This randomized clinical trial evaluates the effects of therapeutic-dose low-molecular-weight heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19.
Key Points
Question
Does thromboprophylaxis with therapeutic-dose low-molecular-weight heparin reduce the incidence of major thromboembolism and death compared with prophylactic/intermediate-dose heparins in inpatients with high-risk COVID-19?
Findings
In this randomized clinical trial of 253 adults, the incidence of major thromboembolism or death was 28.7% with therapeutic-dose vs 41.9% with prophylactic/intermediate-dose heparins, a significant difference—driven by reduction in thromboembolism—that was not seen in critically ill patients. There was no significant difference in major bleeding between groups.
Meaning
Thromboprophylaxis with therapeutic-dose low-molecular-weight heparin reduces a composite outcome of major thromboembolism and death in high-risk inpatients with COVID-19.
Abstract
Importance
Hospitalized patients with COVID-19 are at risk for venous and arterial thromboembolism and death. Optimal thromboprophylaxis dosing in high-risk patients is unknown.
Objective
To evaluate the effects of therapeutic-dose low-molecular-weight heparin (LMWH) vs institutional standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19.
Design, Setting, and Participants
The HEP-COVID multicenter randomized clinical trial recruited hospitalized adult patients with COVID-19 with D-dimer levels more than 4 times the upper limit of normal or sepsis-induced coagulopathy score of 4 or greater from May 8, 2020, through May 14, 2021, at 12 academic centers in the US.
Interventions
Patients were randomized to institutional standard prophylactic or intermediate-dose LMWH or unfractionated heparin vs therapeutic-dose enoxaparin, 1 mg/kg subcutaneous, twice daily if creatinine clearance was 30 mL/min/1.73 m2 or greater (0.5 mg/kg twice daily if creatinine clearance was 15-29 mL/min/1.73 m2) throughout hospitalization. Patients were stratified at the time of randomization based on intensive care unit (ICU) or non-ICU status.
Main Outcomes and Measures
The primary efficacy outcome was venous thromboembolism (VTE), arterial thromboembolism (ATE), or death from any cause, and the principal safety outcome was major bleeding at 30 ± 2 days. Data were collected and adjudicated locally by blinded investigators via imaging, laboratory, and health record data.
Results
Of 257 patients randomized, 253 were included in the analysis (mean [SD] age, 66.7 [14.0] years; men, 136 [53.8%]; women, 117 [46.2%]); 249 patients (98.4%) met inclusion criteria based on D-dimer elevation and 83 patients (32.8%) were stratified as ICU-level care. There were 124 patients (49%) in the standard-dose vs 129 patients (51%) in the therapeutic-dose group. The primary efficacy outcome was met in 52 of 124 patients (41.9%) (28.2% VTE, 3.2% ATE, 25.0% death) with standard-dose heparins vs 37 of 129 patients (28.7%) (11.7% VTE, 3.2% ATE, 19.4% death) with therapeutic-dose LMWH (relative risk [RR], 0.68; 95% CI, 0.49-0.96; P = .03), including a reduction in thromboembolism (29.0% vs 10.9%; RR, 0.37; 95% CI, 0.21-0.66; P < .001). The incidence of major bleeding was 1.6% with standard-dose vs 4.7% with therapeutic-dose heparins (RR, 2.88; 95% CI, 0.59-14.02; P = .17). The primary efficacy outcome was reduced in non-ICU patients (36.1% vs 16.7%; RR, 0.46; 95% CI, 0.27-0.81; P = .004) but not ICU patients (55.3% vs 51.1%; RR, 0.92; 95% CI, 0.62-1.39; P = .71).
Conclusions and Relevance
In this randomized clinical trial, therapeutic-dose LMWH reduced major thromboembolism and death compared with institutional standard heparin thromboprophylaxis among inpatients with COVID-19 with very elevated D-dimer levels. The treatment effect was not seen in ICU patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT04401293
Introduction
Thrombosis, including venous thromboembolism (VTE), such as deep vein thrombosis and pulmonary embolism, and arterial thromboembolism (ATE), such as myocardial infarction and ischemic stroke, is common among hospitalized adults with COVID-19.1,2,3,4 The incidence of VTE—and in particular pulmonary embolism—appears to be elevated in this population, with rates of 5.5% to 14.1% or more and a more than 2-fold increased risk compared with historical matched controls.2,3,4,5 Microvascular thrombosis and intravascular coagulopathy have been implicated in progression to acute respiratory distress syndrome.6 Lastly, autopsy studies have identified unsuspected VTE or in situ pulmonary arterial thrombosis in more than 60% of patients with COVID-19, suggesting that thrombosis contributes to mortality.7,8
Patient comorbidities and immobility, as well as cytokine storm and virus-induced endothelial changes, are some of the proposed risk factors for and mechanisms of COVID-19 thrombosis.9,10,11 Elevated plasma D-dimer levels, especially greater than 4 times the upper limit of normal, predict a more than 2-fold increased risk of VTE or mortality.10,12 Based on low-quality data, universal thromboprophylaxis with standard prophylactic-dose unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) is recommended, but severely ill inpatients with COVID-19 may experience thrombosis despite standard thromboprophylaxis.2,9,13,14,15 Although the multiplatform trials have shown a reduction in organ-support–free days with therapeutic anticoagulation in noncritically ill patients,16 it is unknown whether high-risk inpatients with COVID-19 may benefit from empirical therapeutic-dose heparin as a thromboprophylactic strategy. To date, there are conflicting randomized clinical trial data on this question, with no benefits of escalated or therapeutic-dose anticoagulation seen in key subgroups of patients on medical wards or with critical illness.16,17,18,19
To address this uncertainty, we conducted a randomized clinical trial to test the hypothesis that in patients hospitalized with COVID-19 without critical illness but with high-risk features (D-dimer level >4 times the upper limit of normal) or with critical illness, empirical therapeutic-dose LMWH would reduce the composite outcome of VTE, ATE, and all-cause mortality within 30 days of hospitalization compared with institutional standard prophylactic or intermediate-dose heparins.
Methods
Study Design and Oversight
HEP-COVID was a multicenter, active control randomized clinical trial that enrolled patients from May 8, 2020, through May 14, 2021, at 12 centers in the US. The trial rationale and design have been described previously.20 The study was conducted in accordance with the Declaration of Helsinki, International Committee on Harmonization guidelines for Good Clinical Practice, and local regulatory requirements. The protocol, which included the statistical analysis plan and the statistical reporting plan (Supplement 1), was approved by the institutional review boards at all centers. All patients or their legally authorized representatives gave written informed consent. Study oversight was provided by an Executive Committee (EC) that was blinded to treatment allocation and convened bimonthly. An independent data safety monitoring board reviewed study progress at 25%, 50%, 75%, and 100% of enrollment and made recommendations to the EC. The authors drafted the manuscript, verified the data, submitted the manuscript for publication, and vouch for the completeness of the data, accuracy of the analyses, and fidelity to the protocol. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Patients were screened within 72 hours from hospital admission or transfer from another facility. Eligible patients consisted of hospitalized nonpregnant adults 18 years or older with COVID-19 diagnosed by nasal swab or serologic testing. Inclusion criteria were (1) requirement for supplemental oxygen per investigator judgment and (2) plasma D-dimer level greater than 4 times the upper limit of normal based on local laboratory criteria or a sepsis-induced coagulopathy score of 4 or greater.21 Exclusionary criteria included a physician-determined need for full-dose anticoagulation or dual antiplatelet therapy, bleeding within the past month, active gastrointestinal or intracranial cancer, bronchiectasis or pulmonary cavitation, hepatic dysfunction with baseline international normalized ratio greater than 1.5, creatinine clearance (CrCl) less than 15 mL/min/1.73 m2, platelet count less than 25 000/μL, a history of heparin-induced thrombocytopenia (HIT) within 100 days, and hypersensitivity/intolerance to study drug or components.20
Study Regimen and Follow-up
Treatment began after randomization and concluded at hospital discharge or upon occurrence of a primary efficacy outcome, key secondary outcome, or principal safety outcome requiring study drug discontinuation. All patients without a primary or key secondary outcome event underwent lower extremity Doppler compression ultrasonography at hospital day 10 + 4 or at discharge if sooner. Follow-up continued until 30 ± 2 days after randomization.
Randomization was performed using a secure web application and was stratified based on noncritical care (non–intensive care unit [ICU]) or critical care (ICU) status at the time of randomization. Patients’ ICU status was defined by mechanical ventilation, noninvasive positive pressure ventilation or high-flow nasal cannula, vasopressors, or vital sign monitoring more often than every 4 hours. Participants were randomly assigned 1:1 to therapeutic-dose enoxaparin or institutional standard prophylactic or intermediate-dose heparins. Patients and investigators were blinded to treatment assignment as much as possible. Patients in the therapeutic-dose group received enoxaparin at a dose of 1 mg/kg subcutaneously twice daily if CrCl was 30 mL/min/1.73 m2 or greater or 0.5 mg/kg twice daily if CrCl was 15-29 mL/min/1.73 m2. Patients in the standard-dose group received prophylactic or intermediate-dose heparin regimens per local institutional standard and could include UFH, up to 22 500 IU subcutaneously (divided twice or thrice daily); enoxaparin, 30 mg or 40 mg subcutaneously once or twice daily (weight-based enoxaparin 0.5 mg/kg subcutaneously twice daily was permitted but strongly discouraged); or dalteparin, 2500 IU or 5000 IU subcutaneously daily.22 If CrCl fell below 15 mL/min/1.73 m2, enoxaparin was converted to treatment-dose intravenous UFH until kidney function improved to CrCl greater than 15 mL/min/1.73 m2, when blinded-dose subcutaneous enoxaparin was resumed. Study drug was administered for the duration of hospitalization, including patient transfers to ICU settings.
Demographic characteristics, comorbidities, medications, and laboratory assessments were recorded at randomization. Race and ethnicity, which were self-reported, was collected to evaluate clinical relevance with regard to demographics. Patients underwent laboratory and screening lower extremity compression ultrasonography testing at hospital day 10 + 4, because asymptomatic proximal deep vein thrombosis diagnosed by ultrasonography is associated with death in medically ill inpatients, including those with pneumonia and sepsis.23 Postdischarge anticoagulation was allowed at the discretion of treating physicians. Primary efficacy, principal safety, and secondary outcomes were assessed in clinic or by telephone 30 ± 2 days after randomization.
Outcome Measures
The primary efficacy outcome was VTE (symptomatic upper or lower extremity deep vein thrombosis, asymptomatic lower extremity proximal deep vein thrombosis, symptomatic pulmonary embolism, splanchnic vein thrombosis, or cerebral sinus thrombosis), or ATE (myocardial infarction, ischemic stroke, peripheral or systemic ATE) or death from any cause within 30 ± 2 days after randomization. Secondary outcomes included the composite primary outcome within 14 days after admission, progression to acute respiratory distress syndrome, new-onset atrial fibrillation, acute kidney injury, nonfatal cardiac arrest, endotracheal intubation, extracorporeal membrane oxygenation, and rehospitalization within 30 ± 2 days. The principal safety outcome was major bleeding based on International Society on Thrombosis and Haemostasis criteria within 30 ± 2 days after randomization.24 Outcomes were adjudicated locally by blinded investigators via imaging, laboratory, and other objective health record data. Serious adverse events included hypersensitivity reactions to study drug, hepatotoxicity, HIT as per major professional society definitions,25 and bone marrow toxicity. Locally adjudicated events underwent central quality review.
Statistical Analysis
Based on a 40% relative risk (RR) reduction in the primary efficacy outcome from 42% in the standard-dose group to 25.2% in the therapeutic-dose heparin group, 246 patients (123 per arm) were required to achieve 80% power at a 2-sided significance level of .05. We initially estimated a dropout rate of 20%, yielding a target population of 308 randomized patients,20 but a lower than expected dropout rate allowed a revised target enrollment of 257 patients. The modified intention-to-treat population consisted of patients who received at least 1 dose of study drug and were followed until day 30 ± 2. The per-protocol population comprised patients who received at least 80% of planned study drug doses and were followed to day 30 ± 2 without major protocol deviations.20
The primary analysis was based on the modified intention-to-treat population, followed by the per-protocol population. An O’Brien-Fleming design was used to detect a significant difference in the primary efficacy outcome.26 One interim analysis after enrollment of 50% of the target population was planned with early termination criteria requiring at least 15 or more excess primary efficacy events in the standard-dose group compared with the therapeutic-dose group, corresponding to an absolute risk reduction greater than 25.6%. Because this criterion was not met, enrollment continued to 100% of target enrollment. Prespecified, Bonferroni-adjusted subgroup analyses were carried out for the ICU and non-ICU strata.
Inclusion criteria were adapted twice.20 The D-dimer criterion, initially greater than 6 times the upper limit of normal, was changed to greater than 4 times the upper limit of normal, drawing on large retrospective data.12 The hypoxemia criterion, initially requiring a respiratory rate greater than 20 breaths/min and oxygen saturation less than 92% on room air was changed to any perceived need for supplemental oxygen as per investigator judgment. Amendments are available in the trial protocol (Supplement 1). Statistical analyses were performed using SAS, version 9.4 (SAS Institute).
Results
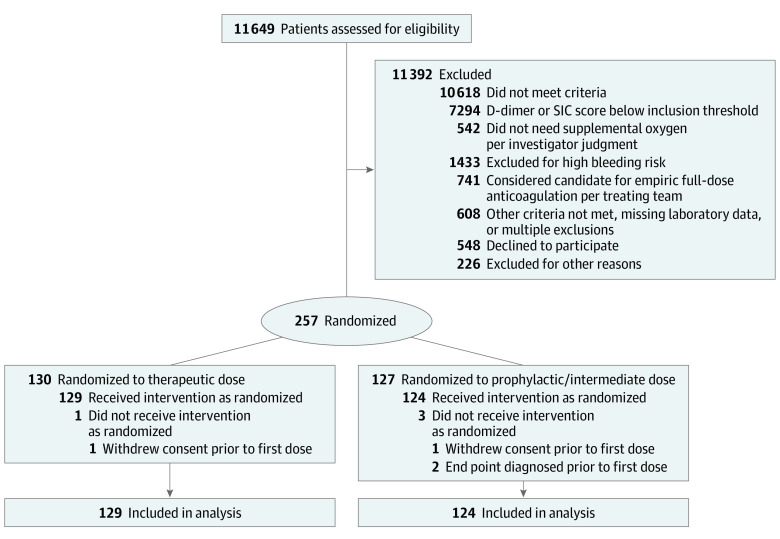
Of 11 649 patients screened, 11 392 were excluded, 548 declined participation, and 226 were not enrolled for other reasons (Figure). Of the 257 participants randomized (130 to the therapeutic-dose and 127 to the standard-dose groups), 4 patients did not receive study drug (2 withdrew consent and 2 reached end points prior to the first dose). Accordingly, there were 253 patients in the modified intention-to-treat population (mean [SD] age, 66.7 [14.0] years; men, 136 [53.8%]; women, 117 [46.2%]; Asian individuals, 25 [9.9%]; Black individuals, 70 [27.7%]; White individuals, 102 [40.3%]; multiracial/unknown race and ethnicity, 56 [22.1%]). The majority of patients (249 [98.4%]) were enrolled using D-dimer criteria. Eighty-three patients (32.8%) were stratified as ICU and 170 patients (67.2%) as non-ICU level of care. In the standard-dose group, 76 patients (61.3%) received prophylactic doses of heparin (enoxaparin, ≤40 mg daily), while 48 patients (38.7%) received intermediate doses of heparin (enoxaparin, 30 mg twice daily, 3 patients [2.4%]; enoxaparin, 40 mg twice daily, 43 patients [34.7%]; enoxaparin, 0.5 mg/kg twice daily, 2 patients [1.6%]). No patients were lost to follow-up.
Figure. CONSORT Study Flow Diagram.
SIC indicates sepsis-induced coagulopathy.
Baseline characteristics and comorbidities were similar in both groups (Table 1). The mean D-dimer level was 3183 ng/mL (median, 1700 ng/mL) in the standard-dose group and 3837 ng/mL (median, 1451 ng/mL) in the therapeutic-dose group. Baseline characteristics of the per-protocol population (215 total patients) are shown in eTable 1 in Supplement 2.
Table 1. Characteristics of Randomized Patients at Baselinea.
| Characteristic | No./total No. (%) | Standardized difference | |
|---|---|---|---|
| Therapeutic dose (n = 129) | Standard dose (n = 124) | ||
| Age, mean (SD), y | 65.8 (13.9) | 67.7 (14.1) | −0.135 |
| Sex, No. (%) | |||
| Male | 68 (52.7) | 68 (54.8) | −0.043 |
| Female | 61 (47.3) | 56 (45.2) | 0.043 |
| BMI, mean (SD) | 31.2 (9.3) | 29.8 (13.6) | 0.116 |
| Race and ethnicity, No. (%)b | |||
| Asian | 11 (8.5) | 14 (11.3) | −0.093 |
| Black | 33 (25.6) | 37 (29.8) | −0.095 |
| White | 56 (43.4) | 46 (37.1) | 0.129 |
| Multiracial/unknown | 29 (22.5) | 27 (21.8) | 0.017 |
| ICU | 45/129 (34.9) | 38/124 (30.6) | 0.090 |
| Comorbidities | |||
| Hypertension | 81/129 (62.8) | 70/123 (56.9) | 0.120 |
| Heart failure | 0 | 2/124 (1.6) | NA |
| Diabetes mellitus | 51/128 (39.8) | 43/124 (34.7) | 0.107 |
| Dyslipidemia | 48/129 (37.2) | 39/124 (31.5) | 0.121 |
| Coronary artery disease | 7/129 (5.4) | 11/124 (8.9) | −0.134 |
| Valvular heart disease | 1/129 (0.8) | 3/124 (2.4) | −0.131 |
| History of ischemic stroke | 5/129 (3.9) | 3/124 (2.4) | 0.084 |
| History of carotid occlusive disease | 0 | 0 | NA |
| Peripheral arterial disease | 4/129 (3.1) | 1/124 (0.8) | 0.166 |
| Chronic kidney disease | 5/129 (3.9) | 4/124 (3.2) | 0.035 |
| Chronic lung disease | 9/129 (7.0) | 8/124 (6.5) | 0.021 |
| Chronic liver disease/cirrhosis | 2/129 (1.6) | 1/124 (0.8) | 0.069 |
| Pulmonary hypertension | 1/127 (0.8) | 2/124 (1.6) | −0.076 |
| VTE risk factors | |||
| Personal history of VTE | 6/129 (4.7) | 2/124 (1.6) | 0.175 |
| History of cancer | 16/129 (12.4) | 10/124 (8.1) | 0.144 |
| Active cancer | 1/129 (0.8) | 4/124 (3.2) | −0.176 |
| Autoimmune disease | 1/128 (0.8) | 2/124 (1.6) | −0.077 |
| Hormonal therapy/oral contraceptives | 1/129 (0.8) | 1/124 (0.8) | −0.004 |
| Known thrombophilia | 0 | 0 | NA |
| Recent stroke with paresis | 1/129 (0.8) | 1/124 (0.8) | −0.004 |
| Clinical scores, mean (SD) | |||
| IMPROVEDD VTE risk score | 4.33 (1.48) | 4.22 (1.36) | 0.076 |
| Sepsis-induced coagulopathy score | 2.35 (0.73) | 2.31 (0.85) | 0.043 |
| Laboratory parameters, mean (SD) | |||
| White blood cell count, /μL | 9600 (5800) | 9800 (8200) | −0.032 |
| Platelets, ×103/μL | 287.7 (119.8) | 269.7 (108.2) | 0.158 |
| Serum creatinine, mg/dL | 0.94 (0.45) | 1.00 (0.50) | −0.117 |
| Prothrombin time, s | 13.5 (1.6) | 13.6 (2.6) | −0.033 |
| D-dimer, ng/mL | |||
| Mean (SD) | 3837 (6166) | 3183 (5409) | 0.113 |
| Lower quartile | 1045 | 1072 | |
| Median | 1451 | 1700 | |
| Upper quartile | 3393 | 2942 | |
| Medications prior to randomization | |||
| Low-molecular-weight heparin | 106/128 (82.8) | 97/124 (78.2) | 0.116 |
| Unfractionated heparin | 18/127 (14.2) | 23/121 (19.0) | −0.130 |
| Remdesivir | 93/129 (72.1) | 85/124 (68.6) | 0.078 |
| Glucocorticoids | 111/127 (87.4) | 93/123 (75.6) | 0.307 |
| Antiplatelets | 40/129 (31.0) | 24/124 (19.4) | 0.271 |
| Oxygen therapy | |||
| Nasal cannula | 80/129 (62.0) | 83/124 (66.9) | −0.103 |
| Nonrebreather mask | 12/129 (9.3) | 11/124 (8.9) | 0.015 |
| Ventilation mask | 4/129 (3.1) | 2/124 (1.6) | 0.098 |
| High-flow or noninvasive positive-pressure ventilation | 20/129 (15.5) | 19/124 (15.3) | 0.005 |
| Invasive mechanical ventilation | 8/129 (6.2) | 5/124 (4.0) | 0.099 |
| Length of hospital stay, mean (SD), d | 12.2 (9.3) | 11.6 (8.2) | 0.073 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ICU, intensive care unit; NA, not applicable; VTE, venous thromboembolism.
SI conversion factors: To convert white blood cell count to ×109/L, multiply by 0.001; platelet count to ×109/L, multiply by 1.0; serum creatinine to μmol/L, multiply by 88.4.
Modified intention-to-treat population.
Race and ethnicity (Asian, Black, White, multiracial) was reported by the patient.
Primary Outcomes
In the modified intention-to-treat population, 89 patients (35.2%) reached a primary efficacy outcome, including 56 deaths (22.1%) and 50 thromboembolic events (19.8%) (Table 2). The incidence of the primary efficacy outcome was 41.9% (28.2% VTE, 3.2% ATE, 25.0% death) in the standard-dose group vs 28.7% (11.7% VTE, 3.2% ATE, 19.4% death) in the therapeutic-dose group (RR, 0.68; 95% CI, 0.49-0.96; P = .03), driven by a reduction in thromboembolism (29.0% vs 10.9%; RR, 0.37; 95% CI, 0.21-0.66; P < .001); the majority of thromboembolic events consisted of symptomatic deep vein thrombosis and nonfatal pulmonary embolism (Table 3); there was no significant difference in death between groups (25.0% vs 19.4%; RR, 0.78; 95% CI, 0.49-1.23; P = .28) (Table 2), a large proportion of which was cardiovascular, with numerically more cardiovascular deaths in the standard-dose group vs therapeutic-dose group (12.1% vs 7.8%; RR, 0.64; 95% CI, 0.30-1.37; P = .25) (Table 3). There were 8 major bleed events (3.2%), 2 (1.6%) in the standard-dose vs 6 (4.7%) in the therapeutic-dose groups (RR, 2.88; 95% CI, 0.59-14.02; P = .17) (Table 2). No major bleed events were fatal (Table 3).
Table 2. Clinical Outcomes During the 30-Day Postrandomization Phase.
| Outcome | No./total No. (%) | RR (95% CI) | P valuea | |
|---|---|---|---|---|
| Therapeutic dose (n = 129) | Standard dose (n = 124) | |||
| Primary efficacy outcome | ||||
| VTE, ATE, or death | 37/129 (28.7) | 52/124 (41.9) | 0.68 (0.49-0.96) | .03 |
| Non-ICU stratum | 14/84 (16.7) | 31/86 (36.1) | 0.46 (0.27-0.81) | .004 |
| ICU stratum | 23/45 (51.1) | 21/38 (55.3) | 0.92 (0.62-1.39) | .71 |
| VTE + ATE | 14/129 (10.9) | 36/124 (29.0) | 0.37 (0.21-0.66) | <.001 |
| Death | 25/129 (19.4) | 31/124 (25.0) | 0.78 (0.49-1.23) | .28 |
| Secondary efficacy outcomes | ||||
| Primary efficacy outcome at day 14 | 30/129 (23.3) | 45/124 (36.3) | 0.64 (0.43-0.95) | .02 |
| Progression to ARDS | 11/127 (8.7) | 6/121 (5.0) | 1.75 (0.67-4.58) | .25 |
| Rehospitalization | 1/129 (0.8) | 3/124 (2.4) | 0.32 (0.03-3.04) | .36 |
| Intubation | 17/122 (13.9) | 21/121 (17.4) | 0.80 (0.45-1.45) | .46 |
| ECMO | 1/129 (0.8) | 1/124 (0.8) | 0.96 (0.06-15.20) | >.99 |
| Nonfatal cardiac arrest | 0 | 2/124 (1.6) | 0.19 (0.01-3.97) | .24 |
| Acute kidney injuryb | 17/129 (13.2) | 12/124 (9.7) | 1.36 (0.68-2.73) | .38 |
| New-onset atrial fibrillation | 4/129 (3.1) | 5/124 (4.0) | 0.77 (0.21-2.80) | .75 |
| Principal safety outcome | ||||
| Major bleeding | 6/129 (4.7) | 2/124 (1.6) | 2.88 (0.59-14.02) | .28 |
| Non-ICU stratum | 2/84 (2.4) | 2/86 (2.3) | 1.02 (0.15-7.10) | >.99 |
| ICU stratum | 4/45 (8.9) | 0 | 7.62 (0.42-137.03) | .12 |
Abbreviations: ARDS, acute respiratory distress syndrome; ATE, arterial thromboembolism; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; RR, relative risk; VTE, venous thromboembolism.
Modified intention-to-treat population (2-sided P value for superiority).
Acute kidney injury defined as (1) increase in serum creatinine by 0.3 mg/dL or greater within 48 hours, (2) increase in serum creatinine by a factor of 1.5 times baseline or greater, or (3) decrease in urine volume to less than 0.5 mg/kg/h for 6 hours per Kidney Disease: Improving Global Outcomes standard definition.
Table 3. Clinical Outcome Components During the 30-Day Postrandomization Phase in the Modified Intention-to-Treat Population.
| Outcome | No./total No. (%) | RR (95% CI) | P valuea | |
|---|---|---|---|---|
| Therapeutic dose (n = 129) | Standard dose (n = 124) | |||
| VTE | ||||
| Symptomatic DVT | 7/129 (5.4) | 19/124 (15.3) | 0.35 (0.15-0.81) | .01 |
| Asymptomatic proximal DVT | 2/129 (1.6) | 3/124 (2.4) | 0.64 (0.11-3.77) | .68 |
| Symptomatic pulmonary embolism | 4/129 (3.1) | 10/124 (8.1) | 0.38 (0.12-1.19) | .08 |
| Other VTEa | 2/129 (1.6) | 3/124 (2.4) | 0.64 (0.11-3.77) | .68 |
| ATE | ||||
| Myocardial infarction | 0 | 3/124 (2.4) | 0.14 (0.01-2.63) | .12 |
| Stroke | 1/129 (0.8) | 1/124 (0.8) | 0.96 (0.06-15.20) | >.99 |
| Major adverse limb event | 2/129 (1.6) | 0 | 4.81 (0.23-99.13) | .50 |
| Other ATEb | 1/129 (0.8) | 0 | 2.88 (0.12-70.13) | >.99 |
| Death, No./total No. (%) | ||||
| Cardiovascular | 10/129 (7.8) | 15/124 (12.1) | 0.64 (0.30-1.37) | .25 |
| Infectious/sepsis | 12/129 (9.3) | 8/124 (6.5) | 1.44 (0.61-3.41) | .40 |
| Other | 3/129 (2.3) | 8/124 (6.5) | 0.36 (0.10-1.33) | .11 |
| Bleeding, No./total No. (%) | ||||
| Decrease in hemoglobin ≥2 g/dL within 24 h | 4/129 (3.1) | 1/124 (0.8) | 3.85 (0.44-33.93) | .37 |
| Transfusion of ≥2 U of packed red blood cells | 0 | 1/124 (0.8) | 0.32 (0.01-7.79) | .49 |
| Critical site bleeding | 2/129 (1.6) | 0 | 4.81 (0.23-99.13) | .50 |
| Fatal bleeding | 0 | 0 | NA | NA |
Abbreviations: ATE, arterial thromboembolism; DVT, deep vein thrombosis of the upper or lower extremities; NA, not applicable; RR, relative risk; VTE, venous thromboembolism.
SI conversion factor: To convert hemoglobin to g/L, multiply by 10.0.
Other VTE includes splanchnic vein thrombosis and cerebral sinus vein thrombosis.
Other ATE includes intracardiac thrombus.
Compared with standard-dose heparins, therapeutic-dose LMWH reduced the incidence of the primary efficacy outcome among patients in the non-ICU stratum (36.1% vs 16.7%; RR, 0.46; 95% CI, 0.27-0.81; P = .004) but not in the ICU stratum (55.3% vs 51.1%; RR, 0.92; 95% CI, 0.62-1.39; P = .71). There was no significant difference in major bleeding between groups in either stratum, although there were numerically more major bleeds among patients in the ICU stratum in the therapeutic-dose compared with the standard-dose group (4 [8.9%] vs 0; RR, 7.62; 95% CI, 0.42-137.03; P = .12) (Table 2).
Secondary Outcomes
For secondary outcomes, therapeutic-dose LMWH reduced the incidence of the primary efficacy outcome at day 14 from hospitalization (36.3% vs 23.3%; RR, 0.64; 95% CI, 0.43-0.95; P = .02). There were no significant differences in other secondary outcomes between groups (Table 2). In each of the 2 groups, there were 3 patients who had more than 1 thromboembolic event. Serious adverse events included 1 case of thrombocytopenia with negative HIT serology results and 1 case of rectus sheath hematoma, both in the therapeutic-dose group.
In the per-protocol population, therapeutic-dose LMWH reduced the incidence of the primary efficacy outcome (48.0% vs 30.1%; RR, 0.63; 95% CI, 0.44-0.89; P = .007), with a reduction in thromboembolism (33.3% vs 10.6%; RR, 0.32; 95% CI, 0.18-0.58; P < .001) vs standard-dose heparins (eTable 2 in Supplement 2).
In the overall population, the number needed to treat to prevent 1 thromboembolic event and death was 8, while in the non-ICU stratum, the number needed to treat was 5. The number needed to harm in the overall population was 33, while in the non-ICU stratum, the number needed to harm was approximately 2000.
Discussion
In this multicenter randomized clinical trial of hospitalized patients with COVID-19 and very elevated D-dimer levels, therapeutic-dose LMWH reduced the risk of thromboembolism and mortality compared with institutional standard prophylactic or intermediate-dose LMWH or UFH for thromboprophylaxis (absolute risk reduction, 13.2%) without increasing major bleeding (absolute incremental risk, 3.0%). This absolute risk reduction was magnified to 17.9% in the per-protocol population. The treatment effect was mainly observed within 14 days of hospitalization. The number of high-risk patients needed to treat to prevent a single thromboembolic event or death was small (8 in the overall population and 5 in the non-ICU population), suggesting a favorable net clinical benefit of the therapeutic-dose LMWH regimen. No benefit accrued to patients receiving ICU care when randomized.
Patients hospitalized with COVID-19—especially those with severe or critical illness—face an elevated risk of thrombosis, especially VTE, despite standard heparin thromboprophylaxis. This raises the important clinical question of whether escalated or therapeutic-dose anticoagulation as primary thromboprophylaxis has potential to decrease thrombotic risk in COVID-19 without substantially increasing major bleed risk.2,4,14,27 Globally, more than 20 trials have been initiated comparing escalated or treatment-dose anticoagulation with standard institutional protocols of heparin thromboprophlaxis.27 Our trial shows a reduction in thromboembolism and mortality from the use of therapeutic-dose LMWH for thromboprophylaxis in high-risk inpatients with COVID-19. Our trial identified non-ICU patients with a very high risk of thromboembolism and mortality (36.1% incidence in the standard-dose group), using a criterion of very elevated D-dimer level, whose course of illness was modified by higher-dose anticoagulant therapy.
Recently published trials in hospitalized patients with COVID-19 reported no improvement in clinical outcomes of either therapeutic-dose anticoagulation (either with rivaroxaban or heparin) or intermediate-dose enoxaparin compared with standard prophylactic-dose heparins.16,17,18,19,28 These divergent results from our trial may relate to study designs, as the HEP-COVID trial used a traditional antithrombotic clinical trial design and selected higher-risk patients. In addition, our trial used therapeutic-dose LMWH whereas 1 previous trial used a lower intermediate dose of LMWH, where this absence of benefit could be explained by a lower dose used in a thrombotic population.17 Lastly, the type of anticoagulant may play a role, as a therapeutic dose of LMWH used in our trial may exert pleiotropic effects such as anti-inflammatory, immunomodulatory, and antiviral effects, in addition to its antithrombotic properties, whereas small-molecule direct oral anticoagulants may lack these properties.18,29,30 The results of the HEP-COVID study are in line with results of the multiplatform trials in moderately ill or medical ward COVID-19 inpatients, in whom therapeutic-dose heparin reduced the need for organ support and possibly in-hospital mortality, suggesting a role in improving the hypoxemia and respiratory failure caused by microvascular thrombosis.6,16
The benefits of therapeutic-dose LMWH in our trial were not seen in patients with COVID-19 requiring ICU-level care, as in previous trials of escalated-dose anticoagulation, and consistent with the recently published results of the multiplatform trials in patients with severe COVID-19.17,18,19 This may indicate that the treatment effects of heparins may only be beneficial early in the course of disease to prevent both macrovessel as well as microvascular thrombosis, before an irreversible hyperinflammatory state and cytokine storm causing thromboinflammation have set in.29,30 There were also numerically more major bleeds in the therapeutic-dose group, consistent with other studies in critically ill patients with COVID-19.17,19
Limitations and Strengths
Our trial has limitations and strengths. Although both investigators and patients were blinded to study drug regimen, other unblinded personnel may have introduced bias affecting outcome ascertainment. We used local adjudication of clinical events. However, the principal outcomes were major thromboembolic events and death, and central quality checks were performed. Our study did not capture nonmajor bleeding events, as we wanted to compare both efficacy and safety events that would influence major outcomes, such as mortality.23,31 The study population who received corticosteroids and other targeted therapies for COVID-19 reflects the current standard of care for this population. Lastly, the absolute risk reductions in the primary outcome using therapeutic LMWH shown by our study in a high-risk subgroup of inpatients with COVID-19 may not be generalizable to hospitalized patients who are less acutely ill.
Conclusions
In the HEP-COVID randomized clinical trial, therapeutic-dose LMWH reduced the composite of thromboembolism and death compared with standard heparin thromboprophylaxis without increased major bleeding among hospitalized patients with COVID-19 with very elevated D-dimer levels. The treatment effect was not seen in ICU patients. Randomized clinical trials in this patient population to assess any further benefits of therapeutic anticoagulation are ongoing, and novel antithrombotic strategies in critically ill patients with COVID-19 are needed.
Trial Protocol
eTable 1. Characteristics of the Patients at Baseline in the Per-Protocol Population
eTable 2. Clinical Outcomes During the 30-Day Post-Randomization Phase in the Per-Protocol Population
Nonauthor Collaborators. The HEP-COVID Investigators nonauthor collaborators
Data Sharing Statement
References
- 1.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799-801. doi: 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148-150. doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2020. doi: 10.1002/rth2.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spyropoulos AC, Weitz JI. Hospitalized COVID-19 patients and venous thromboembolism: a perfect storm. Circulation. 2020;142(2):129-132. doi: 10.1161/CIRCULATIONAHA.120.048020 [DOI] [PubMed] [Google Scholar]
- 5.Poissy J, Goutay J, Caplan M, et al. ; Lille ICU Haemostasis COVID-19 Group . Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184-186. doi: 10.1161/CIRCULATIONAHA.120.047430 [DOI] [PubMed] [Google Scholar]
- 6.Berger JS, Connors JM. Anticoagulation in COVID-19: reaction to the ACTION trial. Lancet. 2021;397(10291):2226-2228. doi: 10.1016/S0140-6736(21)01291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350-361. doi: 10.7326/M20-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268-277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358-1364. doi: 10.1097/CCM.0000000000004458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen SL, Gianos E, Barish MA, et al. ; Northwell Health COVID-19 Research Consortium . Prevalence and predictors of venous thromboembolism or mortality in hospitalized COVID-19 patients. Thromb Haemost. 2021;121(8):1043-1053. doi: 10.1055/a-1366-9656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Capitán C, Barba R, Díaz-Pedroche MDC, et al. Presenting characteristics, treatment patterns, and outcomes among patients with venous thromboembolism during hospitalization for COVID-19. Semin Thromb Hemost. 2021;47(4):351-361. doi: 10.1055/s-0040-1718402 [DOI] [PubMed] [Google Scholar]
- 14.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) . High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098. doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spyropoulos AC, Levy JH, Ageno W, et al. ; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis . Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. doi: 10.1111/jth.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawler PR, Goligher EC, Berger JS, et al. ; ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators . Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021;385(9):790-802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadeghipour P, Talasaz AH, Rashidi F, et al. ; INSPIRATION Investigators . Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630. doi: 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. ; ACTION Coalition COVID-19 Brazil IV Investigators . Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. doi: 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goligher EC, Bradbury CA, McVerry BJ, et al. ; REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators . Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med. 2021;385(9):777-789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldin M, Giannis D, Diab W, et al. Treatment-dose LMWH versus prophylactic/intermediate dose heparins in high-risk COVID-19 inpatients: rationale and design of the HEP-COVID trial. Thromb Haemost. Published online 2021 April 6, 2021. doi: 10.1055/a-1475-2351 [DOI] [PubMed] [Google Scholar]
- 21.Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M; Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis . Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989-1994. doi: 10.1111/jth.14578 [DOI] [PubMed] [Google Scholar]
- 22.Cohoon KP, Mahé G, Tafur AJ, Spyropoulos AC. Emergence of institutional antithrombotic protocols for coronavirus 2019. Res Pract Thromb Haemost. 2020;4(4):510-517. doi: 10.1002/rth2.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raskob GE, Spyropoulos AC, Cohen AT, et al. Association between asymptomatic proximal deep vein thrombosis and mortality in acutely ill medical patients. J Am Heart Assoc. 2021;10(5):e019459. doi: 10.1161/JAHA.120.019459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 25.Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi: 10.1182/bloodadvances.2018024489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson SS, Fleming TR. Symmetric group sequential test designs. Biometrics. 1989;45(3):905-923. doi: 10.2307/2531692 [DOI] [PubMed] [Google Scholar]
- 27.Tritschler T, Mathieu M-E, Skeith L, et al. ; International Network of VENous Thromboembolism Clinical Research Networks INVENT-VTE . Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020;18(11):2958-2967. doi: 10.1111/jth.15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sholzberg M, Tang GH, Rahhal H, et al. Heparin for moderately ill patients with COVID-19. medRxiv. Preprint posted online July 12, 2021. doi: 10.1101/2021.07.08.21259351 [DOI]
- 29.Cardillo G, Viggiano GV, Russo V, et al. ; FondenoxavidStudy Group . Antithrombotic and anti-inflammatory effects of fondaparinux and enoxaparin in hospitalized COVID-19 patients: the FONDENOXAVID study. J Blood Med. 2021;12:69-75. doi: 10.2147/JBM.S285214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gozzo L, Viale P, Longo L, Vitale DC, Drago F. The potential role of heparin in patients with COVID-19: beyond the anticoagulant effect. a review. Front Pharmacol. 2020;11:1307. doi: 10.3389/fphar.2020.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moustafa F, Stehouwer A, Kamphuisen P, et al. ; RIETE Investigators . Management and outcome of major bleeding in patients receiving vitamin K antagonists for venous thromboembolism. Thromb Res. 2018;171:74-80. doi: 10.1016/j.thromres.2018.09.049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Characteristics of the Patients at Baseline in the Per-Protocol Population
eTable 2. Clinical Outcomes During the 30-Day Post-Randomization Phase in the Per-Protocol Population
Nonauthor Collaborators. The HEP-COVID Investigators nonauthor collaborators
Data Sharing Statement