Abstract
Introduction
Decreased libido in middle-aged and elderly men is often difficult to treat, and identifying the risk factors affecting decreased libido is important for the clinical management of decreased libido. However, limited information is available regarding specific risk factors in this population.
Aim
The present study investigated the risk factors for decreased libido among middle-aged and elderly men.
Methods
Patients who attended our male andropausal outpatient clinic between 2009 and 2015 were enrolled. All patients completed a self-administered questionnaire, which included the Aging Male Symptoms (AMS) scale, International Prostate Symptom Score (IPSS), and Sexual Health Inventory for Men (SHIM). Information on waist size, body mass index, present illness, present use of any medication, and lifestyle habits were collected by each attending physician. Blood biochemical data such as free testosterone, total cholesterol, triglyceride, high density lipoprotein-cholesterol (HDL-Chol), and hemoglobin A1c values were assessed. Libido was assessed based on AMS scale question 17, and a score of 4 or higher was defined as severely decreased libido (severe group).
Main Outcome Measure
The clinical factors associated with severely decreased libido were analyzed based on multiple regression analysis.
Results
A total of 292 subjects were included in the analysis, 111 (38%) of which belonged to the severe group. The mean age of study subjects was 66.2 years, and the mean FT value was 7.1 ± 2.2. Comparisons of each variable among the severe and not severe groups showed significant differences in older age, current cigarette smoking, AMS scale, IPSS, frequency of nocturnal voiding, SHIM score, and HDL-Chol value. Multivariate regression analysis revealed that current cigarette smoking, frequent nocturnal voiding, and a low SHIM score were the independent risk factors for severely decreased libido. Furthermore, the frequency of nocturnal voiding significantly increased with severity of decreased libido.
Conclusion
Current cigarette smoking, frequent nocturnal voiding, and a low SHIM score were the independent risk factors for a severely low libido.
K Shigehara, Y Kato, M Iijima, et al. Risk Factors Affecting Decreased Libido Among Middle-Aged to Elderly Men; Nocturnal Voiding is an Independent Risk Factor of Decreased Libido. Sex Med 2021;9:100426.
Key Words: Erectile Dysfunction, Libido, Nocturia, Smoking
INTRODUCTION
A low libido is one of the components of male sexual dysfunction, which include erectile dysfunction (ED), ejaculation disorders, and delayed or inhibited orgasm. Libido, along with appetite and sleep desire, is an instinct necessary for a person to live and is an essential desire to preserving the species. Therefore, this is an important aspect of quality of life (QOL).
Libido varies widely from person to person and may temporarily decrease due to various mental conditions such as fatigue and anxiety.1 In general, libido decreases gradually with age. Indeed, decreased libido is a well-known symptom of late-onset hypogonadism (LOH) syndrome, which is a cluster of various clinical conditions caused by testosterone decline with age.2,3 Indeed, the Aging Male Symptoms (AMS) scale used worldwide for the screening of LOH syndrome includes a question about decreased libido.4 Decreased libido involves reduced frequency of sexual thoughts and fantasies, interest in sexual intercourse, frequency of sexual activity, and sexual stimulation by sight, words, or touch. Persistent decreased libido can occasionally bother couples. In today's aging society, decreased libido can be one of the important problems among elderly men that impair a couple's QOL. In addition, a recent study demonstrated a close relationship between libido and cardiovascular diseases, concluding that maintaining libido could have a beneficial effect on cardiovascular and overall health among men.5
Known causes of decreased libido in men include psychosomatic stress, cranial nerve diseases, endocrine diseases, drugs, old age, and testosterone decline.1 In younger men, the most common cause of decreased libido is psychosomatic stress. Psychological factors such as depression and anxiety can inhibit sexual desire,6,7 and low sexual desire can have an adverse effect on many psychological and social aspects.6 On the other hand, middle-aged and elderly men often have many comorbidities such as testosterone decline, lifestyle-related diseases, urinary disorders, and depression. These comorbidities and some lifestyle habits can be directly and indirectly associated with decreased libido.1 Decreased libido in middle-aged and elderly men is often difficult to treat, and many clinicians often fail to improve it. Therefore, identifying the risk factors affecting decreased libido is important for the clinical management of decreased libido in middle-aged and elderly men. However, only limited information is available regarding specific risk factors in this population.
The aim of the present study is to answer the question, “What are the risk factors for decreased libido among middle to elderly men?” It is the goal of this retrospective, observational, cross-sectional study to address these issues.
MATERIALS AND METHODS
Study Protocol
348 patients who attended our male andropausal outpatient clinic between 2009 and 2015 were enrolled in the study. At the initial visit, all patients completed a self-administered questionnaire that included the AMS scale, International Prostate Symptom Score (IPSS), and Sexual Health Inventory for Men (SHIM). Information on waist size, body mass index (BMI), present illness, present use of any medication, and lifestyle habits including current cigarette smoking, alcohol drinking, and exercise were collected by each attending physician. Blood biochemical data such as free testosterone (FT), total cholesterol (Tchol), triglyceride (TG), high density lipoprotein-cholesterol (HDL-Chol), and hemoglobin A1c (HbA1c) values were assessed. All blood analyses were performed using blood serum collected between 09:00 and 11:00. FT value was measured by radioimmunoassay using a DPC Free Testosterone kit (Mitsubishi Kagaku Iatron), which was a commercial kit approved by the Japanese Ministry of Health, Labor and Welfare. We retrospectively collected these data from the medical records.
We excluded patients with cerebral neurological disorders, psychiatric disorders, malignancies under ongoing treatment, and current use of medicines that could affect libido and erectile function, such as antidepression drugs, selective serotonin reuptake inhibitors, serotonin–noradrenalin reuptake inhibitors, benzodiazepine, androgen supplements, phosphodiesterase-5 inhibitor, finasteride, and dutasteride.
Frequency of nocturnal voiding was evaluated using IPSS question 7, and libido was assessed based on AMS scale question 17 (AMS-17; “Decrease in sexual desire/libido; lacking pleasure in sex, lacking desire for sexual intercourse”; scored 1–5, indicating “none” to “extreme”). A score of 4 or higher on AMS-17 was defined as severely decreased libido (severe group), whereas 3 or less of AMS-17 was considered as none to moderately decreased libido (nonsevere group). We retrospectively investigated and recorded the data described above.
The present study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital (No. 2021-042). The details of this retrospective study were published in writing on our hospital website and were available from the outpatient clinic.
Statistical Analysis
Patient characteristics were compared using the Mann–Whitney U test, whereas categorical data were analyzed using the unpaired chi-squared test. In addition, multivariate regression analysis was performed to identify the independent factors affecting libido, and 95% confidence intervals (CI) were calculated to determine the significance of differences. Correlation between the severity of decreased libido and frequency of nocturnal voiding was evaluated using the Jonckheere–Terpstra trend test. All statistical analyses were performed using SPSS version 22 (SPSS Inc., Chicago, IL, USA). In all analyses, P value < .05 indicated statistical significance.
RESULTS
After removing 56 patients according to the exclusion criteria, a total of 292 subjects were included in the analysis. The mean ± standard deviation (SD) age of study subjects was 66.2 ± 8.8 years, and the mean FT value was 7.1 ± 2.2 pg/mL (Table 1). Of the 292 patients, 44 (15.1%) scored 1 (none) on AMS-17, 43 (14.7%) scored 2 (mild), 94 (32.2%) scored 3 (moderate), 70 (24.0%) scored 4 (severe), and 41 (14.0%) scored 5 (extremely severe), Thus, 111 (38.0%) subjects were classified into the severe group, and 181 (62.0%) were categorized into the nonsevere group.
Table 1.
Patients’ background (n = 292)
| Variables | |
|---|---|
| Age (years, mean ± SD) | 66.2 ± 8.8 |
| Free testosterone (pg/mL) | 7.1 ± 2.2 |
| Exercise habits | 175 (60.0%) |
| Current cigarette smoking status | 64 (21.9%) |
| Alcohol use | 78 (26.7%) |
| Diabetic mellitus status | 91 (31.1%) |
| Patients’ complaints | |
| Physiological symptoms | 245 (83.9%) |
| Psychological symptoms | 97 (33.2%) |
| Sexual symptoms | 244 (83.6%) |
| AMS scale | 36.8 ± 10.0 |
| Physiological domain | 14.4 ± 4.6 |
| Psychological domain | 8.4 ± 3.3 |
| Sexual domain | 14.0 ± 4.2 |
| Decrease in sexual desire/libido | |
| (1) None | 44 (15.1%) |
| (2) Mild | 43 (14.7%) |
| (3) Moderate | 94 (32.2%) |
| (4) Severe | 70 (24.0%) |
| (5) Extremely severe | 41 (14.0%) |
| Frequency of nocturnal voiding | 1.6 ± 1.2 |
| IPSS | 10.2 ± 8.4 |
| SHIM score | 11.2 ± 6.3 |
| Waist size (cm) | 86.8 ± 9.2 |
| BMI | 23.7 ± 3.8 |
| Tchol (mg/dL) | 187 ± 29 |
| TG (mg/dL) | 121 ± 73 |
| HDL-chol (mg/dL) | 56 ± 15 |
| HbA1c (%) | 6.4 ± 3.3 |
Data are expressed as mean ± standard deviation or as percentages.
AMS = Aging Male Symptoms scale; BMI = body mass index; HbA1c = hemoglobin A1c; HDL = high density lipoprotein; IPSS = International Prostatic Symptoms score; SHIM = Sexual Health Inventory for Men; Tchol = total cholesterol; TG = triglyceride.
Patients’ complaints for referring to our andropausal outpatient clinic were 245 cases (83.9%) in physiological symptoms, 97 (33.2%) in psychological symptoms, and 244 (83.6%) in sexual symptoms. Mean total AMS scale was 36.8 ± 10.0, and physiological, psychological, and sexual subdomains of AMS scale were 14.4 ± 4.6, 8.4 ± 3.3, and 14.0 ± 4.2, respectively.
Comparisons on each variable among the subjects in both groups were performed to analyze the risk factors for decreased libido (Table 2). The patients in the severe group were significantly older (P = .014) and were current smokers (P = .002). The severe group had significantly higher AMS scale (P < .001) and IPSS (P = .011) scores and frequency of nocturnal voiding (P < .001). Furthermore, patients in the severe group had significantly lower SHIM scores (P < .001) and HDL-Chol levels (P = .031). No significant differences were observed in FT value, exercise habits, alcohol use, diabetic status, waist size, BMI, Tchol value, TG value, and HbA1c level between the two groups.
Table 2.
Comparisons on each variable among men with severely and nonseverely decreased libido
| Decrease in libido |
|||
|---|---|---|---|
| Severe | Not severe | ||
| Variables | (n = 111) | (n = 181) | P value |
| Age (years) | 67.7 ± 8.1 | 65.3 ± 9.1 | .014 |
| Free testosterone (pg/mL) | 6.9 ± 2.2 | 7.3 ± 2.2 | .127 |
| Exercise habits | 66 (59.4%) | 109 (60.2%) | .897 |
| Current cigarette smoking status | 35 (31.5%) | 29 (16.0%) | .002 |
| Alcohol use | 31 (27.9%) | 47 (26.0%) | .713 |
| Diabetes mellitus status | 41 (36.9%) | 50 (27.6%) | .0953 |
| AMS scale | 42.0 ± 8.7 | 33.6 ± 9.5 | <.001 |
| Frequency of nocturnal voiding | 1.9 ± 1.2 | 1.4 ± 1.1 | <.001 |
| IPSS | 11.7 ± 8.3 | 9.3 ± 8.3 | .011 |
| SHIM score | 7.4 ± 4.6 | 13.1 ± 6.2 | <.001 |
| Waist size (cm) | 86.6 ± 8.8 | 86.9 ± 9.4 | .393 |
| BMI | 23.5 ± 3.2 | 23.7 ± 4.1 | .331 |
| Tchol (mg/dL) | 188 ± 29 | 186 ± 29 | .238 |
| TG (mg/dL) | 129 ± 69 | 116 ± 76 | .082 |
| HDL-chol (mg/dL) | 53 ± 14 | 57 ± 15 | .031 |
| HbA1c (%) | 6.2 ± 1.1 | 6.5 ± 4.0 | .259 |
Data are expressed as mean ± standard deviation or as percentages.
AMS = Aging Male Symptoms; BMI = body mass index; HbA1c = hemoglobin A1c; HDL = high density lipoprotein; IPSS = International Prostatic Symptoms score; SHIM = Sexual Health Inventory for Men; Tchol = total cholesterol; TG = triglyceride.
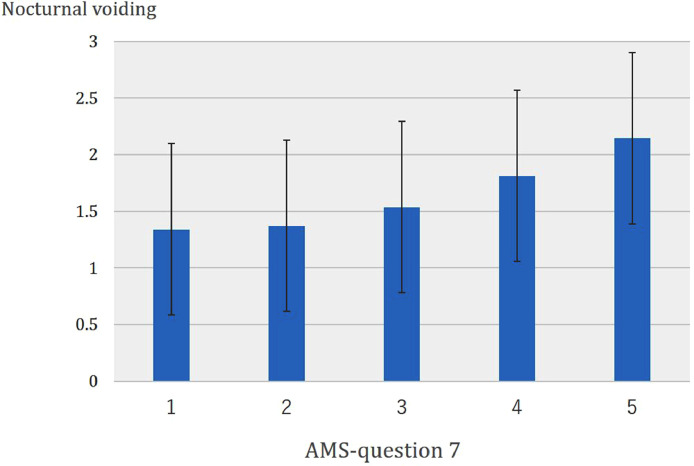
Since the factors with significant differences in univariate analysis may be confounding factors in affecting libido, multivariate regression analysis was performed to identify the independent risk factors (Table 3). Current smoking status, frequent nocturnal voiding, and low SHIM score were the independent risk factors for severely decreased libido. Furthermore, the frequency of nocturnal voiding significantly increased with the severity of decreased libido (P = .0235) (Figure 1).
Table 3.
Risk factors of severe decrease in libido as determined by multivariate regression analysis
| Variables | Odds ratio (95% CI) | P value |
|---|---|---|
| Age | 0.924 (0.842–1.014) | .096 |
| Current cigarette smoking status | 7.168 (1.585–32.30) | .011 |
| AMS scale | 1.038 (0.967–1.115) | .302 |
| Frequency of nocturnal voiding | 2.288 (1.076–4.868) | .032 |
| IPSS | 0.969 (0.876–1.072) | .536 |
| SHIM score | 0.855 (0.750–0.976) | .021 |
| HDL-chol | 0.990 (0.944–1.037) | .659 |
AMS = aging male symptoms; CI = confidence interval; HDL = high density lipoprotein
IPSS = International Prostatic Symptoms Score; SHIM = sexual health inventory for men.
Figure 1.
Correlation between severity of decreased libido and number of nocturnal voiding. Frequency of nocturnal voiding significantly increased with severity of decreased libido (P = .0235).
DISCUSSION
The present study established current cigarette smoking, frequent nocturnal voiding, and low SHIM score as the independent risk factors of severely decreased libido among middle-aged to elderly men. In particular, the finding on the frequency of nocturia as an independent risk factor of decreased libido was likely to be unique. On the other hand, age and FT value, which are well-known risk factors affecting libido, were not found to significantly affect libido in the present study.
It has been widely accepted that lower urinary tract symptoms (LUTS) can have some negative effects on sexual function, and a significant correlation between storage symptoms including nocturia and sexual function has been observed.8, 9, 10, 11 One previous study including 236 men with LUTS demonstrated that overactive bladder symptoms are the risk factors for moderate and severe ED.8 In some previous studies, nocturia, urgency, and incontinence in storage symptoms of LUTS were significantly correlated with ED and sexual dysfunction.9, 10, 11 On the other hand, the relationship between nocturia and libido has been not established.
Nocturia, which is defined as awakening one or more times for voiding during the night, generally becomes more frequent with age and is one of the common clinical symptoms of aging.12 Nocturia causes sleep fragmentation and sleep disturbance, which are significantly associated with depression, anxiety, and decreased daytime activities.13,14 These symptoms can contribute to decrease in sexual satisfaction and libido. In the present study, the patients with severely decreased libido had a higher frequency of nocturia compared with those in the nonsevere group. Furthermore, the frequency of nocturnal voiding significantly increased with the severity of decreased libido. Although we failed to find a significant correlation between total IPSS and a low libido, some previous reports demonstrated that LUTS could have a negative effect on libido.15,16 On the other hand, to our knowledge, little evidence on the correlation between nocturia and libido has been retrieved. Only one previous study that included 5,503 community‐dwelling participants described that low libido was significantly associated with depression and nocturia in men.17
In general, nocturnal awakening due to nocturia decreases testosterone levels.18,19 Testosterone production is closely related to sleep quantity and quality, and testosterone levels have been reported to decrease by 0.142 ng/mL for every count of nocturnal awakening.18 Conversely, testosterone replacement for hypogonadal men with nocturia can improve nocturia and sleep quality.20 The importance of testosterone on libido in men is widely accepted. However, the present study found that FT levels were not significantly correlated with a low libido, which may be due to population bias or low baseline FT levels. Alternatively, among middle-aged and elderly men with lower FT levels than younger men, the frequency of nocturnal voiding may be a much stronger predictor of a low libido compared with FT levels.
Smoking is a well-known risk factor of ED,21 and, as the present study showed, could also have a significant negative effect on libido. Indeed, a previous study involving 18,427 sexually active Australian adults demonstrated that smoking was a significant risk of decreased libido with an odds ratio (OR) of 2.18.22 The accumulation of harmful chemicals in the body that accompanied cigarette smoking directly damages cells and gonadal tissues.23,24 Furthermore, smoking causes the dysregulation of the hypothalamic–pituitary–gonadal axis, which negatively cascades into an imbalance of sex hormones that causes decreased libido.25,26
We also found that a low SHIM score may predict a decreased libido. ED is significantly associated with a low libido; the OR of ED for decreased libido was reported to be 18.2.27 The psychiatric factors associated with ED, such as anxiety, depression, and reduced motivation, are the same with those of decreased libido.28 Lower or severe ED has a negative effect on a couple's sexual life, expressed as sexual bother, disappointment, and anxiety about sexual intercourse, resulting in decreased libido.
Age is significantly associated with decreased libido. Age-related mental and physical changes naturally lead to a decline in libido. A previous study demonstrated that libido in middle-aged men (40–60 years) was three times lower than in younger men (18–29 years).29 In addition, testosterone levels, which play an important role, in maintaining libido and erectile function, generally decrease approximately 1% per year with age.30 Androgen receptors, which are heavily located in the mediobasal hypothalamus and limbic parts of the brain responsible for libido,31 can contribute to decreased libido with age-associated testosterone decline among men over 60.32 As is well known in clinical practice, androgen deprivation therapy for prostate cancer inhibits libido. Conversely, testosterone replacement therapy can improve libido and erectile function in hypogonadal men.33 Nevertheless, the present study found that neither age nor FT levels significantly influenced decreased libido. This may be because the present study enrolled middle-aged to elderly patients with a mean age of 66.2 ± 8.8 years, who were attendees of our andropausal outpatient clinic, indicating lower baseline FT levels and even LOH syndrome.
The present study has some limitations. First, the subjects in this study were patients who attended the andropausal outpatient clinic at our institution, which is evidently a population bias. Patients were older and had lower FT levels at baseline. However, we often encounter many patients with severe decreased libido at andropausal outpatient clinic in our daily practice. Indeed, many (83.6%) of the patients in the present study had any sexual symptoms. Decreased libido in andropausal men is often difficult to treat by only testosterone replacement therapy, and many clinicians often fail to improve it. Therefore, the present findings are likely to be informative for the management of risk factors for severely decreased libido. In addition, this population inevitably scored high on the IPSS with a mean score of 10.2 and scored low on the SHIM with a mean score of 11.2. Therefore, further studies involving a larger population with more diversity in terms of age and FT levels are needed to establish more conclusive findings. In addition, FT levels were measured using a radioimmunoassay kit, which was the only method approved by the Japanese Ministry of Health, Labor and Welfare. Furthermore, this kit was discontinued and is no longer available. As demonstrated in a large epidemiological study in Japan, total testosterone values did not decrease with age, whereas FT showed a gradual decrease.34 Analog FT values showed a good correlation with calculated FT values whose usage has been widely accepted overseas for a diagnosis of hypogonadism.35 Therefore, analog FT values were used to diagnose hypogonadism during this retrospective study. Finally, only question 17 on the AMS scale was used to evaluate libido, which is uncommon, and information on frequency of sexual activity was not collected. The international index of erectile function (IIEF) a 15-item questionnaire is commonly used to explore satisfaction, orgasm, erection, and sexual desire in subjects. However, AMS has similar results with IIEF in evaluating libido.
CONCLUSIONS
This study demonstrates that current smoking status, frequent nocturnal voiding, and low SHIM score were the independent risk factors of severely decreased libido among middle-aged and elderly men who attended a andropausal outpatient clinic. Although more comprehensive studies are needed to verify these findings, these would help inform clinical management of decreased libido and provide baseline data that may support future investigations into effective solutions for the condition.
STATEMENT OF AUTHORSHIP
Conceptualization, K.S. and Y.K.; Data curation, K.S., M.I., S.K., K.I., Y.K., M.N., and A.M.; Formal analysis, K.S. and Y.K.; Supervision, M.N. and A.M.; Original draft, K.S.; Review & editing, A.M. *These authors contributed equally.
ACKNOWLEDGMENTS
We would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Chen L, Shi GR, Huang DD. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Hisasue S. Contemporary perspective and management of testosterone deficiency: Modifiable factors and variable management. Int J Urol. 2015;22:1084–1095. doi: 10.1111/iju.12880. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimura A. The relationship between testosterone deficiency and men's health. World J Mens Health. 2013;31:126–135. doi: 10.5534/wjmh.2013.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinemann LA, Saad F, Zimmermann T. The Aging Males’ Symptoms (AMS) scale: Update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15. doi: 10.1186/1477-7525-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho CH, Wu CC, Chen KC. Erectile dysfunction, loss of libido and low sexual frequency increase the risk of cardiovascular disease in men with low testosterone. Aging Male. 2016;19:96–101. doi: 10.3109/13685538.2015.1129400. [DOI] [PubMed] [Google Scholar]
- 6.Nimbi FM, Tripodi F, Rossi R. Expanding the analysis of psychosocial factors of sexual desire in men. J Sex Med. 2018;15:230–244. doi: 10.1016/j.jsxm.2017.11.227. [DOI] [PubMed] [Google Scholar]
- 7.Lykins AD, Janssen E, Graham CA. The relationship between negative mood and sexuality in heterosexual college woman and men. J Sex Res. 2006;43:136–143. doi: 10.1080/00224490609552308. [DOI] [PubMed] [Google Scholar]
- 8.Amano T, Earle C, Imao T. Are urge incontinence and aging risk factors of erectile dysfunction in patients with male lower urinary tract symptoms? Aging Male. 2016;19:54–57. doi: 10.3109/13685538.2015.1103219. [DOI] [PubMed] [Google Scholar]
- 9.Terai A, Ichioka K, Matsui Y. Association of lower urinary tract symptoms with erectile dysfunction in Japanese men. Urology. 2004;64:132–136. doi: 10.1016/j.urology.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Frankel SJ, Donovan JL, Peters TI. Sexual dysfunction in men with lower urinary tract. J Clin Epidemiol. 1998;51:677–685. doi: 10.1016/s0895-4356(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 11.Ishizuka O, Matsuyama H, Sakai H. Nocturia potentially influences maintenance of sexual function in elderly men with benign prostatic hyperplasia. Low Urin Tract Symptoms. 2013;5:75–81. doi: 10.1111/j.1757-5672.2012.00173.x. [DOI] [PubMed] [Google Scholar]
- 12.Homma Y, Yamaguchi O, Hayashi K, Neurogenic Bladder Society Committee Epidemiologic survey of lower urinary tract symptoms in Japan. Urology. 2006;68:560–564. doi: 10.1016/j.urology.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Stewart RB, Moore MT, May FE. Nocturia: A risk factor for falls in the elderly. J Am Geriatr Soc. 1992;40:1217–1220. doi: 10.1111/j.1532-5415.1992.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 14.Bower WF, Whishaw DM, Khan F. Nocturia as a marker of poor health: Causal associations to inform care. Neurourol Urodyn. 2017;36:697–705. doi: 10.1002/nau.23000. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Kim HH. The complex relationship between lower urinary tract symptoms and sexual health. Curr Urol Rep. 2019;20:58. doi: 10.1007/s11934-019-0930-4. [DOI] [PubMed] [Google Scholar]
- 16.Martin SA, Atlantis E, Lange K. Florey Adelaide Male Ageing Study. Predictors of sexual dysfunction incidence and remission in men. J Sex Med. 2014;11:1136–1147. doi: 10.1111/jsm.12483. [DOI] [PubMed] [Google Scholar]
- 17.Rosen RC, Link CL, O'Leary MP. Lower urinary tract symptoms and sexual health: The role of gender, lifestyle and medical comorbidities. BJU Int. 2009;103:42–47. doi: 10.1111/j.1464-410X.2009.08370.x. [DOI] [PubMed] [Google Scholar]
- 18.Shigehara K, Izumi K, Mizokami A. Testosterone deficiency and nocturia: A review. World J Mens Health. 2017;35:14–21. doi: 10.5534/wjmh.2017.35.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeh SU, Yoon S, Seo DH. Relationship between serum testosterone and nocturia in men without benign prostate enlargement. Andrology. 2017;5:58–62. doi: 10.1111/andr.12270. [DOI] [PubMed] [Google Scholar]
- 20.Shigehara K, Konaka H, Koh E. Effects of testosterone replacement therapy on nocturia and quality of life in men with hypogonadism: A subanalysis of a previous prospective randomized controlled study in Japan. Aging Male. 2015;18:169–174. doi: 10.3109/13685538.2015.1038990. [DOI] [PubMed] [Google Scholar]
- 21.Kałka D, Domagała Z, Rakowska A. Modifiable risk factors for erectile dysfunction: An assessment of the awareness of such factors in patients suffering from ischaemic heart disease. Int J Impot Res. 2016;28:14–19. doi: 10.1038/ijir.2015.26. [DOI] [PubMed] [Google Scholar]
- 22.Wen LM, Rissel C, Cheng Y. Tobacco smoking and sexual difficulties among Australian adults: A cross-sectional study. Sex Health. 2017;14:313–319. doi: 10.1071/SH17005. [DOI] [PubMed] [Google Scholar]
- 23.Nampoothiri LP, Gupta S. Simultaneous effect of lead and cadmium on granulosa cells: A cellular model for ovarian toxicity. Reprod Toxicol. 2006;21:179–185. doi: 10.1016/j.reprotox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Pandya C, Pillai P, Nampoothiri LP. Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 25.Weisberg E. Smoking and reproductive health. Clin Reprod Fertil. 1985;3:175–186. [PubMed] [Google Scholar]
- 26.Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152:491–499. doi: 10.1530/eje.1.01867. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi S, Yamane K, Kamei N. Erectile dysfunction is strongly linked with decreased libido in diabetic men. Aging Male. 2004;7:113–119. doi: 10.1080/13685530412331284713. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen HMT, Gabrielson AT, Hellstrom WJG. Erectile dysfunction in young men-a review of the prevalence and risk factors. Sex Med Rev. 2017;5:508–520. doi: 10.1016/j.sxmr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Sternbach H. Age-associated testosterone decline in men: Clinical issues for psychiatry. Am J Psychiatry. 1998;155:1310–1318. doi: 10.1176/ajp.155.10.1310. [DOI] [PubMed] [Google Scholar]
- 30.Pantalone KM, Faiman C. Male hypogonadism: More than just a low testosterone. Cleve Clin J Med. 2012;79:717–725. doi: 10.3949/ccjm.79a.11174. [DOI] [PubMed] [Google Scholar]
- 31.Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6:1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- 32.Johannes CB, Araujo AB, Feldman HA. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- 33.Shigehara K, Konaka H, Kato Y. Effect of testosterone replacement therapy on sexual function and glycemic control among hypogonadal men with type 2 diabetes mellitus. Int J Impot Res. 2019;31:25–30. doi: 10.1038/s41443-018-0065-z. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto T, Yanase T, Horie H. Late-onset hypogonadism (LOH) and androgens: Validity of the measurement of free testosterone levels in the diagnostic criteria in. Japan Int J Urol. 2009;16:168–174. doi: 10.1111/j.1442-2042.2008.02203.x. [DOI] [PubMed] [Google Scholar]
- 35.Taya M, Koh E, Izumi K. Comparison of testosterone fractions between Framingham Heart Study participants and Japanese participants. Int J Urol. 2014;21:689–695. doi: 10.1111/iju.12393. [DOI] [PubMed] [Google Scholar]