Abstract
Introduction
The use of current available treatment for male erectile dysfunction (ED) has some limitations that are related to efficacy and adverse effects. Nanotechnology has been used as a new tool in medicine to improve these limitations and new medications potentially to alleviate and cure ED.
Aim
To review the currently literature on new nano medications for ED based on scientific and clinical studies, efficacy, safety, mechanisms of action, and to identify gaps for future research.
Methods
A comprehensive literature review was conducted via Google Scholar, Science Direct, and PubMed on English publications using different keywords such as “erectile dysfunction”, “emerging treatments”, “nanotechnology”, and “herbal medicine”. The retrieved papers were organized into groups according to the sections covered in this review paper.
Main Outcomes Measures
We reviewed novel ED treatments such as nanotechnological phosphodiesterase inhibitors, papaverine hydrochloride, sialorphin, adipose tissue-derived stem cells, sonic hedgehog, and herbal medicine.
Results
Numerous preclinical studies have addressed novel phosphodiesterase 5 inhibitors nanoparticle, and their recent delivery systems. Nitric oxide, sialorphin, sonic hedgehog, and herbal medicine loaded nanoparticles and nano adipose tissue-derived stem cells as a potential new treatment for ED. In addition, papaverine-containing nanoparticles have been reported. A limited number of randomized clinical studies have determined the mechanism of these treatments.
Conclusion
A literature review on the application of nanotechnology in ED therapy was successfully conducted. New nano medications are promising to treat ED. However, further studies are warranted to further assess their efficacy and safety. Masuku NP, Unuofin JO, Lebelo SL. Advances in Nanoparticle Delivery System for Erectile Dysfunction: An Updated Review. Sex Med 2021;9:100420.
Key Words: Erectile Dysfunction, Herbal Medicine, Nanotechnology, Nitric Oxide, Papaverine Hydrochloride, Phosphodiesterase 5 Inhibitors, Sialorphin, Sonic Hedgehog, Stem Cells
Abbreviations: ADSCs, Adipose tissue-derived stem cells; AEs, Adverse effects; ALA, Alpha-lipoic acid; AUC, Area under plasma concentration; AVA, avanafil; CAT, Catalase; CC, Corpus cavernosum; BP, Blood pressure; cGMP, Cyclic guanosine monophosphate; Cmax, Maximum plasma concentration; CN, Cavernous nerve; CURC, Curcumin; ED, Erectile dysfunction; EHS, Erection hardness score; GAQ, Global Assessment Questionnaire; ICI, Intracavernous injection; ICP, Intracavernous pressure; IIEF, International Index of Erectile Function; IIT, Intention to treat; GI, Gastrointestinal; NPs, Nanoparticles; FDA, Food and Drug Administration; HCl, Hydrochloride; HPMC, Hydroxyl methylcellulose; MNPs, Magnetic nanoparticles; MAP, Mean arterial pressure; MRT, Mean residence time; NCD, Novel curcumin derivative; NGF, Nerve growth factor; NLCs, Nanostructure lipid carriers; NO, Nitric oxide; NOS, Nitric oxide synthase; LLC, Lyotropic liquid crystal; Pa, Peptide amphiphiles; PaHCl, Papaverine hydrochloride; PDE5, Phosphodiesterase 5; PLGA, Poly-lactic-co-glycolytic acid; RP, Radical prostatectomy; SEDDSs, Self-emulsifying drug delivery systems; SEP, Sexual Encounter Profile; SMEDDSs, Self-microemulsifying drug delivery systems; SNEDDs, Self-nanoemulsifying drug delivery systems; SLNs, Solid lipid nanoparticles; SOD, Superoxide dismutase; SSH, Sonic hedgehog; t1/2, half-life; Tmax, Maximum plasma concentration corresponding time; TPH, Transfersomal papaverine hydrochloride; TSS, Treatment Satisfaction Scale; VDN, Vardenafil
INTRODUCTION
Male erectile dysfunction (ED) or impotence can be defined as incompetence to reach and retain sufficient penile tumescence for sexual intercourse.1,2 Worldwide, there are more than 152 million men affected by ED and are expected to be 322 million in 2025.1,2 The likelihood of developing ED increases with age, it is most prevalent in men aged 40 years old and above.3, 4, 5 ED has been reported to be associated with diabetes6 and radical prostatectomy (RP) used in the treatment of prostate cancer.7, 8, 9 Other risk factors for ED include hypertension, cardiovascular diseases, obesity, alcohol intake, cigarette smoking, drug abuse, and poor physical activity.2,10
Currently, oral phosphodiesterase type 5 (PDE5) inhibitors for example, sildenafil have been endorsed for use by the United States Food and Drug Administration (FDA) as the initial medications for the alleviation and treatment of male ED.2,11,12 This drug class plays an essential role in penile erection. Sexual stimulation leads to the synthesis of nitric oxide (NO) from L-arginine and oxygen by nitric oxide synthase (NOS). NO is released from endothelium and nitrergic nerves then diffuses through the cell membrane into corpora cavernosa smooth muscle and activates the guanylate cyclase. This catalyzes the conformational modification of guanosine-5’-triphosphate (GTP) to form a second messenger called 3’-5’ cyclic guanosine monophosphate (cGMP). In turn, cGMP activates the cGMP-dependent protein kinase (PKG) which causes decreased levels of intracellular calcium, smooth muscle relaxation and vasodilation, and subsequently penile erection. An enzyme, PDE5 degrades the cGMP leading to smooth muscle contraction, vasoconstriction, and penile detumescence. Oral treatment for ED, PDE5 inhibitors bind to the catalytic site of PDE enzymes and block the degradation of 3’,5’-cyclic guanosine monophosphate (cGMP), thus prevents premature penile detumescence and ED.2,11,13,14
PDE5 inhibitors have some limitations. The drugs are not effective, safe, and tolerated by all patients diagnosed with ED.12,13 They are associated with several adverse effects (AEs) attributed to poor aqueous solubility, and low bioavailability.15 The most dominant AEs including headache, dizziness, stuffy nose, feverish, and indigestion.2,11,13,15 PDE5 inhibitors should not be administered with organic nitrates, due to their synergic effect may result in hypotension and fatal.16,17 Besides, some patients discontinued using these drugs.13 For these reasons, PDE5 inhibitors should be modified.10,12 Also, there is a need for more effective and safe treatment options.2,10,18
Nanotechnology has emerged as a common tool used in medicine, to improve the efficacy and safety of the treatments including minimizing their AEs. It involves the application of nanoparticles (NPs). These have exclusive properties such as possess different shapes, small size (1–100 nm), high surface area to volume ratio, chemical, and physical properties.19, 20, 21 Encapsulation of drugs into NPs enhance drugs solubility, reduce toxicity, facilitate delivery at the specific site, control and sustain their release.20,22 At the moment, there are numerous scientific reports on the different nanoparticle delivery systems for the treatment and recuperation of male ED. However, what is insufficient is the comprehensive review that assembles the experimental evidence and logical comments that provides recommendation for the future research in the drug development. Therefore, this article aims to review the current literature on new nano medications for ED based on scientific and clinical studies, efficacy, safety, mechanisms of action, and identify gaps for future research.
NANOMEDICINE FOR MEN ERECTILE DYSFUNCTION
PDE5 Inhibitors NPs
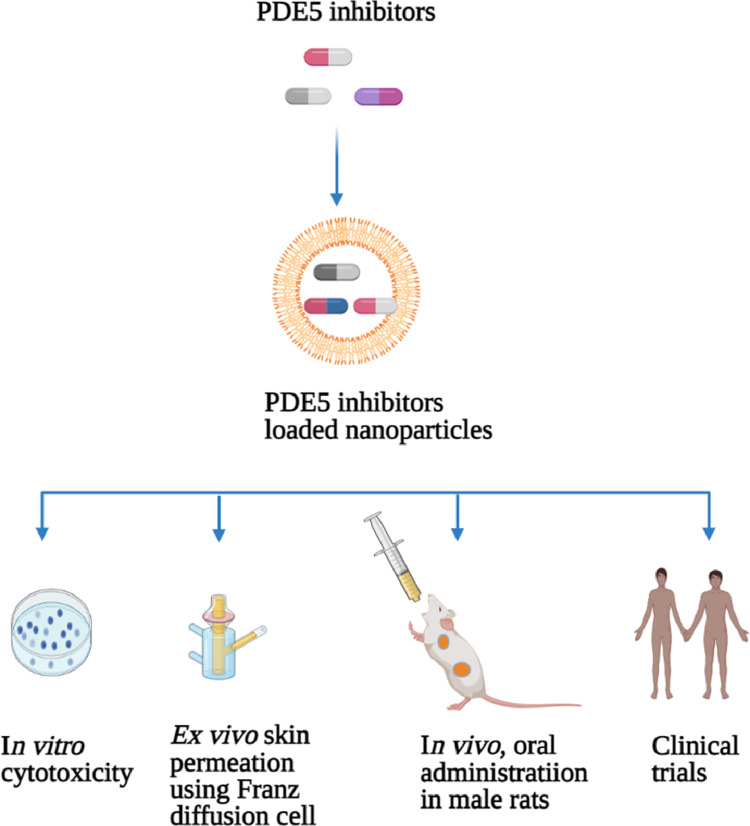
Phosphodiesterase 5 (PDE5) inhibitors are commonly used treatment for ED include avanafil, sildenafil, tadalafil, and vardenafil have been approved for the recuperation and treatment of male ED.2,11 Recently, novel PDE5 inhibitors loaded NPs and various drug delivery systems have been developed. Hosny and Aldawsari23 developed liposomes as multilamellar vesicles composed of cholesterol and more solid phospholipids containing avanafil to optimize the delivery of this drug across the skin (Table 1). Incorporation of avanafil in liposomes improved the avanafil encapsulation efficiency, up to 95.61% EE achieved. The ex vivo skin permeation using the Franz diffusion cell method revealed a significant increase in the absorption with avanafil liposomal suspension (25%) compared to avanafil aqueous suspension (4%). The in vivo pharmacokinetic findings from direct application of the avanafil liposomal and avanafil aqueous suspensions on the shaved patch at the back of male rats showed a significant increase in the bioavailability (7-fold) compared to avanafil aqueous suspension. Avanafil liposome formulation displayed a significantly higher value of Cmax and AUC, longer mean residence time (MRT) (9.2 hours), and Tmax (3.5 hours) compared to aqueous suspension (1, 5 hour, and 2.15 hours, respectively). The authors concluded that the avanafil liposomal transdermal delivery system is promising for ED therapy.23 However, to date there are no clinical trials have confirmed the efficacy and safety of transdermal delivery of avanafil liposomes are available. Therefore, well-designed clinical studies are needed.
Table 1.
Summary of therapeutic agents used for male ED using nanoparticles
| Therapeutic agents | Nanoparticles used | Experiment model | Route and dosage of administration used | Significant findings | Year | References |
|---|---|---|---|---|---|---|
| Avanafil | Liposomes | Male Wistar rats abdominal skin. Male Sprague-Dawley rats |
Franz diffusion cell, 50 mg. Topical 10 mg/kg. |
Liposomes enhanced skin permeation of avanafil compared with avanafil alone. Avanafil liposomal resulted in a sevenfold increase in bioavailability compared to the avanafil group. |
2015 | Hosny and Aldawsari23 |
| Self-nanoemulsifying drug delivery system (SNEDDS) | Male Wistar rats | Oral 10 mg/kg/2 mL. | SNEDDS increased avanafil bioavailability by 1.4-fold compared to the avanafil group. | 2014 | Fahmy et al.24 | |
| Solid lipid nanoparticles (SLNs) hydrogel films (HPMC and chitosan) | Male Wistar rats’ skin | Franz diffusion cells, 150 µg. | Avanafil SLNS HPMC showed a significant increase in skin penetration compared to chitosan film. | 2016 | Kurakula et al25 | |
| Polymeric nanoparticles (NPs) poly (lactic-co-glycolic acid) (PLGA) | Healthy males | Single oral dose 50 mg. | NPs PLGA improved avanafil solubility, bioavailability, and reduced the frequency of administration. | 2020 | Aldawsari et al26 | |
| Sildenafil citrate (SLD) | Solid lipid nanoparticles | Albino male rats | Oral 50 mg/kg. | SLD SLNs showed >1.8-fold increase in bioavailability compared to the SLD group. | 2014 | Hosny and Aljaeid27 |
| Nano-transfersomal films | Wistar male rats Rat abdominal skin |
Oral 10 mg/kg. Franz diffusion cell. |
Nano-transfersomal films improved the oral bioavailability of SLD. Nano-transfersomal films caused a 1.54-fold increase in SLD penetration through the skin compared with the SLD group. |
2016 | Badr-Eldin and Ahmed28 | |
| Tadalafil | Nanoparticles (NPs) | Aging-induced ED Sprague-Dawley rats | Topical 10 mg gel. | Electrostimulation of CN elicited an upsurge in the ICP/BP ratio and a visible penile erection after an hour of tadalafil NPs treatment. | 2010 | Han et al29 |
| Nanostructured lipid carrier (NLC) | Sprague-Dawley rats’ skin. HaCaT cells |
Franz diffusion cells. Cytotoxicity 0.001-10 µM gel |
Tadalafil NLC with ethanol and limonene exhibited increased permeation through the skin compared to the drug alone. Exposure to tadalafil NLC various concentrations were safe and tolerated by cells. |
2015 | Baek et al30 | |
| Alpha-lipoic acid (ALA) Self-nanoemulsion drug delivery system (SNEDD) | Rats’ skin. EA. hy926 |
Franz diffusion cells. Cytotoxicity 0.001-10 mg/mL. |
Tadalafil-ALA showed a significant increase in percutaneous absorption and greater fluorescence intensity compared to the drug alone. Exposure to tadalafil-ALA formulation was safe, and defensive against glucose stress. |
2018 | Fahmy and Aljaeid31 | |
| Vardenafil | Nanoethosomes (NEs) film | Wistar male rats Rat skin. |
Topical 2 mg/kg. Franz diffusion cells. |
Vardenafil NEs films caused a 3.05-fold increase in skin penetration and a 2-fold increase in bioavailability as compared to vardenafil powder film and oral aqueous vardenafil suspension. Vardenafil NEs film showed accelerated diffusion through the skin compared to unprocessed film. |
2015 | Fahmy32 |
| Lipomers | New Zealand albino male rabbits | Oral 5 mg/kg. | Vardenafil lipomers showed a considerable increase in bioavailability than the vardenafil group. | 2018 | Tawfik et al33 | |
| Self-microemulsifying drug delivery system (SMEDDS) | Male Sprague-Dawley rats’ small intestine. Male Sprague-Dawley rats’ Caco2 cell line |
Intestinal drug absorption, 10 mg/kg. Cytotoxicity, 10, 50, 100, and 500 µg/mL. Oral 1.027 mg/kg. |
SMEDDS improved vardenafil penetration through the intestinal epithelium. Exposure to vardenafil SMEDDs was safe and nontoxic. SMEDDS enhanced bioavailability of vardenafil. |
2019 | Parikh and Sawant34 | |
| Zein-alpha-lipoic acid (ALA) nanospheres | Healthy male subjects | Oral 10 mg. | Zein-ALA nanosphere vardenafil subjects showed a .5-fold increase in bioavailability compared to the control group. | 2018 | Ahmed35 | |
| Papaverine hydrochloride (PaHCl) | Lyotropic liquid crystal (LLC) | Synthetic membrane and human epidermis | Franz diffusion cell 2.5, 3, and 4 w/w%. |
Both synthetic membrane and human epidermis revealed the highest diffusion and permeation at 2.5 w/w% PaHCl concentration. | 2018 | Berkó et al43 |
| Transferosomes | ED male patients | Topical 1-2 g gel. | 33% of patients achieved a score 3 and 11% score 4 of erection and no changes in the control group. No serious adverse effects were noticed. |
2015 | Ali et al44 | |
| Sialorphin | Nanoparticles | Aging-induced ED rats (Sprague-Dawley) | Topical 1 mg gel. | Sialorphin NPs gel elicits a spontaneous increase in ICP/BP and a visible spontaneous erection in the absence of CN stimulation. | 2010 | Han et al.29 |
| Nitric oxide (NO) | Nanoparticle gel | Aging-induced ED Sprague-Dawley rats | Topical 10 nMoles gel. | NO-NPs gel initiate an increase in ICP/BP and a spontaneous penile erectile response without CN stimulation. | 2010 | Han et al29 |
| Nanoparticles | Radical prostatectomy-induced ED rats (Sprague Dawley) | Topical 1.49 g /200 µL gel. | NO-NPs gel results in an increase in ICP/BP and spontaneous penile erection. | 2014 | Tar et al49 | |
| Nanoemulsions (NEs) | Male beagle dogs | Topical 800 mM. | NO-NEs improved erection, increased penis vasodilation, and diameter. No clinical signs of methemoglobinemia occurred. |
2018 | Nam et al50 |
A study performed by Fahmy et al24 prepared a self-nanoemulsifying drug delivery system (SNEDDS) for avanafil delivery across the gastrointestinal (GI) tract membrane (Table 1). The in vivo pharmacokinetic study was evaluated using male rats and were given an oral dose of avanafil SNEDDS and avanafil powder in an aqueous. The avanafil-SNEDDS displayed a considerable increase in GI tract bioavailability by 1.4-fold, an increase in maximum avanafil plasma concentration (Cmax) and reduction Cmax corresponding time (Tmax) compared to pure avanafil. Delivery as nanoemulsion formulation improved the onset of action of avanafil previously reached about 30–45 minutes (avanafil powder) and 15 minutes when prepared as avanafil-SNEDDS, enhanced drug half-life by 45% and area under the plasma concentration-time curve (AUC) by 18%. The study concluded that SNEDDS improved avanafil bioavailability.24 However, there limited data confirm the efficacy of oral delivery of avanafil using SNEDDS.
Kurakula et al25 developed solid lipid nanoparticles (SLNs) incorporated in transdermal hydrogel-based films such as hydroxyl methylcellulose (HPMC) and chitosan for transdermal delivery of avanafil (Table 1). The ex vivo skin permeation of hydrogel films was assessed using Franz diffusion cells. Herein, avanafil SLNs loaded in HPMC displayed 65.48 and 47.23% in chitosan after 24 hours confirmed enhanced permeation of avanafil from SLNs HPMC.25 Therefore, more research investigating the therapeutic dose and toxicity of avanafil SLNs hydrogel film is needed.
A clinical study by Aldawsari et al.26 prepared optimized avanafil-biodegradable polymeric NPs (AVA NPs) loaded in poly (lactic-co-glycolic acid) (PLGA) and evaluated its efficacy in male healthy volunteers (Table 1). Twelve (12) healthy male volunteers age between 25 and 45 years were randomized to receive a single oral dose of AVA PLGA NPs formulation and pure AVA. Formulation of AVA as PLGA NPs improved AVA solubility, and bioavailability, and reduced frequency of administration. The results showed a significantly higher maximum AVA plasma concentration (Cmax) (1.3-fold), area under the AVA plasma concentration-time curve (AUC) (1.68-fold), half-life (t1/2) (12.14 hours), and Tmax (1.25 hour) compared to pure AVA. It was concluded that optimization and preparation of AVA as PLGA NPs successfully improved AVA bioavailability.26 However, further clinical trials are required to confirm the long-term efficacy and safety of AVA-NPs PLGA in male patients with ED.
Hosny and Aljaeid27 prepared sildenafil citrate (SLD) encapsulated in SLNs to enhance oral delivery of SLD (Table 1). Albino male rats were used in their study. Oral administration of SLD SLNs resulted in a significant increase in bioavailability (>1.8-fold), a significant increase in maximum plasma concentration (Cmax), an extended corresponding time (Tmax) (1.5 hour), and a higher MRT (17 hours) compared to that of commercially available SLD tablets. The study concluded that SLNs sustained the release of SLD as well as enhancing the bioavailability of SLD. It has the potential to be used as a novel drug delivery system.27 However, to date there are no clinical studies investigated the efficacy and safety of SLD containing SLNs. Thus, to use SLD SLNs, more studies are needed to prevent their toxicity during ED therapy.
Research by Badr-Eldin and Ahmed28 prepared optimized nanotransfersomal films for SLD delivery (Table 1). In an ex vivo study, direct application of SLD nanotransfersomal film (7 mL in phosphate buffer) on the abdominal skin of rats induced a significant increase in drug permeation through the skin (1.54-fold) compared to SLD films. In the in vivo study, oral administration of optimized SLD nanotransfersomal film in male Wistar rats improved the oral bioavailability, showed an increase in drug plasma concentration (Cmax), longer time to reach maximum plasma concentration (Tmax) (15 hours), and a significantly higher area under the curve (AUC). This study concluded that optimization of nano-transfersomal films displayed improved transdermal permeation and oral bioavailability of SLD compared with SLD films.28 To date, there are no published clinical trials to substantiate the efficacy and safety of SLD nano-transfersomal films.
Han et al29 developed a gel containing tadalafil with NPs and it was evaluated in a rat model of ED caused by aging (Table 1). Tadalafil nanoparticle gel was applied on the penis glans and shaft of the anesthetized male rats (>650 g). The intracavernous pressure/blood pressure (ICP/BP) ratio demonstrated increased after electrostimulation of the cavernous nerve (4 mA), the average peak of ICP/BP was 0.737 ± 0.029. A visible erection was observed an hour after treatment. The authors concluded that nanoparticle gel is promising for transdermal delivery of erectogenic agents.29 To date, no randomized clinical studies have assessed the efficacy and safety of tadalafil nanoparticle gel.
A study conducted by Baek et al30 also prepared tadalafil loaded nanostructured lipid carrier (NLC) as a gel for the transdermal delivery of tadalafil (Table 1). The in vitro skin permeation study was carried out using skin cut from the dorsal shaved patch on rats and confirmed by Franz diffusion cells. Ethanol and ethanol with limonene were used as permeation enhancers. The tadalafil-NLC dispersion with ethanol and limonene enhancers showed higher permeation (∼ 4.8-fold) than tadalafil alone. The cytotoxicity of tadalafil loaded NLC was assessed on keratinocyte cell lines (HaCaT cells) using MTT dye for 72 hours. The results confirmed that all tadalafil formulations were nontoxic to HaCaT cells, all exhibited >50% cell viability. This study concluded that NLC plus ethanol and limonene as skin permeation enhancers are promising for transdermal delivery of tadalafil.30 At present, there are no clinical studies confirmed the efficacy and safety of tadalafil-NLC have been found.
Fahmy and Aljaeid31 developed tadalafil-loaded Alpha-lipoic acid (ALA) in the form of a self-nanoemulsion drug delivery system (SNEDD) as a transdermal patch to improve the percutaneous absorption of tadalafil (TFL) for the treatment of ED in diabetic patients to prevent the penile cavernous endothelium from oxidative damage attribute to high glucose level (Table 1). The solubility of TFL ALA was studied, TFL and ALA were mixed with each of these, anise oil, Tween 20, polyethylene glycol (PEG 200). Anise oil and Tween 20 displayed the highest solubility for both TFL and ALA. The in vitro release study was evaluated by Franz diffusion cell and fluorescent (Rhodamine B dye) imaging. The results exhibited a higher percentage of TFL release from the TFL-ALA patch (80%) compared to plain (TFL) film (20%). TFL-ALA displayed greater fluorescence intensity after an hour confirmed the enhanced TFL percutaneous absorption in the rat skin layers. Finally, the protective activity and cytotoxicity of TFL- ALA SNEDDS on endothelial cells (EA. hy926) under glucose stress for 72 h were assessed through MTT assay, the results showed 88.54% cell viability confirmed no-toxic and protective effect on endothelial cells. The study concluded that TFL-ALA SNEDDS as a transdermal patch could be used in the alleviation of ED in patients with diabetes.31 However, there are no published clinical trials that validated the use of TFL-ALA SNEDDS in the treatment of ED in diabetic patients, therefore more studies are needed.
In another study by Fahmy32 developed vardenafil-incorporated nanoethosomes (VRD-NE) film for delivery of vardenafil across the skin (Table 1). The ex vivo skin permeation results using Franz diffusion cell showed fast diffusion with VRD (∼30%) from NE film compared to VRD (4%) from the unprocessed film after an hour. The pharmacokinetic investigations, topical application of VRD-NE film on the shaved patch at the back of male Wistar rats showed a significant increase in penetration (3.05-fold) compared with that of VRD powder film. In comparison with oral administration of aqueous suspension of VRD (2 mg/kg), the application of VRD NE film revealed a 2-fold increase in bioavailability, extended half-life (t1/2) (14 hours), and higher MRT (17 hours), reduction in corresponding time (Tmax) (2 hours), and a significant increase in AUC. These confirmed that enhanced drug deposition occurred through the skin. The results were concluded that a low dosage of VRD incorporated in NE film can be used in the treatment of ED and used over for a prolonged period, enhance patient compliance and minimize the side effects.32 Currently, there are no clinical trials to substantiate the use of VRD-NE films for the treatment of male ED.
Tawfik et al33 have developed vardenafil hydrochloride incorporated lipomers for oral delivery of vardenafil (Table 1). The in vivo pharmacokinetic results from oral treatment with vardenafil lipomers in male albino rabbits demonstrated a significantly higher bioavailability (170%), reduction in Cmax, delayed Tmax (2 hours), and extended MRT (11.37 hours) compared to an aqueous suspension of powdered Levitra or vardenafil tablets. They concluded that lipomers displayed efficient delivery of vardenafil compared with pure drugs.33 However, there are no clinical data to support this finding; therefore, more studies are needed to confirm the efficacy as well as safety.
Parikh and Sawant34 developed a self-microemulsifying drug delivery system (SMEDDS) using Cremophor EL and Tween 20 as surfactants for the delivery of vardenafil (VDN) hydrochloride (HCl) trihydrate (Table 1). The ex vivo permeation study was assessed using the intestinal gut method. Filled up of intestine tissue with VDN SMEDDS showed rapid absorption through the intestinal epithelium than VDN suspension. The Caco2 cell line (Homo sapiens colon colorectal cancer cells) and MTT dye were used to confirm the in vitro cytotoxicity of VDN SMEDDS. The exposure of the Caco2 cell line to increasing doses of VDN SMEDDS displayed nontoxicity, cell viability above 80%. The in vivo study was assessed via crypto sectioning of the intestinal tissue isolated from a rat after an oral dose of VDN SMEDDS and fluorescent imaging exhibited enhanced drug permeation for Tween 20 than Cremophor EL. The in vivo pharmacokinetics, oral administration of VDN SMEDDS showed higher Cmax, longer t1/2 (3 hours), and higher AUC. The authors concluded that SMEDDS can be used to enhance oral drug bioavailability as well as their therapeutic efficiencies.34 However, further clinical studies investigating the efficacy and safety of VDN SMEDDS are required.
Ahmed35 performed a clinical study. The author developed zein and alpha-lipoic acid (ALA) nanospheres incorporated vardenafil (VRD) using liquid-liquid phase separation and evaluated their efficacy in 20 healthy Egyptian male subjects aged ranges between 25 and 43 years (Table 1). Patients were randomized into 2 groups to receive an oral dose of zein-ALA nanosphere VRD formulation and marketed VRD tablets. Oral administration of zein-ALA nanosphere VRD formulation enhanced the oral bioavailability (2.5-fold), showed higher Cmax, delayed Tmax (2 hours), a significantly higher AUC, longer t1/2 (9 hours), and MRT (12 hours) compared to pure VRD tablets. The author concluded that zein-ALA encapsulated VRD could be useful in the alleviation of ED.35 However, further well-designed large-scale randomized clinical studies are needed to evaluate the long-term efficacy and safety in men with ED. Figure 1 provides an overview of the methods used to improve phosphodiesterase 5 inhibitors.
Figure 1.
Overview of the methods used to improve phosphodiesterase 5 inhibitors. PDE5 = Phosphodiesterase 5. Figure 1 was created with www.biorender.com.
Papaverine Hydrochloride NPs
Papaverine hydrochloride is a benzylisoquinoline alkaloid isolated from Papaver somniferum (opium poppy). It is a vasodilator drug that has been used clinically for the treatment of impotence. It has been used as an option for patients with poor response to oral PDE5 inhibitors.11,36,37 It is intracavernous self-injection therapy. Papaverine not FDA approved for intracavernous injection; but, it continues to be used in the alleviation of ED.11,37 The mechanism of action, papaverine increases the production of cAMP and cGMP by activation of intracellular adenyl cyclase, leading to decreased levels of calcium, thereby resulting in relaxation of cavernous smooth muscle, and penile erection.11,38
Papaverine has been used as a monotherapy, their therapeutic efficacy and safety have been evaluated in a study conducted with 135 men aged between 27 and 69 years with ED who received ICI papaverine treatment, 91 (67.4%) patients reported satisfactory and 44 (32.6%) discontinued using treatment due to several AEs. The most common AEs have been reported including abnormalities in liver enzymes in 4 (3%) men, urethral bleeding in 3 (2.2%), penile hematoma in 37 (27.4%) men, prolonged erections by 15 (11.1%), and penile nodes in 15 (11.1%) men.39
As a combination therapy, papaverine has been used with other vasoactive agents such as phentolamine, prostaglandin E1 plus phentolamine, or atropine to improve its efficacy.38,40 The efficacy and safety of drug combination therapy are evaluated in the comparative studies. A study by Shenfeld et al41 compared the efficacy of a mixture of papaverine + phentolamine (2-drug mixtures) and papaverine + phentolamine + prostaglandin E1 (3-drug mixtures) in 20 patients with ED. The treatment with combinations significantly improved erection, 73% of patients (3-drug combinations) and 28% patients (2-drug combinations) showed penile erection hard enough for penetration. The side effects include prolonged penile erection in 1 patient injected with 2-drug combinations and 2 patients who received 3-drug combinations. Severe pain was observed in 1 patient injected 3-drug combinations.41 In another study engaged 60 male patients with ED who were administered papaverine + phentolamine (2-drug mixture) and prostaglandin E1 (alone) showed that a 2-drug combination is more effective than prostaglandin E alone. Of the patients injected, 54% (2-drug combinations) and 50% (prostaglandin E1) had a full rigid penile erection. The most common side effects reported include prolonged penile erection (18 and 15%, respectively), and pain (12 and 35%, respectively).42
Owing to side effects associated with ICI of papaverine has been ceased in clinics for the treatment.43 Recently, topical application of papaverine was introduced as a noninvasive therapy option to replace the papaverine ICIs. Papaverine gel and or formulations have been developed, were combined with NPs to enhance the absorption of papaverine (Figure 2).43,44 Berkó et al43 developed and evaluated the efficiency of topical application of papaverine (PaHCl) incorporated in lyotropic liquid crystal (LLC) formulation as a delivery system (Table 1). The ex vivo drug permeation through the skin using the Franz diffusion cell method and in vivo drug penetration through the excised human epidermis was studied. The treatment with PaHCl-LLC formulation induced better permeation through the skin at the lower concentration (2.5%) compared with other concentrations. The permeation of PaHCl-LLC through the skin decreases as the concentration increases. The results of in vitro synthetic membrane and ex vivo human epidermis skin layer demonstrated similar permeation. The study concluded that PaHCl LLC formulation may be effective and safe, hence could be used as an alternative for injectable formulations.43 Therefore, future clinical trials that investigate the efficacy and safety of PaHCl LLC formulation will be helpful in the development of new noninvasive treatments for ED with fewer or no side effects.
Figure 2.
Relaxation of corpora cavernosa by topical application of papaverine hydrochloride nanoparticles gel. NPs = nanoparticles. Figure 2 was created with www.biorender.com.
Ali et al44 conducted a clinical research to investigate the efficacy and safety of topical application of transfersomal gel as a delivery system for papaverine hydrochloride across the penile skin, scrotum, and perineum in 9 male patients aged ranges from 32–60 years with a 1-month history of ED or organic ED (Table 1). They were grouped to receive topical application of transfersomal papaverine hydrochloride (TPH) gel on the penis, scrotum, and perineum on day 1, free PH gel on day 4, and gel without PH (placebo) at day 8. Color flow Doppler ultrasound was used to assess the blood flow. Penile erection was evaluated using score 1 to 5: no effect (i); tumescence without rigidity (ii); tumescence with 70 degrees or less of erection (iii); tumescence with 70–90 degrees of erection (iv); and tumescence with more than 90 degrees of erection (v). For safety profile, patients were checked for signs of AEs such as skin irritation, rash on the face, dizziness, erythema, facial flushing, dizziness, as well as pain was gel applied. Topical application of TPH gel improved erectile function, 3 (33%) patients had penile tumescence with 70 degrees, and 1 (11%) patient had penile tumescence with 70–90 degrees. Five (5%) patients showed no changes of erection in response to the application of placebo. It was concluded that TPH gel can be used in the diagnosis and attenuation of male ED.44 However, to use TPH gel for medical care, large-scale randomized clinical trials are required to confirm its effectiveness and safety before approved to treat men with ED.
Sialorphin NPs
Sialorphin is also known as SMR1-pentapeptide is the final matured peptide derived from submandibular rat1 protein (SMR1).45 The peptide is produced and released into the blood circulation in response to androgen steroid and environmental stress. It is also found in the saliva.45,46 Sialorphin is encoded by variance coding sequence a 1 (Vcsa1) gene also called submandibular rat1 gene. The expression of the Vcsa1 has been reported to be higher in the prostate gland, submandibular gland, and corpora cavernosa of adult rats.45, 46, 47 It was reported that a decrease in the expression of Vcsa1 in the corpora cavernosa is associated with ED. Based on this, the downregulated Vcsa1 is often used in rat models of ED.46,47 Davies et al47 evaluated the efficiency of sialorphin in the recovery of erectile function in aging rats by relaxation of corporal smooth muscles. Intracorporal injection of sialorphin (100 µg) into retired breeder male Sprague-Dawley rats (>500 g) significantly increased the ICP/BP ratio (∼6.0) when measured from 55 to 65 minutes after injection. After contraction of corporal smooth muscle tissue by phenylephrine, treatment with sialorphin (1 µg/mL) increased the relaxation (2.5-fold) of corporal smooth muscle tissue by activation of C-type natriuretic peptide. The results were concluded that sialorphin enhances erectile function by relaxation of corporal smooth muscle tissue. Tong et al46 proposed that the Vcsa1 gene regulates male rat erectile function by acting on G-protein coupled receptor (GCPR). In corporal smooth muscle, inhibition of neutral endopeptidase by sialorphin results in decreased GCPR expression. In contrast, suppression of Vcsa1 results in activation of GCPR.46 Moreover, the findings of Messaoudi et al48 suggested that sialorphin control male rat sexual behavior by regulating the balance of excitation and inhibition mechanism.
Recently, a study conducted by Han et al29 developed novel sialorphin-containing nanoparticle gel and investigated its efficacy in a rat model of ED induced by aging (Table 1). In their study, sialorphin nanoparticle gel was applied directly on the anesthetized male rats (>650 g) penis glans and shaft. The application of gel initiated a spontaneous increase in ICP/blood pressure (BP) in the absence of electrostimulation of the cavernous nerve, and the authors suggested that erectile response was induced by sialorphin nanoparticle gel. The average peak of the ICP/BP ratio was 0.72 ± 0.13. A visible spontaneous erection was observed at an average of 4.5 minutes, with a duration of action of persisted for 8 minutes, and ICP/BP of 0.6 after administration of gel.29 Considering the results, sialorphin nanoparticle gel is promising for the treatment of ED. However, there have been no clinical data found to substantiate the use of sialorphin for sexual dysfunction.
NO-Loaded NPs
NO is a free radical and effective vasodilator produced from endothelium. It plays an essential function in penile erection. The release of NO stimulates a pathway leading to relaxation of the corpus cavernosum and subsequent erection.2,14 Han et al29 developed a gel containing NO with NPs and evaluated the efficacy of this gel in a rat model of ED caused by aging (Table 1). They applied a gel on the penile shaft and glans of the 7 anesthetized male rats (>650 g). The ICP/blood pressure (BP) spontaneously increased in the absence of electrostimulation of cavernous nerve in 5 rats, and the authors suggested that erectile response was induced by NO nanoparticle gel. The first visible erection was observed at an average of 4.5 minutes (~ 5 minute) after the application of gel, which persisted for a duration of <2 minutes, the average was 1.42 minutes. The average peak ICP/BP of the first erection was 0.67 ± 0.14. Other several erections were observed showed diminishing.
Tar et al.49 prepared NO-NPs in dimethylsulfoxide (DMSO) gel or coconut oil and investigated the effects of NO-NPs on ED in a rat model of radical prostatectomy (Table 1). Male rats underwent bilateral transection of the cavernous nerve. After one week, they applied the NO-NPs on the penile shaft of rats. The ICP/BP significantly increased in 8 of 10 rats were applied NO-NPs in DMSO gel, from the average peak of 0.182 ± 0.03 to 0.317 ± 0.12 after treatment. The spontaneous erections were observed in 6 of 10 rats with a duration of 1 minute and the average peak ICP/BP ratio was >0.6. The time to onset of spontaneous erections was observed at the average of 15 ± 11 after treatment. Similar findings were observed in rats treated with coconut oil. NO without NPs treated rats did not produce spontaneous erections. The study suggested that NO-NPs should be used in the recovery of penile erectile function after radical prostatectomy.49 However, there have been no published clinical studies found to confirm the efficiency. Shortly, we should conduct clinical research that evaluates the efficacy and safety of this novel NO nanoparticle gel for the mitigation of male ED.
In another study by Nam et al50 prepared NO loaded water-in-oil (w/o) nanoemulsions (NEs) and examined the safety and efficacy of topical application of NO-NEs on the penile skin of anesthetized and unanaesthetised 4 male dogs aged 7 and 8 years old (∼ middle-aged male) (Table 1). The diameter of the penis, redness of skin, blood NOx concentration in the penis was used to assess the penile erection. Topical application of NO-NEs on penis skin showed increased diameter by 15% compared with diameter before applications. The redness of the skin corresponds with the dilation of the blood vessels on the mucous membrane. Application of NO-NEs increased the degree of redness (35%) than NE without NO (12%). Blood NOx level collected from the penile corpus cavernosum, and jugular vein was 3-fold higher than after application with NE without NO. However, NOx concentration was lower than blood nitrate level cause methemoglobinemia. Methemoglobinemia is a condition characterized by elevated methemoglobin in the blood. It is one of the common side effects of nitric oxide. It prevents the binding of oxygen to hemoglobin. The clinical signs include dyspnea, penile cyanosis, tachycardia, and bradycardia. Application of NEs with NO to dogs did not show clinical signs of methemoglobinemia. The proposed mechanism of action topically applied NO-NEs absorbed through the penile skin into the CC. Then NO is released from the NO and activates the guanyl cyclase-cGMP pathway leading to penile erection. The study concluded that NO-NEs could be used as a topical drug delivery system for the treatment of ED patients and those who have poor drug reactions to phosphodiesterase 5 inhibitors.50 This study is promising, but there is limited evidence on its use for the treatment of ED in humans. However, more clinical research is needed to evaluate the therapeutic efficacy and safety before is recommended for humans.
Adipose Tissue-Derived Stem Cells-NPs
The use of adipose tissue-derived stem cells (ADSCs) has been promising treatment of ED for tissue engineering and as a regenerative medicine.51, 52, 53 Numerous preclinical studies have been published, evaluated the use of ADSCs in the treatment of ED in animal models of cavernous nerve (CN) injury and diabetes. In these studies, the intracavernous injection or injection of ADSCs into the corpus cavernosum (CC) revealed a significant improvement in erectile functions for a period of several days or weeks after injections compared to untreated controls. The ICP/BP ratio was used to assess the erectile function, increased in ICP/BP in response to electrostimulation of the corpus nerve (CN) confirmed the enhanced erectile function (after 4 weeks). The injections with ADSCs resulted in increased smooth muscle content in the penis, decreased apoptosis, and prevention of CC fibrosis. Cell tracking with fluorescent dyes was used to monitor cell movement and locations. The results revealed a small number of cells that remained in the CC after injection, confirmed that cells are carried by systemic blood to other parts of the body, for instance, bone marrow and major pelvic ganglia. The mechanism of action is not clear; however, it was proposed that the recovery of erectile function might be due to paracrine factors such as growth factors and cytokines secreted by cells stimulated repair in injured CC.53,54 Also, clinical studies involved a small number of patients have been published to affirm the efficacy and safety of ADSCs treatment. They revealed a positive effect of ADSCs treatment on ED patients. Patients who received ADSCs therapy demonstrated improved erectile function and maintained this for several months (maximum 12 months) without serious AEs.10,18,55, 56, 57
Recently, interesting studies have explored the usage of NPs to retain ADSCs in the CC, prevent them from migrating or carried by the blood to other body parts after injection. Kim et al52 prepared human ADSCs (hADSC) with nerve growth factor-incorporated hyaluronic acid-based hydrogel (NGF-hydrogel) and assessed its effects of their direct administration in a rat model of bilateral CN crush injury (Table 2). Administration of the PKH26-labeled hADSCs and NGF-hydrogel on the injured CN showed enhanced regeneration of CN after 4 weeks. The treatment demonstrated increased ICP/mean arterial pressure (MAP) ratio in ADSCs, NGF-hydrogel, and hADSCs NGF-hydrogel groups compared with control, these confirmed improved erectile function. Also, the hADSCs were discovered around the CN surgery after 4 weeks. The study did not discuss the mechanism of actions underlying CN regeneration; however, it was proposed that recovery is due to paracrine effects of cytokines and growth factors. The study concluded that the use of hADSCs with NGF-hydrogel is promising for the rehabilitation of erectile function after radical prostatectomy surgery.52 Yet, no clinical trials have been found.
Table 2.
Summary of regenerative therapy for male ED using nanoparticles
| Therapy | Nanoparticles used | Experiment model | Route and dosage of administration used | Significant findings | Year | References |
|---|---|---|---|---|---|---|
| Human ADSC (hADSC) | Hyaluronic acid-based hydrogel | CN crush injury-induced ED rats (Sprague-Dawley) | CN injury administration 1 × 106 cells |
Enhance regeneration of the CN after 4 wks. ADSCs was observed around the CN 4 weeks after treatment. Increase ICP/BP ratio and improvement of erectile response. |
2013 | Kim et al52 |
| ADSCs | NanoShuttle magnetic nanoparticles | Sprague Dawley male rats | CC injection 1 × 106 cells |
ADSCs were retained in the CC for up to 3 days compared to the control group. Enhance ICP/BP ratio and improve penile erectile response. |
2016 | Lin et al58 |
| ADSCs | Superparamagnetic iron oxide nanoparticles | Diabetes-induced ED rats (Sprague-Dawley) | Intracavernosal injection 1 × 106 cells |
Promote proliferation of ADSCs and retaining in the CC after injection. Increased ICP/BP ratio and improved erectile response. |
2016 | Zhu et al59 |
| ADSCs | NanoShuttle | Male Sprague-Dawley rats | Intracavernosal injection 2 × 105 cells |
Enhance mean ICP and ICP/MAP ratio and total ICP. Retain ADSCs in the CC. |
2018 | Wu et al60 |
| Sonic Hedgehog |
Peptide amphiphiles nanofiber hydrogel | Male Sprague-Dawley rats | CN injury treatment 4.54 µg |
Prevent apoptosis, neurodegenerative and also inspires regeneration of CN. | 2017 | Choe et al 12 |
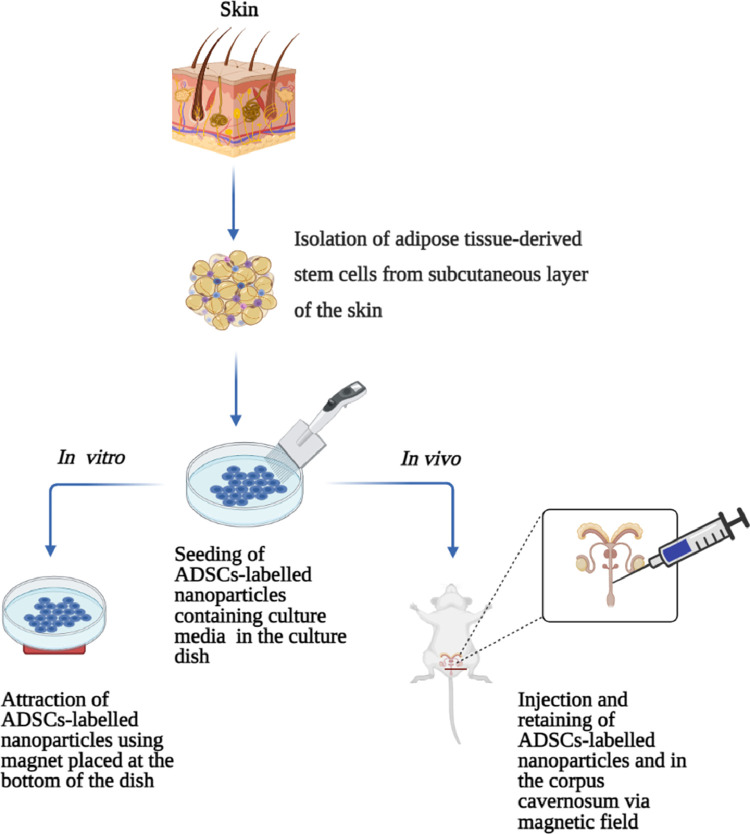
Lin et al58 used NanoShuttle magnetic NPs to retain stem cells in the CC after intracavernous injection in rats (Table 2). The in vitro study, ADSCs were cultured with a medium containing NanoShuttle for 24 h and labelled with CellTracker green CMFDA for 30 minutes. The magnet was placed under the cell culture dish. The NanoADSCs shifted toward the magnet. In the in vivo study, rats underwent bilateral CN crush. After, labeled ADSCs, and NanoADSCs were injected in the CC. Then magnet was placed outside under the injected CC site of the NanoADSCs group for 6 hours (Figure 3). The results exhibited NanoADSCs moved toward the magnetic field. The cells were retained in the CC for up to 3 days compared to ADSCs without a magnetic field. The electrostimulation of CN showed a significantly higher ICP/MAP ratio in NanoADSCs treated group, showed improvement in erectile function. The study concluded that NanoShuttle magnetic NPs can retain ADSCs in the CC and recover the erectile function in male rats.58 However, there are no clinical studies available to support these findings.
Figure 3.
Potential treatment of cavernous nerve-induced male erectile dysfunction with magnetized adipose tissue-derived stem cells by retaining in the corpus cavernosum. ADSCs = adipose tissue derived stem cells. Figure 3 was created with www.biorender.com.
Another similar research conducted by Zhu et al,59 the authors labelled ADSCs with superparamagnetic iron oxide nanoparticles (SPION) and examine their effects on the erectile function in a rat model of streptozotocin-induced diabetes using the external magnetic field. (Table 2) The in vitro study, ADSCs-labelled SPION containing culture medium were seeded in a dish, and the magnet was placed at the bottom of the dish for 24 hours. The SPION-labeled ADSCs more toward the magnet. In the in vivo study, rats underwent bilateral CN crush, then ADSCs and ADSCs-labeled SPION were injected in the CC. The magnet was placed outside underneath the injected CC site of the ADSCs-labeled SPION group for 30 minutes (Figure 3). The results showed a significantly increased ADSCs-labeled SPION in the CC, suggesting that cells were retained in the CC by magnetic force compared to the ADSCs group without the magnet underneath. ADSCs were identified in the CC of rats 4 weeks after ICI. The measurement of erectile function, ADSCs-labeled SPION group showed a significantly higher ICP/MAP ratio than ADSCs group, confirmed greater improvement in erectile function. The mechanism of action, ADSCs paracrine factors might be responsible for the functional and structural recovery of CN. The study concluded that NanoADSCs plus magnetic field therapy is an effective approach for the treatment of diabetes-induced ED.59 However, there are no published clinical trials have been conducted to validate this finding.
The study by Wu et al60 further determined the possible small concentration of ADSCs for intracavernous injection could be used to improve ED and simultaneously prevent the likelihood of developing cancer (Table 2). Intracavernous injection of NanoShuttle-ADSCs in phosphate buffer improved the erectile function. Electrostimulation of CC was used to determine the efficacy after 28 days. The stimulation induced a significant increase in mean ICP and ICP/MAP ratio, and total ICP compared to ADSCs and corpus cavernous nerve injury groups. The study also revealed that more Nano-ADSCs were retained in the CC by a magnet from day 0 to 3 than other groups and the number of cells declined at day 7. It has been concluded that nanotechnology can be used to optimize the efficacy of a small dose of ADSCs to alleviate ED. They suggest that the mechanism of erectile function may be involved the revitalization of the smooth muscle, nerve as well as endothelium tissues.60
Based on the findings, ADSCs can be effective in the treatment of damaged CN-induced ED. NPs and magnetic fields can help to retain them in the CC. Yet, there is limited data on clinical trials. Therefore, further well-designed studies are needed to evaluate their long-term efficacy and safety in ED patients.15 Besides, we still need a very effective and permanent way to retain ADSCs in the CC, promote regeneration, and cure ED.
Sonic Hedgehog NPs
Sonic hedgehog (SHH) is a protein expressed in the pelvic ganglia (PG) neurons and Schwann cells of the cavernous nerve (CN). It plays a significant role in the regeneration of CN injury and the prevention of ED. Increased levels of SHH in the PG and Schwann cells inhibit the degeneration and apoptosis of penile smooth muscles. Depletion of this protein has been found associated with CN injury.12,61 Choe and co-workers12 prepared a peptide amphiphiles (PA) nanofiber hydrogel for delivery of SHH protein and evaluated its effects in a rat model of CN crush injury. (Table 2) Male rats underwent bilateral CN crush injury and CN was treated with SHH PA hydrogel. SHH PA treatment resulted in retrograde transport of SHH to the periglomerular (PG) neurons and prevented PG neurons from apoptosis. The study concluded that SHH treatment by PA prevents neurodegenerative and also encourages regeneration of CN through sustaining normal communication between PG neurons and satellite glial cells.12 So far, there are no clinical trials have been found assessing the efficacy and safety of SHH PA-gel in the treatment of ED. Therefore, more studies are needed to evaluate the treatment of SHH in ED patients.
Herbal Medicine NPs
Herbal medicine are used worldwide to maintain good health, to prevent and treat myriad diseases.62, 63, 64 Plant extracts are the principal sources of ingredients in the pharmaceutical industry.64,65 They are natural, superabundant, sufficient, easy harvest, cost-effective, and associated with negligible or no adverse events.2,64,66 Plants embrace several diverse phytochemicals such as polyphenols, terpenoids, alkaloids, and steroids. Such are found largely in fruits, vegetables, seeds, teas, and nuts.2,67,68 Nanotechnology has been used to enhance the efficacy of herbal medicine and or their phytochemicals.69 NPs deliver herbal medicine to the specific targeted site in a controlled manner and reduce the concentration of plant extracts.19,69 Encapsulation of medicinal plant extracts and bioactive compounds in NPs is more effective than their pure counterparts.70, 71, 72 A few studies have been published that investigate the use of NPs encapsulated plant extracts for the mitigation and treatment of male ED.
Curcumin
Curcumin (CURC) or diferuloylmethane is a bioactive compound produced by Curcuma longa.2,70,73,74 Curcuma longa is commonly called turmeric is a member of the Zingiberaceae family. The plant has been used medicinally to treat dermatitis, diabetes, acquired immune deficiency, and erectile dysfunction.2,70,75,76 Curcumin is the well-known active ingredient for its pharmacological activity on the penile erection, it has anti-ED action.2,76 The therapeutic effects of curcumin are limited by poor aqueous solubility, low bioavailability, and fat metabolism.70,73,74,77 To improve the efficiency of curcumin, water-soluble curcumin derivatives have been developed.76,78 Abdel Aziz et.al76 investigated a novel water-soluble curcumin derivative. In their study, oral administration of synthesized water long-acting CURC derivative (10 mg/kg) resulted in a significant improvement in the activity of cavernous tissue heme oxygenase enzyme-1 (HO- 1) and cGMP, and prolonged duration of action compared to sildenafil (4 mg/kg), pure CURC (10 mg/kg), and water-soluble long-acting CURC derivative (2 mg/kg). Intake of water-soluble curcumin derivatives resulted in improvement of erectile function was confirmed by a significant increase in ICP. Another research by Abdel Aziz et al78 compared the efficacy of novel curcumin derivative (NCD) with that of natural curcumin (CURC) and tadalafil (Cialis) in an animal diabetic model of ED. The study involved 60 adult male albino rats, of which 10 were used for control and 50 were diabetic rats caused by a single intraperitoneal injection of streptozotocin (65 mg/kg body weight). Rats were randomized to receive oral intake of CURC (10 mg/kg body weight), NCD (2 mg/kg body weight), tadalafil in distilled water (1.6 mg/kg ~ 20 mg dose in adult), and NCD plus tadalafil. Heme oxygenase-1 (HO-1) enzyme activity, NOS activity, cGMP, and ICP/MAP were used to assess the efficacy of the treatment. All therapies resulted in a significant increase in HO-1 activity, NOS activity, cGMP, and ICP/MAP. The proposed mechanism of action, HO-1 enzyme catalyzed the generation of carbon monoxide resulted in erectile function. NOS stimulated the production of endothelium NO resulted in an increase in cGMP levels and subsequent erectile function. The erectile function was confirmed by electrical stimulation of CN and the results showed an increase in ICP/MAP. The study concluded that novel curcumin derivative is more effective than pure curcumin and tadalafil.78 However, there are no published clinical studies to support the efficacy and safety of curcumin derivatives in the mitigation of ED. Therefore, clinical trials are essential in the development of effective and safe medication.
Recently, research conducted by Draganski et al79 developed a novel curcumin-loaded nanoparticles (CURC-np) and investigated its potential treatment of the topical application in the rat model of diabetes-induced ED (Figure 4 and Table 3). Direct application of CURC-np mixed with coconut oil on the male rats’ abdomen skin resulted in a significantly improved erectile response after 24 hrs of administration on the skin as compared to pure curcumin. ICP and systemic blood pressure (BP) ratio from electrostimulation of the corpus nerve were used to assess erectile function. The results showed a significant increase in ICP/BP confirmed enhanced erectile function. The study concluded that direct application of CURC-np could be used for the treatment of ED in type 2 diabetics. The study suggests that more research should be done to optimize protocols and determine the toxicity of curcumin-loaded NPs.79 The topical application of curcumin NPs emerges to have the great potential to be produced as a natural noninvasive medicine for ED treatment. However, there are no clinical data. Therefore, clinical trials confirming its efficacy and safety for the treatment of male impotence are needed.
Figure 4.
Potential treatment of male erectile dysfunction with medicinal plant extracts containing nanoparticles LH = luteinizing hormone; FSH = follicle-stimulating hormone; SOD = superoxide dismutase; CAT = catalase. Figure 4 was created with www.biorender.com.
Table 3.
Summary of medicinal plants and phytochemicals used to treat male ED using nanoparticles
| Plant or compound name | Nanoparticles used | Experiment model | Route and dosage of administration | Significant findings | Year | References |
|---|---|---|---|---|---|---|
| Curcumin or diferuloylmethane | Nanoparticles | Diabetes-induced ED rats (Zucker diabetic fatty male rats) | Topical 4 mg. | Enhance ICP/BP ratio and improvement of erectile function. | 2018 | Draganski et al79 |
| Myricitrin or myricetin-3-0-α-rhamnoside |
Solid lipid nanoparticles | Diabetes-induced ED mice (Naval Medical Research male mice) | Intraperitoneal injection 1, 3, and 10 mg/kg. |
Upsurge in plasma levels of SOD, CAT, and total antioxidant activity. Upsurge in levels of plasma FSH, LH, and testosterone, as well as spermatogenesis. Prevents oxidative stress, reduction of antioxidant activity, diabetes-induced ED. |
2019 | Oroojan et al71 |
| Panax ginseng | Poly-lactic-co-glycolytic acid (PLGA) nanoparticles | Albino male rats | Intragastric administration 4, 10, 20 mg/kg. |
Rejuvenation of pituitary gland and improvement of LH, FSH, and testosterone secretion. | 2015 | Linjawi86 |
Myricitrin
Myricitrin (myricetin-3-0-α-rhamnoside) is a flavanol glycoside isolated from Myrica rubra. Myricitrin possesses biological activities such as antioxidant, anti-inflammatory, and antifibrotic activities.71,80 It was reported that the bioavailability of myricitrin is low and NPs can improve the efficacy of myricitrin.71 A study by Oroojan et al71 developed a novel myricitrin-loaded SLNs to enhance the delivery of myricitrin and evaluated the effects of myricitrin-loaded SLNs versus pure myricitrin on reproductive system disorders caused by diabetes in male mouse (Figure 4 and Table 3). A single dose of nicotinamide and streptozotocin was injected intraperitoneally to male mice for induction of type 2 diabetes mellitus. Intraperitoneal injection of myricitrin and myricitrin SLNs showed an increase in plasma levels of superoxide dismutase, catalase, and total antioxidant activity compared with the control group. Administration caused an increase in the follicle-stimulating hormone, luteinizing hormone, testosterone, as well as spermatogenesis. In conclusion, the study proposed that oxidative stress and reduction in antioxidant activity are major causes of type 2 diabetes-inducing reproductive disorder,2,71 hence oral intake of myricitrin and myricitrin SLNs can prevent the ED. However, this was more significant with solid nanoparticle myricitrin.71 There are no clinical data has been found. Therefore, more clinical trials are needed to further investigate the efficacy and safety of myricitrin SLNs for the treatment of ED.
Panax Ginseng
Panax ginseng belongs to the Araliaceae family. The plant has been used in the treatment of diabetes, neurological disorders, and as an aphrodisiac for erectile dysfunction.2,81, 82, 83 Ginsenosides are a class of triterpenoid saponins isolated from P. ginseng.82,83 Ginsenosides including steroidal saponins, protopanaxatriols, and protopanaxadiols are believed to be responsible for the healing properties of P. ginseng.81, 82, 83 It has been reported that the absorption rate of ginseng saponins is low when given orally because of their poor membrane permeability and extensive metabolism in the gastrointestinal tract.84 Also, the use of different methods to process ginseng can change the rate and extent of absorption of ginsenosides.85 A study by Linjawi86 revealed that ginseng containing NPs were more efficacious than natural ginseng (Table 3). The authors prepared a novel Panax ginseng loaded PLGA NPs and evaluated their protective effect for the treatment of reproductive disorders induced by nicotine. The intragastric administration of nicotine (1.5 mg/kg) for 4 weeks in adult albino male rats resulted in reduced secretion of serum-free luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone that vital for erectile function.2,86 Also, the exposure to nicotine caused a decrease in the expression of cytochrome P19 genes in the testis, and LH and FSH genes in the pituitary glands encoding these genes. However, the intragastric treatment of male rats with nicotine (1.5 mg/kg) plus various concentrations of P. ginsengNPs (4, 10, 20 mg/kg) for 4 weeks induced an increase in serum-free LH, FSH, and testosterone compared to control and nicotine alone. A significantly increased in these hormones was observed with 10 mg/kg and 20 mg/kg P. ginseng NPs. In conclusion, the study proposed that encapsulation of P. ginseng in PLGA enhanced the ability of ginsenosides, the major active compounds from P. ginseng to activate the hypothalamic-pituitary-testicular axis and increasing the secretion of LH, FSH, and testosterone (Figure 4).86 The results are promising for the treatment of ED-induced hypogonadism. However, well-designed clinical studies are necessary to evaluate the efficacy and safety of the treatment of male ED.
CONCLUSION
The application of nanotechnology in ED medicine can revolutionize the treatment of ED. The findings of preclinical studies demonstrated a tremendous potential therapeutic effect against ED. PDE5 inhibitors, papaverine hydrochloride, sialorphin, nitric oxide, adipose tissue-derived stem cells, sonic hedgehog, and medicinal plant extracts or their phytochemicals are promising for ED therapy. However, few clinical studies evaluated the efficacy and safety of these therapies. Therefore, future well-designed large-scale randomized clinical trials of long-term effects are useful in the development of new and adequate medications for ED treatment.
STATEMENT OF AUTHORSHIP
Conceptualization, N.P.M., J.O.U and L.S.L.; Methodology, N.P.M. and J.O.U.; Investigation, N.P.M., J.O.U and L.S.L.; Writing – Original Draft, S.C.P. and S.Y.W.; Writing – Review & Editing, N.P.M. and J.O.U.; Funding Acquisition, L.S.L.; Resources, J.O.U and L.S.L.; Supervision, J.O.U and L.S.L.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Life and Consumer Sciences and the College of Agriculture and Environmental Sciences (CAES) through the University of South Africa (UNISA) for support.
Footnotes
Conflict of Interest: The authors declared no competing interest.
Funding: This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Eleazu C, Obianuju N, Eleazu K. The role of dietary polyphenols in the management of erectile dysfunction–Mechanisms of action. Biomed Pharmacother. 2017;88:644–652. doi: 10.1016/j.biopha.2017.01.125. [DOI] [PubMed] [Google Scholar]
- 2.Masuku NP, Unuofin JO, Lebelo SL. Promising role of medicinal plants in the regulation and management of male erectile dysfunction. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110555. [DOI] [PubMed] [Google Scholar]
- 3.Nicolosi A, Moreira ED, Shirai M. Epidemiology of erectile dysfunction in four countries: Cross-national study of the prevalence and correlates of erectile dysfunction. Adult Urol. 2003;61:201–206. doi: 10.1016/s0090-4295(02)02102-7. [DOI] [PubMed] [Google Scholar]
- 4.de Klerk H, de Villiers PJT, Isaacs S. Prevalence arld characteristics of erectile dysfunction in black and mixed race primary care populations of the cape flats and Helderberg Basin area of the Western Cape, South Africa. South African Fam Pract. 2003;45:14–20. [Google Scholar]
- 5.Lockhat Y, Ross A, Ramlachan P. The prevalence of erectile dysfunction at a primary healthcare clinic in durban, kwazulu-natal. South African Fam Pract. 2013;55:289–293. [Google Scholar]
- 6.DeLay KJ, Haney N, Hellstrom WJ. Modifying risk factors in the management of erectile dysfunction: a review. World J Mens Health. 2016;34:89–100. doi: 10.5534/wjmh.2016.34.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura Y, Honda M, Teraoka S. Impact of penile rehabilitation with phosphodiesterase-5 inhibitors on recovery of erectile function in patients undergoing robot-assisted radical prostatectomy: A propensity score-matched analysis. Int J Urol. 2021;28:637–642. doi: 10.1111/iju.14527. [DOI] [PubMed] [Google Scholar]
- 8.Haga N, Miyazaki T, Tsubouchi K. Comprehensive approach for preserving cavernous nerves and erectile function after radical prostatectomy in the era of robotic surgery. Int J Urol. 2021;28:360–368. doi: 10.1111/iju.14491. [DOI] [PubMed] [Google Scholar]
- 9.Albers LF, Tillier CN, van Muilekom E. Sexual satisfaction in men suffering from erectile dysfunction after robot-assisted radical prostatectomy for prostate cancer: An observational study. J Sex Med. 2021;18:339–346. doi: 10.1016/j.jsxm.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Cho MC, Cho SY. Novel emerging therapies for erectile dysfunction. World J Mens Health. 2020;39:48–64. doi: 10.5534/wjmh.200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatzimouratidis K, Salonia A, Adaikan G. Pharmacotherapy for erectile dysfunction: Recommendations from the fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:465–488. doi: 10.1016/j.jsxm.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Choe S, Bond CW, Harrington DA. Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomed Nanotechnol Biol Med. 2017;13:95–101. doi: 10.1016/j.nano.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porst H, Burnett A, Brock G. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med. 2013;10:130–171. doi: 10.1111/jsm.12023. [DOI] [PubMed] [Google Scholar]
- 14.Hotta Y, Kataoka T, Mori T. Review of a potential novel approach for erectile dysfunction: Light-controllable nitric oxide donors and nanoformulations. Sex Med Rev. 2020;8:297–302. doi: 10.1016/j.sxmr.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Liu SZ, Feng DC, Liu ZH. Development of nanotechnology in andrology. Transl Androl Urol. 2020;9:702–708. doi: 10.21037/tau.2020.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim A, Ali M, Kiernan TJ. Erectile dysfunction and ischaemic heart disease. Eur Cardiol Rev. 2018;13:98–103. doi: 10.15420/ecr.2017.21.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail EA, El-Sakka AI. Innovative trends and perspectives for erectile dysfunction treatment: A systematic review. Arab J Urol. 2016;14:84–93. doi: 10.1016/j.aju.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Protogerou V, Chrysikos D, Karampelias V. Erectile dysfunction treatment using stem cells: A review. Medicines. 2021;8:2. doi: 10.3390/medicines8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovais M, Khalil AT, Raza A. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine. 2016;12:3157–3177. doi: 10.2217/nnm-2016-0279. [DOI] [PubMed] [Google Scholar]
- 20.Simos YV, Spyrou K, Patila M. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J Pharm Sci. 2021;16:62–76. doi: 10.1016/j.ajps.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilczewska AZ, Niemirowicz K, Markiewicz KH. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64:1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 22.Bilal M, Barani M, Sabir F. Nanomaterials for the treatment and diagnosis of Alzheimer’s disease: An overview. NanoImpact. 2020;20 [Google Scholar]
- 23.Hosny KM, Aldawsari HM. Avanafil liposomes as transdermal drug delivery for erectile dysfunction treatment: Preparation, characterization, and in vitro, ex vivo and in vivo studies. Trop J Pharm Res. 2015;14:559–565. [Google Scholar]
- 24.Fahmy UA, Ahmed OAA, Hosny KM. Development and evaluation of avanafil self-nanoemulsifying drug delivery system with rapid onset of action and enhanced bioavailability. AAPS PharmSciTech. 2014;16:53–58. doi: 10.1208/s12249-014-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurakula M, Ahmed OAA, Fahmy UA. Solid lipid nanoparticles for transdermal delivery of avanafil: Optimization, formulation, in-vitro and ex-vivo studies. J Liposome Res. 2016;26:288–296. doi: 10.3109/08982104.2015.1117490. [DOI] [PubMed] [Google Scholar]
- 26.Aldawsari HM, Fahmy UA, Abd-Allah F. Formulation and optimization of avanafil biodegradable polymeric nanoparticles: A single-dose clinical pharmacokinetic evaluation. Pharmaceutics. 2020;12:596. doi: 10.3390/pharmaceutics12060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosny KM, Aljaeid BM. Sildenafil citrate as oral solid lipid nanoparticles: A novel formula with higher bioavailability and sustained action for treatment of erectile dysfunction. Expert Opin Drug Deliv. 2014;11:1015–1022. doi: 10.1517/17425247.2014.912212. [DOI] [PubMed] [Google Scholar]
- 28.Badr-Eldin SM, Ahmed OAA. Optimized nano-transfersomal films for enhanced sildenafil citrate transdermal delivery: Ex vivo and in vivo evaluation. Drug Des Devel Ther. 2016;10:1323–1333. doi: 10.2147/DDDT.S103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han G, Tar M, Kuppam DSR. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med. 2010;7:224–233. doi: 10.1111/j.1743-6109.2009.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek JS, Pham CV, Myung CS. Tadalafil-loaded nanostructured lipid carriers using permeation enhancers. Int J Pharm. 2015;495:701–709. doi: 10.1016/j.ijpharm.2015.09.054. [DOI] [PubMed] [Google Scholar]
- 31.Fahmy UA, Aljaeid BM. Tadalafil transdermal delivery with alpha-lipoic acid self nanoemulsion for treatment of erectile dysfunction by diabetes mellitus Usama Ahmed Fahmy and Bader Mubarak Aljaeid. Int J Pharmacol. 2018;14:945–951. [Google Scholar]
- 32.Fahmy UA. Nanoethosomal transdermal delivery of vardenafil for treatment of erectile dysfunction: Optimization, characterization, and in vivo evaluation. Drug Des Devel Ther. 2015;9:6129–6137. doi: 10.2147/DDDT.S94615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tawfik MA, Tadros MI, Mohamed MI. Lipomers (lipid-polymer hybrid particles) of vardenafil hydrochloride: A promising dual platform for modifying the drug release rate and enhancing its oral bioavailability. AAPS PharmSciTech. 2018;19:3650–3660. doi: 10.1208/s12249-018-1191-0. [DOI] [PubMed] [Google Scholar]
- 34.Parikh KJ, Sawant KK. Solubilization of vardenafil HCl in lipid-based formulations enhances its oral bioavailability in vivo: A comparative study using Tween - 20 and Cremophor - EL. J Mol Liq. 2019;277:189–199. [Google Scholar]
- 35.Ahmed OAA. Development and single dose clinical pharmacokinetics investigation of novel zein assisted- alpha lipoic acid nanoencapsulation of vardenafil. Sci Rep. 2018;8:15802. doi: 10.1038/s41598-018-34235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yafi FA, Jenkins J, Albersen M. Erectile dysfunction. Nat Rev Dis Prim. 2016;2:16003. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatzimouratidis K, Amar E, Eardley I. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–814. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Duncan C, Omran GJ, Teh J. Erectile dysfunction : A global review of intracavernosal injectables European Association of Urology Japanese Society of Sexual Medicine. World J Urol. 2019;37:1007–1014. doi: 10.1007/s00345-019-02727-5. [DOI] [PubMed] [Google Scholar]
- 39.Weidner W, Schroeder-Printzen I, Fischer C. Experience in the treatment of erectile dysfunction using the intracavernosal self-injection of papaverine. World J Urol. 1992;10:179–182. [Google Scholar]
- 40.Yiou R, Bütow Z, Parisot J. Is it worth continuing sexual rehabilitation after radical prostatectomy with intracavernous injection of alprostadil for more than 1 year? Sex Med. 2015;3:42–48. doi: 10.1002/sm2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenfeld O, Hanani J, Shalhav A. Papaverine-phentolamine and prostaglandin E1 versus papaverine-phentolamine alone for intracorporeal injection therapy: A clinical double-blind study. J Urol. 1995;154:1017–1019. [PubMed] [Google Scholar]
- 42.Bechara A, Casabé A, Chéliz G. Comparative study of papaverine plus phentolamine versus prostaglandin E1 in erectile dysfunction. J Urol. 1997;157:2132–2134. [PubMed] [Google Scholar]
- 43.Berkó S, Zsikó S, Deák G. Papaverine hydrochloride containing nanostructured lyotropic liquid crystal formulation as a potential drug delivery system for the treatment of erectile dysfunction. Drug Des Devel Ther. 2018;12:2923–2931. doi: 10.2147/DDDT.S168218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali MFM, Salem HF, Abdelmohsen HF. Preparation and clinical evaluation of nano-transferosomes for treatment of erectile dysfunction. Drug Des Devel Ther. 2015;9:2431–2447. doi: 10.2147/DDDT.S81236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rougeot C, Messaoudi M, Hermitte V. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci U S A. 2003;100:8549–8554. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong Y, Tiplitsky SI, Tar M. Transcription of G-protein coupled receptors in corporeal smooth muscle is regulated by the endogenous neutral endopeptidase inhibitor sialorphin. J Urol. 2008;180:760–766. doi: 10.1016/j.juro.2008.03.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies KP, Tar M, Rougeot C. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431–435. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messaoudi M, Desor D, Nejdi A. The endogenous androgen-regulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684–691. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Tar M, Cabrales P, Navati M. Topically applied no-releasing nanoparticles can increase intracorporal pressure and elicit spontaneous erections in a rat model of radical prostatectomy. J Sex Med. 2014;11:2903–2914. doi: 10.1111/jsm.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nam E, Yoo S, Kim HY. Transdermal water-in-oil nanocarriers of nitric oxide for triggering penile erection. Sci Rep. 2018;8:7312. doi: 10.1038/s41598-018-25786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HJ, Jeong H, Park YH. Adipose tissue-derived stem cell therapy for cavernous nerve injury-induced erectile dysfunction in the rat model: A systematic review and meta-analysis using methodological quality assessment. Int J Stem Cells. 2019;12:206–217. doi: 10.15283/ijsc18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim IG, Piao S, Lee JY. Effect of an adipose-derived stem cell and nerve growth factor-incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng - Part A. 2013;19:14–23. doi: 10.1089/ten.tea.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albersen M, Fandel TM, Lin G. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albersen M, Lin CS, Lue T. Stem-cell therapy for erectile dysfunction. Arab J Urol. 2013;11:237–244. doi: 10.1016/j.aju.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MG G. Intracavernous administration of adipose stem cells: A new technique of treating erectile dysfunction in diabetic patient, preliminary report of 6 cases. MOJ Cell Sci Rep. 2015;2:8–11. [Google Scholar]
- 56.Protogerou V, Beshari S El, Michalopoulos E. The combined use of stem cells and platelet lysate plasma for the treatment of erectile dysfunction: A pilot study–6 months results. Medicines. 2020;7:14. doi: 10.3390/medicines7030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Protogerou V, Michalopoulos E, Mallis P. Administration of adipose derived mesenchymal stem cells and platelet lysate in erectile dysfunction: A single center pilot study. Bioengineering. 2019;6:21. doi: 10.3390/bioengineering6010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin H, Dhanani N, Tseng H. Nanoparticle improved stem cell therapy for erectile dysfunction in a rat model of cavernous nerve injury. J Urol. 2016;195:788–795. doi: 10.1016/j.juro.2015.10.129. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr Diab Rep. 2016;16:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu H, Tang W-H, Zhao L-M. Nanotechnology‑assisted adipose‑derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury: In vivo cell tracking, optimized injection dosage, and functional evaluation. Asian J Androl. 2018;20:442–447. doi: 10.4103/aja.aja_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angeloni N, Bond C, Tang Y. 876 Cavernous nerve regeneration by sonic hedgehog. J Urol. 2010;183:e343–e344. [Google Scholar]
- 62.Rasethe MT, Semenya SS, Maroyi A. Medicinal plants traded in informal herbal medicine markets of the Limpopo province, South Africa. Evidence-Based Complement Altern Med. 2019;2019:2609532. doi: 10.1155/2019/2609532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masuku NP, Unuofin JO, Lebelo SL. Phytochemical content, antioxidant activities and androgenic properties of four South African medicinal plants. J HerbMed Pharmacol. 2020;9:245–256. [Google Scholar]
- 64.Masuku NP, Lebelo SL. Investigation of the effects of Kigelia Africana (Lam.) Benth. Extracts on Tm3 Leydig cells. Asian J Pharm Clin Res. 2019;12:87–92. [Google Scholar]
- 65.Wadood A, Ghufran M, Jamal SB. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2:1–4. [Google Scholar]
- 66.Manukumar HM, Shiva Kumar J, Chandrasekhar B. Evidences for diabetes and insulin mimetic activity of medicinal plants: present status and future prospects. Crit Rev Food Sci Nutr. 2017;57:2712–2729. doi: 10.1080/10408398.2016.1143446. [DOI] [PubMed] [Google Scholar]
- 67.Yadav RNS, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3:10–14. [Google Scholar]
- 68.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barani M, Mirzaei M, Torkzadeh-Mahani M. Evaluation of Carum-loaded niosomes on breast cancer cells:physicochemical properties, in vitro cytotoxicity, flow cytometric, DNA fragmentation and cell migration assay. Sci Rep. 2019;9:7139. doi: 10.1038/s41598-019-43755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krausz AE, Adler BL, Cabral V. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine Nanotechnology, Biol Med. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oroojan AA, Ahangarpour A, Paknejad B. Effects of myricitrin and solid lipid nanoparticle-containing myricitrin on reproductive system disorders induced by diabetes in male mouse. World J Mens Health. 2019;39:147–157. doi: 10.5534/wjmh.190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Unuofin JO, Oladipo AO, Msagati TAM. Novel silver-platinum bimetallic nanoalloy synthesized from Vernonia mespilifolia extract: antioxidant, antimicrobial, and cytotoxic activities. Arab J Chem. 2020;13:6639–6648. [Google Scholar]
- 73.Zamarioli CM, Martins RM, Carvalho EC. Nanoparticles containing curcuminoids (Curcuma longa): development of topical delivery formulation. Rev Bras Farmacogn. 2015;25:53–60. [Google Scholar]
- 74.Adusumilli NC, Mordorski B, Nosanchuk J. Curcumin nanoparticles as a photoprotective adjuvant. Exp Dermatol. 2021;30:705–709. doi: 10.1111/exd.14282. [DOI] [PubMed] [Google Scholar]
- 75.Karri VVSR, Kuppusamy G, Talluri SV. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol. 2016;93:1519–1529. doi: 10.1016/j.ijbiomac.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 76.Abdel Aziz MT, El Asmer MF, Rezq A. Novel water-soluble curcumin derivative mediating erectile signaling. J Sex Med. 2010;7:2714–2722. doi: 10.1111/j.1743-6109.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 77.Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today. 2017;22:1582–1592. doi: 10.1016/j.drudis.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Abdel Aziz TM, Rezq AM, Atta HM. Molecular signalling of a novel curcumin derivative versus Tadalafil in erectile dysfunction. Andrologia. 2015;47:616–625. doi: 10.1111/and.12309. [DOI] [PubMed] [Google Scholar]
- 79.Draganski A, Tar MT, Villegas G. Topically applied curcumin-loaded nanoparticles treat erectile dysfunction in a rat model of type-2 diabetes. J Sex Med. 2018;15:645–653. doi: 10.1016/j.jsxm.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Domitrović R, Rashed K, Cvijanović O. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem Biol Interact. 2015;230:21–29. doi: 10.1016/j.cbi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 81.Leung KW, Wong AS. Ginseng and male reproductive function. Spermatogenesis. 2013;3:e26391. doi: 10.4161/spmg.26391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ratan ZA, Haidere MF, Hong YH. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2020;45:199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bai L, Gao J, Wei F. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front Pharmacol. 2018;9:423. doi: 10.3389/fphar.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi L-W, Wang C-Z, Du G-J. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818–822. doi: 10.2174/138920011797470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J, Li M, Chen L. Effects of processing method on the pharmacokinetics and tissue distribution of orally administered ginseng. J Ginseng Res. 2018;42:27–34. doi: 10.1016/j.jgr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Linjawi SAA. Evaluation of the protective effect of panax ginseng nanoparticles against nicotineinduced reproductive disorders in male rats. Int J Pharm Sci Rev Res. 2015;32:38–45. [Google Scholar]