Abstract
Purpose:
With anecdotal observations of atypical recurrences following minimally-invasive surgery (MIS) and alongside new concerns following cervical cancer surgery, there is a need to evaluate cancer-specific outcomes for MIS kidney cancer surgery using national data. We sought to evaluate cancer-specific outcomes following MIS versus open surgery for early-stage kidney cancer
Materials and Methods:
We performed a retrospective population-based cohort study using data from the Surveillance, Epidemiology and End Results (SEER) program linked with Medicare claims that included beneficiaries, at least 66 years old, diagnosed between 2004 and 2013 with early-stage, non-urothelial kidney cancer who underwent surgical resection within a year of diagnosis. We compared overall survival, disease-specific survival, rate of second kidney cancer surgery, and rate of post-operative systemic cancer therapy based on whether surgery was MIS or an open resection. Multivariable regression was used to account for confounders.
Results:
5,150 patients were included and 3,062 (59.5%) underwent MIS. On multivariable analysis, MIS was not associated with differences in overall survival (HR 0.94, 95% CI 0.84–1.06) or disease-specific survival (HR 0.96, 95% CI 0.83–1.11). MIS recipients were more likely to receive systemic cancer therapy (HR 1.31, 95% CI 1.09–1.59). No difference in the rate of second surgery associated with surgical approach was observed.
Conclusions:
Use of MIS for early-stage kidney cancer was not associated with differences in overall or disease-specific survival, or the rate of second kidney cancer surgery. MIS recipients received more post-operative systemic therapy. This could represent a disparate cancer-specific outcome associated with MIS.
Keywords: Kidney Cancer, minimally-invasive surgery, outcomes research
Introduction
Minimally-invasive kidney cancer surgery has been widely embraced due to purported benefits of diminished peri-operative morbidity and convalescence.1 This technology has rapidly diffused into broad adoption,2,3 however, the literature supporting these changes has limitations. For instance, much of the data used to support broader adoption of minimally-invasive surgery (MIS) for kidney cancer has been based on reports of short-term safety4 and non-randomized series from pioneering institutions.5–8
The rapid uptake of MIS for kidney cancer has been mirrored across surgical oncology9, and there is recent evidence that raises concerns regarding oncologic efficacy of these approaches for certain types of cancer.10–12 For instance, a randomized trial demonstrated an 11% decrease in overall survival and more locoregional recurrences for early-stage cervical cancer managed with MIS as compared to open approaches.13 This report and a simultaneous study using a national database,10 has led to modification of national guidelines to caution against MIS for cervical cancer and to cessation of MIS cervical cancer surgery at some institutions.14 Oncologic concerns for one type of cancer may not translate across all types, however, among patients with kidney cancer, there are published reports of atypical recurrences following MIS15–17 and we have noticed these in our practice as well. Thus, there is a need to better understand this scope of this issue across a broader population.
In this context, using population-based data, we compare cancer-specific outcomes among patients with localized, early-stage kidney cancer (i.e., T1N0) based on whether initial surgical resection was performed via an open or MIS approach. We utilize Surveillance, Epidemiology and End Results (SEER) Medicare linked data to evaluate whether patients receiving MIS radical or partial nephrectomy have differences in overall survival, cancer-specific survival, rates of second kidney cancer surgery, or rates of subsequent systemic cancer therapy relative to patients undergoing open partial or radical nephrectomy for the same diagnosis.
Materials and Methods:
Data
We used linked data from the SEER registry and the Centers for Medicare and Medicaid Services (Medicare data) to identify patients with non-metastatic, T1N0 kidney cancer diagnosed between 2004 and 2013. The SEER registry contains information on incidence, disease characteristics (e.g., histologic subtype, grade, stage), treatment, and mortality across 18 distinct geographic regions capturing approximately 30% of the United States population.18 The Medicare linkage provides additional claims-based information about patient comorbidities and health services rendered. The study was considered exempt research by the institutional review board of Memorial Sloan Kettering Cancer Center, and it was conducted in adherence with a Data Use Agreement from the National Cancer Institute.
Cohort
We created an initial cohort of patients who were at least 66 years old, diagnosed with non-metastatic, non-urothelial kidney cancer with stage T1N0 (<7 cm) as their first and only site of cancer between 2004–2013. This cohort was limited to only include patients with Medicare fee-for-service coverage for a continuous period spanning at least twelve months before and twelve months after diagnosis. Patients were excluded if they had bilateral tumors, received neoadjuvant therapy, had an unknown diagnosis date, were diagnosed on death certificate, diagnosed at autopsy, or if patients did not have a cancer directed surgery as a first treatment (i.e. radical nephrectomy or partial nephrectomy) within one year of diagnosis.
Treatment type
The Medicare provider analysis and review (MEDPAR) file and physician claims were used to identify a specific surgical procedure received for primary treatment. A previously-published, validated claims-based algorithm was used to determine the specific surgical procedure for each patient.19 This algorithm was followed as outlined with two small amendments: 1) any claim containing ICD-9 procedure code 17.42 (robotic-assisted procedure of trunk region) was classified as a MIS procedure; and 2) the previously outlined rule to classify patients with length of stay < 2 days as recipients of MIS was omitted from our algorithms. MIS procedures were not further sub-divided to delineate whether procedure was robotically-assisted due to known issues reliably identifying robotically-assisted surgery in claims data. After identifying treatment type, patients were subdivided into two study groups for comparison based on whether surgery was open or MIS.
Covariates
SEER was used to identify demographic information and clinicopathologic (e.g., tumor grade, histology, stage) features for each patient. Medicare claims from the twelve months preceding diagnosis and previously published algorithms were used to identify comorbid conditions and assign a Charlson comorbidity score to each patient.20
Outcomes
We compared four outcomes across study groups. Overall and cancer-specific survival were evaluated based on SEER cause of death variables. Deceased patients were classified as cancer-specific if SEER COD codes were 29020, 37000, and 38000, and the presence of any other COD was classified as ‘other’ cause death. Medicare claims were used to evaluate the rates of second cancer directed surgery and receipt of post-operative systemic cancer therapy. Patients were defined as receiving a second cancer directed surgery if there was any claims-based evidence of a second surgery for kidney cancer. Second surgery was defined as a surgical claim >30 days from the initial surgical claim for either a radical nephrectomy, a partial nephrectomy, or an ablative procedure on the kidney using the following procedure codes (CPT codes: 49203, 49204, 49205, 50592, 50593, or these additional ICD-9 codes: 55.32, 55.33, 55.34). Receipt of post-operative systemic cancer therapy was identified from Medicare inpatient, outpatient, and Part D pharmacy claims. A patient was listed as receiving systemic cancer therapy if they had a claim at any point in their post-operative period for either a cancer therapy infusion or for a medication for treatment of cancer (specific codes available in Appendix).
Statistical analyses
Patient characteristics were described and compared across groups with univariate statistics (Mann-Whitney-U and Chi-squared tests for continuous and categorical variables, respectively). Next, a Cox-proportional hazards model was fit to compare overall survival. Model covariates included MIS versus open surgical approach, radical versus partial nephrectomy, Charlson index (0, 1, 2, 3+), age at diagnosis (66–69, 70–74, 75–79, and 80+ years), diagnosis year (2004–2013), receipt of systemic cancer therapy (time-dependent covariate), receipt of second surgery (time-dependent covariate), tumor grade (unknown, poorly-differentiated, well-differentiated), and tumor histology (clear cell; papillary; chromophobe; renal-cell carcinoma not otherwise specified; other). A Fine-Gray competing risk model was used to assess disease-specific survival. This model included the same co-variates as the overall survival model. Due to rarity of second surgical events, stratified Kaplan-Meier analysis was used to evaluate for unadjusted differences in rate of repeat surgery among patients who either underwent MIS or open surgery. Finally, a Cox model was used to assess rate of systemic therapy after surgery. Included covariates were: open surgery vs MIS, radical vs partial nephrectomy, age at diagnosis, tumor grade, and tumor histology. All analyses were performed using SAS 9.4 (Cary, NC) and R version 3.3.3 (2017).
Results
During the evaluated period, 2,088 patients underwent open surgery and 3,062 underwent MIS for stage T1N0 kidney cancer. The median time from diagnosis to surgery was 21 days amongst MIS recipients and 19 days amongst recipients of open surgery. Baseline characteristics stratified by surgical approach are presented in Table 1. MIS procedures were increasingly common over time (Table 1). Recipients of MIS more commonly had clear cell histology (59.5% versus 53.4%; p<0.001) and were more likely to undergo radical nephrectomy (66.9% vs. 59.3 % for open surgery, p<0.001).
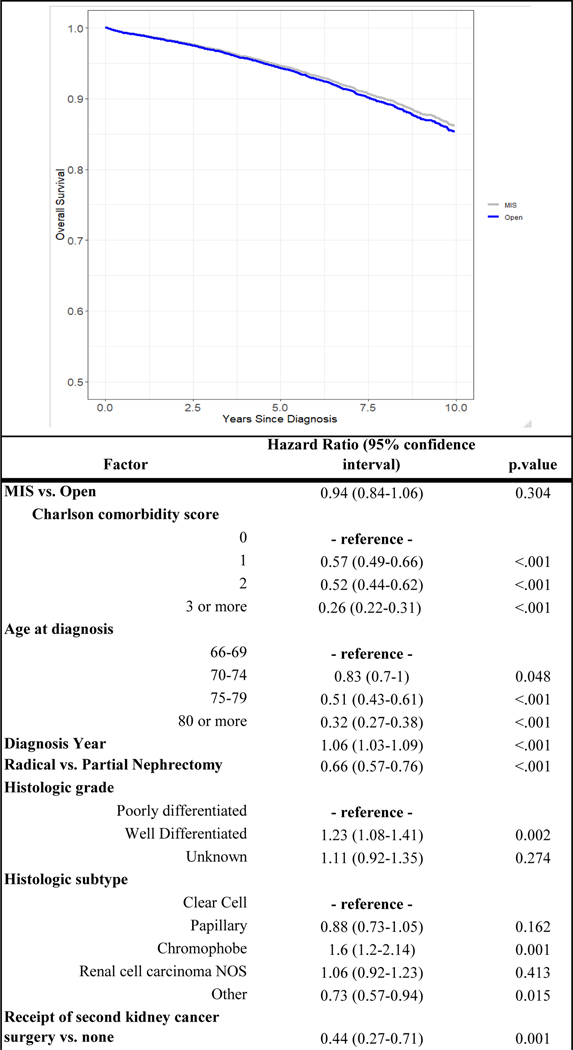
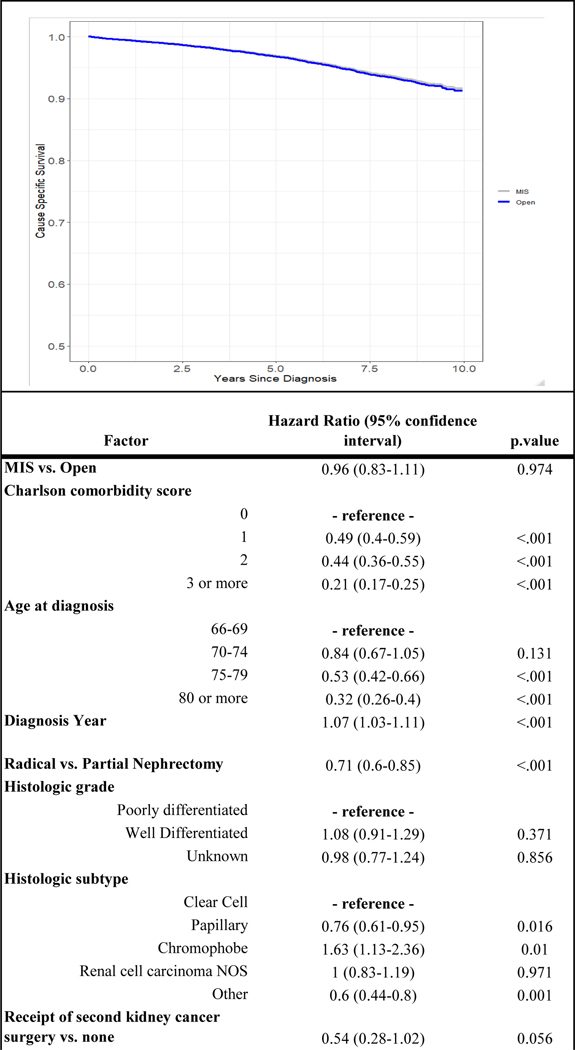
Over a median follow up of 57.2 months [32.3–86.9 IQR], 551 (26.4 %) patients died from any cause after open kidney cancer surgery whereas 649 (21.2%) died of any cause after minimally-invasive kidney cancer surgery. Kidney cancer-specific death was observed in 101 (4.9%) patients undergoing open surgery as compared to 102 (3.3%) following MIS. After adjusting for potential confounders, there was no difference in overall survival associated with surgical approach (HR 0.94, 95% CI 0.84–1.06) (Figure 1). As Figure 2 demonstrates, surgical approach was not associated with differences in disease-specific survival after accounting for covariates.
Figure 1.
Adjusted overall survival curve from Cox regression stratified by surgical approach and relationship between factors included in model and overall survival
Figure 2.
Adjusted cancer-specific survival curve from Fine-Gray competing risk model stratified by surgical approach and relationship between factors included in model and survival
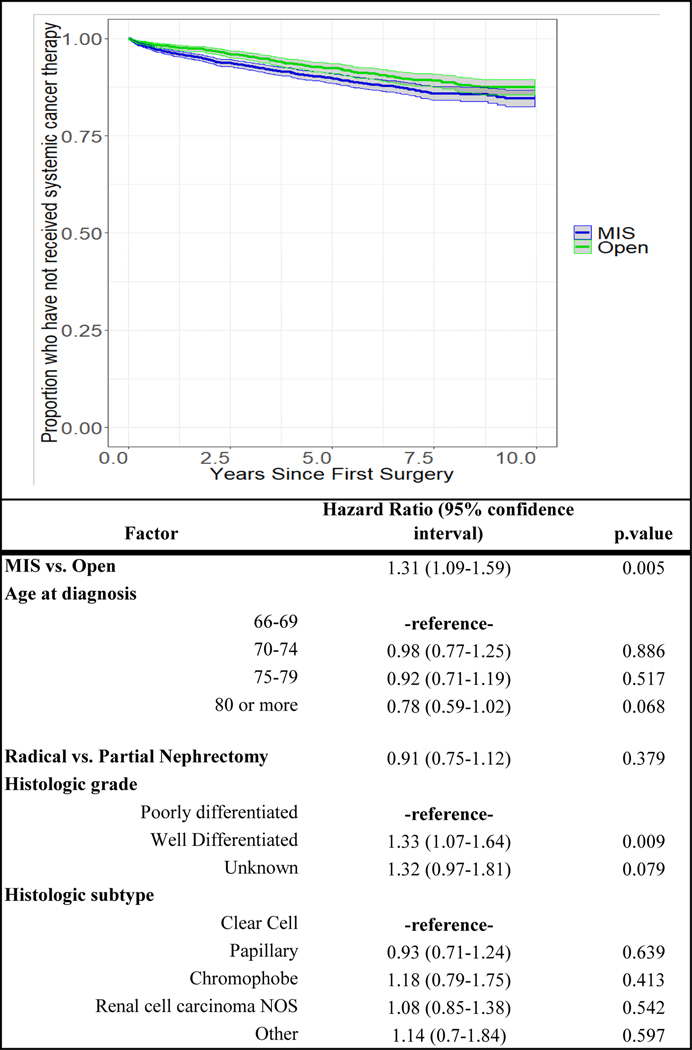
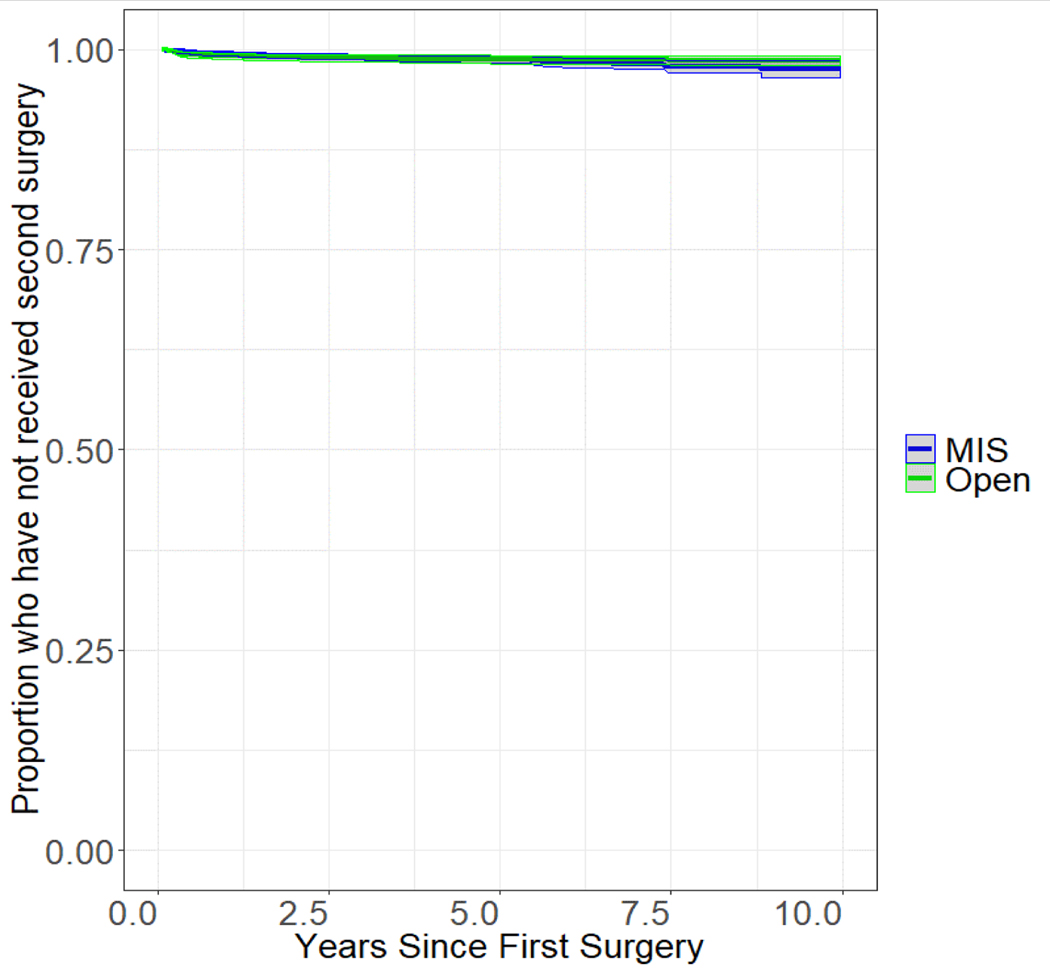
After open surgery 130 (6.2%) patients subsequently received systemic cancer therapy as compared to 214 (6.9%) patients treated initially with MIS. After accounting for covariates, MIS was significantly associated with a higher likelihood of receiving secondary systemic cancer therapy (HR 1.31, 95% CI 1.09–1.59) (Figure 3). Second surgical events were rare in both groups and there were no observed differences in rate of second surgery across groups (Figure 4).
Figure 3.
Adjusted Cox regression curve for rate or receipt of systemic cancer stratified by surgical approach and relationship between factors included in model and systemic cancer therapy
Figure 4.
Kaplan-Meier curve for rate of survival free from second kidney cancer surgery stratified by surgical approach
Discussion
Using a national cancer registry, we compared survival and cancer-specific outcomes in patients with early-stage (T1) kidney cancer who underwent their initial operation utilizing MIS or open approaches. We found no association between type of surgery and overall survival, disease-specific survival, or rate of second kidney cancer surgery. However, we did find that patients who underwent MIS were more likely to receive subsequent systemic therapy for kidney cancer.
Our findings inform practice in an area where, to date, there has been no prospective randomized comparison of oncologic outcomes. MIS for kidney cancer has been rapidly adopted.2 The safety and efficacy of this approach has largely been evaluated via non-randomized case-series.5,21–23 Although such reports are encouraging, there are limitations. First, it is well recognized that results from retrospective, single or multi-center case series may be subject to biases that influence results.22 Further, as technology diffuses beyond sites of early adoption, it is possible that outcomes achieved in every day practice environments deviate from those of leading centers who publish initial experiences. Finally, the diffusion of MIS, especially robotically-assisted MIS, did not occur in a vacuum. Device manufacturers have been heavily involved in the process of training and credentialing surgeons to use their devices24. These same manufacturers often sponsor research and/or the researchers that explore the efficacy and safety of MIS25 and have widely marketed the benefits of their devices prior to thorough objective evaluation of outcomes. In addition, hospitals have invested large sums of money on these technologies and likely have a natural inclination to recuperate those investments. The influence of marketing and financial factors on research can be hard to quantify, however, these elements need to be closely scrutinized as the body of evidence supporting new technologies is evaluated. Echoing such concerns, the U.S. Food and Drug Administration (FDA) recently issued a safety communication pointing out that the evidence supporting robotic surgery for cancer is limited for many disease sites.26
In the absence of a prospective, randomized trial, our population-based evaluation, inclusive of patients undergoing operations in varied practice environments, represents a meaningful way to compare outcomes after MIS and open kidney cancer surgery. The current investigation was pursued in the context of anecdotal experience of increasing early, atypical, and often catastrophic, kidney cancer recurrences following MIS at a large academic cancer hospital. Admittedly, the population seeking care at our center comes with obvious biases (e.g., likely enriched for patients who have had negative outcomes after a first surgical event). Further, in an era where more patients are undergoing MIS, it is logical that there would be an increase in the observed number of recurrences following MIS. It would be of greater concern if adverse outcomes occurring across a population are more commonly associated with a specific surgical approach. In this population-based analysis, we importantly do not demonstrate any differences in overall survival, cancer-specific survival, or survival free of secondary procedures for kidney cancer attributable to surgical approach. However, our finding that patients undergoing MIS were significantly more likely to require systemic therapy is of potential concern. With no FDA approved adjuvant treatments for early-stage kidney cancer, systemic therapy after surgery would only be expected among patients who develop metastatic or recurrent disease.27,28 This may be an indication of an adverse cancer-specific outcome associated with MIS and could translate to worse survival with longer follow-up. Given the relatively modest difference in receipt of systemic therapy (i.e., HR=1.3), it is also possible this difference is influenced by unmeasured elements such as institutional differences in utilization of adjuvant systemic therapy, differences in surgical margin status, or other factors that may not reflect true differences in rate of recurrence.
These findings should be taken in the context of several limitations. First, this is an observational study, based on claims and registry data, and it is possible that confounding, unmeasured differences, or coding errors could explain the results. We are, therefore, unable to determine if there is a causal relationship between surgical approach and the differences in needing subsequent systemic therapy. Second, two of our outcome measures (i.e., rate of secondary surgery and rate of systemic therapy) are proxy measures that may not perfectly estimate our true outcome of interest—the rate of disease recurrence after initial surgery. Within the limitations of the dataset, these measures, devised to ascertain if patients are undergoing treatment for a disease recurrence, are our best approximation of the rate of recurrence within the confines of the data. Finally, some of the data from the early 2000’s may have been in the early phase of MIS adoption meaning surgeons may have been on a learning-curve as they incorporate newer technologies. A subgroup analysis, not included due to space limitations, of all patients who received surgery in 2010 or later (a period thought to be far enough along in the continuum of adoption of MIS techniques to limit the impact of learning curve), demonstrated no tangible differences in outcomes as compared to the results presented herein. Thus, our conclusion is there is not a major impact of learning curve on the results of this investigation.
These limitations notwithstanding, our findings have important implications in the context of increasingly common use of MIS techniques for kidney cancer surgery. The diffusion of MIS techniques has been rapid and may have outpaced the accumulation of data demonstrating comparative oncologic outcomes relative to traditional open surgery. Recent data showing adverse survival associated with MIS for cervical cancer certainly augments concerns.10,13 The mechanisms for these differences remain unknown (e.g., related to cervical cancer biology or MIS hysterectomy techniques, to surgeon learning curve, or factors related to MIS more broadly), but these findings necessitate a wider evaluation of MIS oncologic surgery with contemporary data. Our investigation did not detect differences in overall or disease-specific, or rates of second cancer surgery associated with surgical approach for early-stage kidney cancer. We did observe a modest increase in the rate of systemic therapy after MIS. This may be an early sign of an association between MIS techniques and disease recurrence that warrants close observation and further investigation.
Ultimately, post-operative disease recurrences can be catastrophic and complete and durable remission is extremely rare in late-stage kidney cancer regardless how the disease spread. As surgeons, all attempts to determine oncological efficacy of newer approaches to cancer surgery must be made while limiting focus on short term recovery metrics. As new technologies become available a dedication to prospective, unbiased evaluation of effectiveness is paramount to ensure best outcomes for patients requiring cancer surgery.
Conclusions
Use of MIS for early-stage kidney cancer was not associated with differences in overall or disease-specific survival, or the rate of second kidney cancer surgery. MIS recipients received more post-operative systemic therapy. This could represent a disparate cancer-specific outcome associated with MIS requiring further investigation.
Supplementary Material
Table 1. Patient characteristics
Abbreviations:
- MIS
minimally-invasive surgery
- SEER
Surveillance, Epidemiology and End Results
References
- 1.Ng AM, Shah PH, Kavoussi LR. Laparoscopic Partial Nephrectomy: A Narrative Review and Comparison with Open and Robotic Partial Nephrectomy. Journal of endourology / Endourological Society. 2017;31(10):976–984. [DOI] [PubMed] [Google Scholar]
- 2.Banegas MP, Harlan LC, Mann B, Yabroff KR. Toward greater adoption of minimally invasive and nephron-sparing surgical techniques for renal cell cancer in the United States. Urologic oncology. 2016;34(10):433 e439–433 e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh QD, Menon M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the nationwide inpatient sample. The Journal of urology. 2014;191(4):907–912. [DOI] [PubMed] [Google Scholar]
- 4.Veeratterapillay R, Addla SK, Jelley C, et al. Early surgical outcomes and oncological results of robot-assisted partial nephrectomy: a multicentre study. BJU international. 2017;120(4):550–555. [DOI] [PubMed] [Google Scholar]
- 5.Lane BR, Campbell SC, Gill IS. 10-year oncologic outcomes after laparoscopic and open partial nephrectomy. The Journal of urology. 2013;190(1):44–49. [DOI] [PubMed] [Google Scholar]
- 6.Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. The Journal of urology. 2007;178(1):41–46. [DOI] [PubMed] [Google Scholar]
- 7.Springer C, Hoda MR, Fajkovic H, et al. Laparoscopic vs open partial nephrectomy for T1 renal tumours: evaluation of long-term oncological and functional outcomes in 340 patients. BJU international. 2013;111(2):281–288. [DOI] [PubMed] [Google Scholar]
- 8.Pierorazio PM, Hyams ES, Lin BM, Mullins JK, Allaf ME. Laparoscopic radical nephrectomy for large renal masses: critical assessment of perioperative and oncologic outcomes of stage T2a and T2b tumors. Urology. 2012;79(3):570–575. [DOI] [PubMed] [Google Scholar]
- 9.Fantus RJ, Cohen A, Riedinger CB, et al. Facility-level analysis of robot utilization across disciplines in the National Cancer Database. J Robot Surg. 2019;13(2):293–299. [DOI] [PubMed] [Google Scholar]
- 10.Melamed A, Margul DJ, Chen L, et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. The New England journal of medicine. 2018;379(20):1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. The New England journal of medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 12.Bochner BH, Dalbagni G, Marzouk KH, et al. Randomized Trial Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: Oncologic Outcomes. European urology. 2018;74(4):465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. The New England journal of medicine. 2018;379(20):1895–1904. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer 4.2019. [Google Scholar]
- 15.Kreshover JE, Richstone L, Kavoussi LR. Renal cell recurrence for T1 tumors after laparoscopic partial nephrectomy. Journal of endourology / Endourological Society. 2013;27(12):1468–1470. [DOI] [PubMed] [Google Scholar]
- 16.Shimokihara K, Kawahara T, Takamoto D, et al. Port site recurrence after laparoscopic radical nephrectomy: a case report. J Med Case Rep. 2017;11(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Kim E, Mobley J, et al. Port site metastasis after surgery for renal cell carcinoma: harbinger of future metastasis. The Journal of urology. 2014;192(2):364–368. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Ma Y, Wang S, Han X, Gao D. Laparoscopic Versus Open Radical Nephrectomy for Renal Cell Carcinoma: a Systematic Review and Meta-Analysis. Transl Oncol. 2017;10(4):501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. European urology. 2012;61(5):972–993. [DOI] [PubMed] [Google Scholar]
- 23.Hemal AK, Kumar A, Kumar R, Wadhwa P, Seth A, Gupta NP. Laparoscopic versus open radical nephrectomy for large renal tumors: a long-term prospective comparison. The Journal of urology. 2007;177(3):862–866. [DOI] [PubMed] [Google Scholar]
- 24.Pradarelli JC, Campbell DA Jr., Dimick JB. Hospital credentialing and privileging of surgeons: a potential safety blind spot. Jama. 2015;313(13):1313–1314. [DOI] [PubMed] [Google Scholar]
- 25.Patel SV, Yu D, Elsolh B, Goldacre BM, Nash GM. Assessment of Conflicts of Interest in Robotic Surgical Studies: Validating Author’s Declarations With the Open Payments Database. Annals of surgery. 2018;268(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Administration USFaD. Caution When Using Robotically-Assisted Surgical Devices in Women’s Health including Mastectomy and Other Cancer-Related Surgeries: FDA Safety Communication. In:2019. [Google Scholar]
- 27.NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer 2.2019. [DOI] [PubMed] [Google Scholar]
- 28.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. The New England journal of medicine. 2017;376(4):354–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Patient characteristics