every 12 weeks thereafter. Bone scans were performed at baseline and, if bone lesions were identified, every 24 weeks thereafter. Tumor assessment continued until disease progression, initiation of new anticancer therapy, or withdrawal from the study, whichever came first. Laboratory tests were performed on days 1 and 14 of the first 2 cycles and on day 1 of subsequent cycles. Vital signs were assessed on day 1 of every cycle. Common Terminology Criteria for Adverse Events, version 4.0 was used to grade toxicity at each cycle.
Outcomes
The primary end point of this study was investigator-assessed PFS, defined as the time from study randomization to disease progression per RECIST, version 1.1 or death due to any cause, whichever occurred first. Patients alive without disease progression were censored at the date of last disease evaluation (eTable 3 in Supplement 1). Secondary end points included objective response rate per RECIST, version 1.1; duration of response; clinical benefit rate; time to progression; time to response; OS; and safety and tolerability (eMethods in Supplement 1).
Statistical Analysis
Efficacy was assessed in the intent-to-treat set, which included all patients who had undergone randomization. Safety was assessed in the safety-analysis set, which included all patients who received at least 1 dose of study drug.
The PARSIFAL trial was initially designed to randomize approximately 304 patients. During the trial, enrollment was expanded to 486 patients based on findings from the phase 3 Palbociclib: Ongoing Trials in the Management of Breast Cancer 2 (PALOMA-2)12 and FALCON trials.11
Investigator-assessed PFS was analyzed using a Cox regression proportional-hazards model adjusted for stratification factors, ie, type of disease presentation and presence of visceral involvement. An HR of less than 1 would indicate a result favoring fulvestrant-palbociclib.
Sample size was based on a superiority test of PFS when 254 PFS events were observed. The 2-sided log-rank test had 80% power to detect a 9.3-month increase in median PFS over a 22-month median PFS for the letrozole-palbociclib group. Hence, we planned to detect an HR less than or equal to 0.70 in favor of fulvestrant-palbociclib, using a 2-sided log-rank of level 0.05. Based on a 52% ratio between PFS events and patients, the target sample size was 486 patients. We estimated a 24-month accrual period and a 12-month treatment period, for a total 36-month follow-up period.
If the superiority objective was not achieved, noninferiority analysis would be conducted, the margin of which, defined in terms of HR, was 1.21 and corresponded to the combined effect of PALOMA-113 and PALOMA-212 lower boundary (HR, 1.79; 95% CI, 1.47-2.18) adjusted to retain 50% of the historical effect of the active control compared with placebo.14
The Lan-DeMets O’Brien-Fleming spending function was used to control the type I error in the interim and final analyses of efficacy. Interim analysis was conducted after 28 months with 89 investigator-assessed PFS events (35% of expected). The 2-sided nominal α errors for testing the null hypothesis within the interim and final analyses were set at 0.001 and 0.0498, respectively.
Survival estimates for each time-to-event end point were estimated using the Kaplan-Meier method and 95% CIs. The HRs for the treatment effect, P values, and 95% CI were estimated as defined for PFS. The P values and 95% CIs were calculated using the likelihood-ratio test and the profile-likelihood method, respectively. The Breslow method for tie handling in survival analysis was used. Comparison of objective response and clinical benefit–response rates between treatment groups was performed using Fisher exact test.
The consistency-of-treatment effect was assessed across prespecified stratification factors. It was tested by a Cox model for PFS with a treatment-by-factor interaction term set at a 2-sided 0.1 α level. The proportionality of hazards was assessed using cumulative sums of martingale-based residuals. Data analysis was carried out from February 11 to May 15, 2020, using SAS software, version 9.4 (SAS Institute).
Results
Recruitment and Patient Disposition
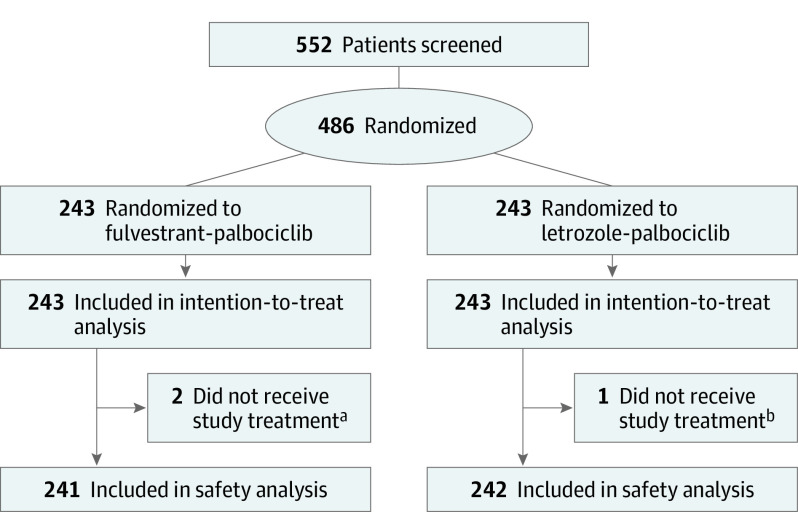
Between July 30, 2015, and January 8, 2018, 486 women (median age [range], 63 years [25-90 years]; 3 Asian women [0.6%]; 4 Black women [0.8%]; 461 White women [94.9%]; 18 women of unknown race [3.7%]) were randomly assigned to receive fulvestrant plus palbociclib (n = 243) or letrozole plus palbociclib (n = 243). Data cutoff was January 31, 2020, when the target number of PFS events (n = 256) was met. A total of 483 patients received at least 1 dose of study treatment; 3 patients did not start study treatment because of investigator decision (n = 1), withdrawal of consent (n = 1), and protocol violation (n = 1) (Figure 1).
Figure 1. Consolidated Standards for Reporting Trials (CONSORT) Patient Diagram.
All randomized patients were included in the intention-to-treat population; randomized patients who received at least 1 dose of study treatment were included in the safety population.
aOf 2 patients who did not receive study treatment, 1 patient was mistakenly included because she did not meet the selection criterion (presence of uncontrolled brain metastases), and 1 patient was discontinued per the investigator’s decision.
bOne patient did not receive study treatment because the patient withdrew consent.
Baseline Characteristics
Baseline characteristics of the intent-to-treat population were balanced between treatment groups (Table 1). Visceral disease was present in 233 patients (47.9%), 198 (40.7%) presented with de novo ABC, 462 (95.1%) had an Eastern Cooperative Oncology Group score of 0 to 1, and 224 (46.1%) had previously received adjuvant endocrine therapy.
Table 1. Patient Demographic Details at Baseline.
| Variable | No. (%) | ||
|---|---|---|---|
| All patients (n = 486) | Fulvestrant-palbociclib (n = 243) | Letrozole-palbociclib (n = 243) | |
| Age, median (range), y | 63 (25-90) | 64 (25-88) | 62 (35-90) |
| Race | |||
| Asian | 3 (0.6) | 2 (0.8) | 1 (0.4) |
| Black | 4 (0.8) | 3 (1.2) | 1 (0.4) |
| White | 461 (94.9) | 231 (95.1) | 230 (94.7) |
| Unknown | 18 (3.7) | 7 (2.9) | 11 (4.5) |
| ECOG performance statusa | |||
| 0 | 275 (56.6) | 151 (62.1) | 124 (51.0) |
| 1 | 187 (38.5) | 80 (32.9) | 107 (44.0) |
| 2 | 24 (4.9) | 12 (4.9) | 12 (4.9) |
| Menopausal status | |||
| Premenopausal | 37 (7.6) | 17 (7.0) | 20 (8.2) |
| Postmenopausal | 449 (92.4) | 226 (93.0) | 223 (91.8) |
| Type of disease | |||
| De novo | 198 (40.7) | 102 (42.0) | 96 (39.5) |
| Recurrent | 288 (59.3) | 141 (58.0) | 147 (60.5) |
| Disease site | |||
| Visceral | 233 (47.9) | 115 (47.3) | 118 (48.6) |
| Nonvisceral | 253 (52.1) | 128 (52.7) | 125 (51.4) |
| No. of disease sites | |||
| <3 | 274 (52.1) | 141 (58.0) | 133 (51.4) |
| ≥3 | 212 (47.9) | 102 (42.0) | 110 (48.6) |
| Measurable disease | |||
| Yes | 376 (77.4) | 195 (80.2) | 181 (74.5) |
| No | 110 (22.6) | 48 (19.8) | 62 (25.5) |
| Previous treatment in early setting | |||
| Neoadjuvant chemotherapy | 46 (9.5) | 25 (10.3) | 21 (8.6) |
| Adjuvant chemotherapy | 144 (29.6) | 73 (30.0) | 71 (29.2) |
| Tamoxifen only | 107 (22.0) | 48 (19.8) | 59 (24.3) |
| Aromatase inhibitors only | 47 (9.7) | 26 (10.7) | 21 (8.6) |
| Tamoxifen and aromatase inhibitors | 70 (14.4) | 39 (16.0) | 31 (12.8) |
Eastern Cooperative Oncology Group (ECOG) performance status is graded as follows: 0, fully active; 1, restricted in strenuous activity but capable of light house and office work; 2, ambulatory and capable of self-care but unable to carry out work activities; 3, capable of only limited self-care; 4, cannot perform self-care; 5, dead.
Treatment
At final analysis cutoff, 72 patients (29.6%) receiving fulvestrant-palbociclib and 88 (36.2%) receiving letrozole-palbociclib were continuing treatment. Treatment discontinuation was primarily due to disease progression, which occurred in 122 patients (50.2%) in each study group.
Median relative dose intensity was 99.2% (IQR, 97.3%-100%) for fulvestrant and 91.7% (IQR, 76%-97.6%) for palbociclib in the fulvestrant-palbociclib group and 98.8% (IQR, 96.3%-99.9%) for letrozole and 90.0% (IQR, 77.4%-98.3%) for palbociclib in the letrozole-palbociclib group. Palbociclib dose was reduced according to protocol in 85 of the 241 patients (35.3%) in the fulvestrant-palbociclib group and in 108 of the 242 patients (44.6%) in the letrozole-palbociclib group (eTable 4 in Supplement 1).
Primary Outcome
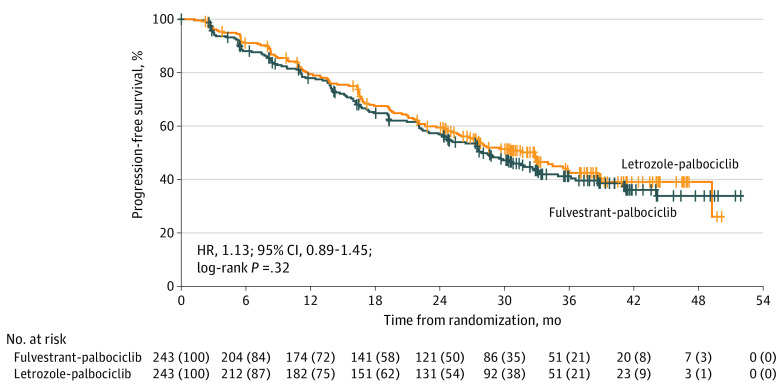
Final analysis occurred after 256 PFS events (131 [53.9%] in the fulvestrant-palbociclib group and 125 [51.4%] in the letrozole-palbociclib group). Median follow-up was 32 months (IQR, 24.2-39.7 months). Median investigator-assessed PFS was 27.9 months (95% CI, 24.2-33.1 months) in the fulvestrant-palbociclib group vs 32.8 months (95% CI, 25.8-35.9 months) in the letrozole-palbociclib group (HR, 1.13; 95% CI, 0.89-1.45; P = .32) (Figure 2), demonstrating no significant difference between treatment groups. The noninferiority margin (1.21) was included in the 95% CI.
Figure 2. Kaplan-Meier Curve for Investigator-Assessed Progression-Free Survival in the Intent-to-Treat Population.
HR indicates hazard ratio.
Secondary Outcomes
Efficacy
Overall survival data were immature at data cutoff, with 51 deaths (21.0%) in both treatment groups. Estimated 3-year OS was 79.4% (95% CI, 73.1%-84.4%) in the fulvestrant-palbociclib group vs 77.1% (95% CI, 70.2%-82.5%) in the letrozole-palbociclib group (HR, 1.00; 95% CI, 0.68-1.48; P = .99) (eFigure 1 in Supplement 1).
The objective response rate was achieved in 113 of 243 patients (46.5%; 95% CI, 40.1%-53.0%) in the fulvestrant-palbociclib group and in 122 of 213 patients (50.2%; 95% CI, 43.7%-56.7%) in the letrozole-palbociclib group (P = .41). In patients with measurable disease (n = 376; 77.4%), the objective response occurred in 110 of 195 patients (56.4%; 95% CI, 49.1%-63.5%) in the fulvestrant-palbociclib group and in 119 of 181 patients (65.7%; 95% CI, 59.3%-72.6%) in the letrozole-palbociclib group. Median duration of response was 34 months (95% CI, 23.3 months to not estimable) in the fulvestrant-palbociclib group and 30.2 months (95% CI, 26.7 months to not estimable) in the letrozole-palbociclib group. In the intent-to-treat population, clinical benefit was achieved in 172 of 243 patients (70.8%; 95% CI, 64.6%-76.4%) in the fulvestrant-palbociclib group and in 168 of 243 patients (69.1%; 95% CI, 62.9%-74.9%) in the letrozole-palbociclib group (P = .69). Median time to progression was 28.9 months (95% CI, 24.6-36.2 months) in the fulvestrant-palbociclib group vs 32.8 months (95% CI, 26.0-38.6 months) in the letrozole-palbociclib group (HR, 1.09; 95% CI, 0.85-1.40; P = .49), and median time to response was 5.3 months (95% CI, 3.7-5.5 months) in the fulvestrant-palbociclib group vs 5.2 months (95% CI, 2.9-5.5 months) in the letrozole-palbociclib group (HR, 0.9; 95% CI, 0.7-1.2) (eTable 5 in Supplement 1).
Subgroup analyses of PFS according to the stratification factors showed no significant differences between treatment groups across all prespecified subgroups. The P values for treatment-by-visceral involvement and treatment-by-type of disease interaction tests were 0.28 and 0.98, respectively (eFigure 2 in Supplement 1).
Safety
The most common adverse events (AEs) of any grade reported in both groups were neutropenia, asthenia, arthralgia, anemia, and diarrhea. With the exception of neutropenia and leukopenia, most AEs were of grade 1 or 2. Nearly all of the most frequent AEs were deemed possibly related to study treatment. Neutropenia was the most common grade 3 or 4 AE in both treatment groups. Decrease in neutrophil counts of grade greater than or equal to 3 occurred in 159 of 241 patients (66.0%) in the fulvestrant-palbociclib group and in 165 of 242 (68.2%) in the letrozole-palbociclib group. Febrile neutropenia was reported in only 3 patients (1.2%) in the fulvestrant-palbociclib group and 1 patient (0.4%) in the letrozole-palbociclib group (Table 2).
Table 2. Summary of Adverse Events of Any Grade Occurring in More Than 15% of Patients in Either Study Group.
| Variable | No. (%) | |||||
|---|---|---|---|---|---|---|
| Fulvestrant-palbociclib (n = 241) | Letrozole-palbociclib (n = 242) | |||||
| Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 | |
| Any AE | 240 (99.6) | 167 (69.3) | 28 (11.6) | 240 (99.2) | 168 (69.4) | 22 (9.1) |
| Hematologic AEsa | ||||||
| Neutropenia | 198 (82.2) | 141 (58.5) | 18 (7.5) | 207 (85.5) | 153 (63.2) | 12 (5.0) |
| Leukopenia | 60 (24.9) | 16 (6.6) | 1 (0.4) | 61 (25.2) | 14 (5.8) | 0 |
| Anemia | 55 (22.8) | 6 (2.5) | 0 | 68 (28.1) | 6 (2.5) | 0 |
| Thrombocytopenia | 49 (20.3) | 3 (1.2) | 0 | 39 (16.1) | 1 (0.4) | 1 (0.4) |
| Nonhematologic AEs | ||||||
| Asthenia | 90 (37.3) | 7 (2.9) | 0 | 87 (36.0) | 5 (2.1) | 0 |
| Diarrhea | 65 (27.0) | 4 (1.7) | 0 | 60 (24.8) | 3 (1.2) | 0 |
| Arthralgia | 62 (25.7) | 1 (0.4) | 0 | 80 (33.1) | 1 (0.4) | 0 |
| Fatigue | 62 (25.7) | 4 (1.7) | 0 | 63 (26.0) | 4 (1.7) | 0 |
| Back pain | 57 (23.7) | 7 (2.9) | 0 | 49 (20.2) | 1 (0.4) | 0 |
| Nausea | 57 (23.7) | 3 (1.2) | 0 | 45 (18.6) | 0 | 0 |
| Alopecia | 56 (23.2) | 0 | 0 | 61 (25.2) | 0 | 0 |
| Cough | 54 (22.4) | 0 | 0 | 42 (17.4) | 0 | 0 |
| Hot flush | 41 (17.0) | 0 | 0 | 46 (19.0) | 0 | 0 |
| Stomatitis | 40 (16.6) | 0 | 0 | 48 (19.8) | 2 (0.8) | 0 |
| Vomiting | 35 (14.5) | 2 (0.8) | 0 | 39 (16.1) | 2 (0.8) | 0 |
| Constipation | 34 (14.1) | 0 | 0 | 40 (16.5) | 3 (1.2) | 0 |
Abbreviation: AEs, adverse events.
Five patients died due to unrelated AEs, 3 of whom (1.2%) were randomized to fulvestrant-palbociclib and 2 (0.8%) to letrozole-palbociclib.
Incidence of grade 3 or 4 toxicity and serious AEs was similar in both treatment groups (grade 3 or 4 AEs, 80.9% vs 78.5% and serious AEs, 29.9% vs 21.1% in the fulvestrant-palbociclib group vs the letrozole-palbociclib group, respectively). Permanent discontinuation of study treatment due to AEs occurred in 13 patients (5.3%) in the fulvestrant-palbociclib group and in 5 patients (2.1%) in the letrozole-palbociclib group (eFigure 3 in Supplement 1). No treatment-related deaths were reported.
Regarding the AEs of special interest, pulmonary embolism occurred in 12 of 241 patients (5.0%) in the fulvestrant-palbociclib group and in 6 of 242 patients (2.5%) in the letrozole-palbociclib group. Six patients (2.5%) in each group had interstitial lung disease or pneumonitis of any grade; 2 of 241 patients (0.8%) in the fulvestrant-palbociclib group had grade 3 pneumonitis, and 3 of 242 patients (1.2%) in the letrozole-palbociclib group had grade 3 pneumonitis (eTable 6 in Supplement 1).
Discussion
To our knowledge, PARSIFAL is the only randomized clinical trial to directly compare the therapeutic efficacy of fulvestrant or letrozole in combination with the CDK4/6 inhibitor palbociclib in patients with previously untreated, endocrine-sensitive, hormone receptor–positive, ERBB2-negative ABC. Despite the significant antitumor activity of fulvestrant-palbociclib, this combination showed no superiority in PFS over the standard letrozole-palbociclib in this patient population. The study also failed to prove fulvestrant-palbociclib noninferiority, because the 95% CI contained the noninferiority margin.
In spite of the limitations of making indirect comparisons between studies, the median PFS reported in PARSIFAL for the control group was numerically better than in the last reports from the 4 pivotal phase 3 trials exploring the combination of a CDK4/6 inhibitor with NSAI.2,3,4,15 Patients treated with fulvestrant-palbociclib had a slightly inferior median PFS compared with that achieved in the Fulvestrant/Palbociclib vs Fulvestrant/Placebo as First-Line Therapy in Postmenopausal Women with Hormone Receptor+/ERBB2– Endocrine-Sensitive Advanced Breast Cancer (FLIPPER)16 and Study of Efficacy and Safety of LEE011 in Postmenopausal Women With Advanced Breast Cancer 3 (MONALEESA-3)6 trials.
In the FALCON trial, a larger treatment effect with fulvestrant compared with anastrozole was identified in some subgroups, particularly in patients with nonvisceral disease.11 However, PARSIFAL showed no differences between fulvestrant-palbociclib and letrozole-palbociclib across all prespecified stratification factors, possibly owing to the addition of palbociclib that counteracted the superiority of fulvestrant over letrozole in the absence of CDK4/6 inhibition.
Overall, the toxicity profile was similar between both groups and was consistent with the known safety profile reported in the PALOMA trials,3,8,12,13,17 although more dose reductions were observed in patients treated with letrozole-palbociclib. While this study was under way, interstitial lung disease or pneumonitis and venous thromboembolic events were identified as potentially related to CDK4/6 inhibitor–based regimens.18 Incidence and severity of interstitial lung disease or pneumonitis was low (2.5% in both groups) and mild (<1.0% grade 3 and 0% grade 4). Nevertheless, the incidence of pulmonary embolism was higher than that reported previously3,8 and especially in patients treated with fulvestrant-palbociclib (5.0% vs 2.5%).
The optimal strategy for systemic treatment of hormone receptor–positive, ERBB2-negative ABC remains debatable. Gains in OS achieved with CDK4/6 inhibitors in the endocrine-resistant population9,10 have been partially confirmed for endocrine-sensitive patients.6,15 An OS analysis of the first-line regimen with a longer follow-up and mature data from the 3 pivotal studies2,3,4 is highly anticipated. However, meaningful improvement in median PFS associated with a higher objective response rate along with absence of a negative effect on quality of life have established the CDK4/6 inhibitor and NSAI regimen as the preferred strategy for patients with endocrine-sensitive, hormone receptor–positive, ERBB2-negative ABC.
Limitations
This study has some limitations. First, despite the randomized design and well-balanced population, any interpretation of the results should consider the open-label design. Second, the primary end point was not confirmed by independent central review, which usually results in reexamination of all disease progression events of all patients. Although the local-investigator evaluation provided a reliable estimate of treatment effect, potential evaluation bias in reading PFS events between the treatment groups could have been introduced. Third, the OS analysis was not powered to show statistical significance, and OS data were immature at the time of data cutoff. An additional limitation was the low number of participants who were Asian or Black.
Conclusions
This international, randomized, open-label, phase 2 clinical trial demonstrated no improvement in PFS with fulvestrant-palbociclib over letrozole-palbociclib among patients receiving initial systemic treatment for endocrine-sensitive, hormone receptor–positive, ERBB2-negative ABC. These findings confirm NSAI as the preferred palbociclib partner for effective treatment with a tolerable safety profile in this patient population.
eTable 1. Trial Randomizing Sites, Principal Investigators, and Patient Numbers
eTable 2. Selection Criteria
eTable 3. Criteria for Defining Progression-Free Survival Events and Censoring
eTable 4. Relative Dose Intensity and Drug Discontinuation
eTable 5. Tumor Best Response According to RECIST version 1.1
eTable 6. Adverse Events of Special Interest between Arms According to CTCAE version 4.0
eFigure 1. Overall Survival
eFigure 2. Kaplan-Meier Curves for Investigator-Assessed Progression-Free Survival in Patients without (A) and with (B) Visceral Disease, and with Recurrent (C) and de novo (D) Metastatic Disease in the Intent-To-Treat Population
eFigure 3. Summary of All Adverse Events After Randomization According to CTCAE version 4.0
eMethods. Protocol-Specified Outcomes and Measurements and Relative Dose Intensity (RDI)
eTable 7. Committee Members
Trial Protocol
Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28(1):16-33. doi: 10.1093/annonc/mdw544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541-1547. doi: 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 3.Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719-729. doi: 10.1007/s10549-018-05125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi: 10.1038/s41523-018-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594-4600. doi: 10.1200/JCO.2010.28.8415 [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465-2472. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 7.Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. doi: 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514-524. doi: 10.1056/NEJMoa1911149 [DOI] [PubMed] [Google Scholar]
- 10.Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116-124. doi: 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997-3005. doi: 10.1016/S0140-6736(16)32389-3 [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936. doi: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35. doi: 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration . Non-inferiority clinical trials to establish effectiveness guidance for industry. Accessed March 30, 2020. https://www.fda.gov/media/78504/download
- 15.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904-915. doi: 10.1016/S1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 16.Albanell J, Martinez MTM, Ramos M, et al. LBA19 GEICAM/2014-12 (FLIPPER) study: First analysis from a randomized phase II trial of fulvestrant (F)/palbociclib (P) versus (vs) F/placebo (PL) as first-line therapy in postmenopausal women with HR (hormone receptor)+/HER2– endocrine sensitive advanced breast cancer (ABC). Ann Oncol. 2020;31(S4):S1151. doi: 10.1016/j.annonc.2020.08.2247 [DOI] [Google Scholar]
- 17.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926-1936. doi: 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. FDA warns about rare but severe lung inflammation with Ibrance, Kisqali, and Verzenio for breast cancer. December 20, 2019. Accessed December 11, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-severe-lung-inflammation-ibrance-kisqali-and-verzenio-breast-cancer
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Trial Randomizing Sites, Principal Investigators, and Patient Numbers
eTable 2. Selection Criteria
eTable 3. Criteria for Defining Progression-Free Survival Events and Censoring
eTable 4. Relative Dose Intensity and Drug Discontinuation
eTable 5. Tumor Best Response According to RECIST version 1.1
eTable 6. Adverse Events of Special Interest between Arms According to CTCAE version 4.0
eFigure 1. Overall Survival
eFigure 2. Kaplan-Meier Curves for Investigator-Assessed Progression-Free Survival in Patients without (A) and with (B) Visceral Disease, and with Recurrent (C) and de novo (D) Metastatic Disease in the Intent-To-Treat Population
eFigure 3. Summary of All Adverse Events After Randomization According to CTCAE version 4.0
eMethods. Protocol-Specified Outcomes and Measurements and Relative Dose Intensity (RDI)
eTable 7. Committee Members
Trial Protocol
Statistical Analysis Plan
Nonauthor Collaborators
Data Sharing Statement