Abstract
Objective
To investigate the association between changes in long term residential exposure to ambient fine particulate matter (PM2.5) and premature mortality in Canada.
Design
Population based quasi-experimental study.
Setting
Canada.
Participants
663 100 respondents to the 1996, 2001, and 2006 Canadian censuses aged 25-89 years who had consistently lived in areas with either high or low PM2.5 levels over five years preceding census day and moved during the ensuing five years.
Interventions
Changes in long term exposure to PM2.5 arising from residential mobility.
Main outcome measures
The primary outcome was deaths from natural causes. Secondary outcomes were deaths from any cardiometabolic cause, any respiratory cause, and any cancer cause. All outcomes were obtained from the national vital statistics database.
Results
Using a propensity score matching technique with numerous personal, socioeconomic, health, and environment related covariates, each participant who moved to a different PM2.5 area was matched with up to three participants who moved within the same PM2.5 area. In the matched groups that moved from high to intermediate or low PM2.5 areas, residential mobility was associated with a decline in annual PM2.5 exposure from 10.6 μg/m3 to 7.4 and 5.0 μg/m3, respectively. Conversely, in the matched groups that moved from low to intermediate or high PM2.5 areas, annual PM2.5 increased from 4.6 μg/m3 to 6.7 and 9.2 μg/m3. Five years after moving, individuals who experienced a reduction in exposure to PM2.5 from high to intermediate levels showed a 6.8% (95% confidence interval 1.7% to 11.7%) reduction in mortality (2510 deaths in 56 025 v 4925 deaths in 101 960). A greater decline in mortality occurred among those exposed to a larger reduction in PM2.5. Increased mortality was found with exposure to PM2.5 from low to high levels, and to a lesser degree from low to intermediate levels. Furthermore, the decreases in PM2.5 exposure were most strongly associated with reductions in cardiometabolic deaths, whereas the increases in PM2.5 exposure were mostly related to respiratory deaths. No strong evidence was found for the changes in PM2.5 exposure with cancer related deaths.
Conclusions
In Canada, decreases in PM2.5 were associated with lower mortality, whereas increases in PM2.5 were associated with higher mortality. These results were observed at PM2.5 levels considerably lower than many other countries, providing support for continuously improving air quality.
Introduction
Ambient fine particulate matter ≤2.5μm in diameter (PM2.5) is a leading cause of death and disability worldwide.1 Globally, exposure to PM2.5 is responsible for about four million deaths annually.1 The detrimental effects of PM2.5 on mortality have been found in regions with PM2.5 levels below the current World Health Organization air quality guideline of 10 µg/m3 per annum,2 3 suggesting that lowering PM2.5 from current concentrations could further reduce mortality and other outcomes among hundreds of millions of people worldwide. Despite this evidence, critics argue that the associations between PM2.5 and mortality alone might be inadequate to draw a direct inference about actual health benefits from lowering PM2.5 levels.4 5 6 A major concern is that previous studies often relied on hypothetical changes in exposure to extrapolate dose-response relations, rather than actual changes in exposure. To reduce the uncertainty associated with the previous findings and to support future regulations, interest in identifying improvements in key indicators of health (eg, mortality) arising from actual reductions in PM2.5 is mounting 7
Considering alternative study designs and triangulating these findings becomes highly relevant in this context. Although experimental designs are hardly conceivable in such settings,6 it is possible to capitalise on natural experiments and related quasi-experimental methods to infer the influence of PM2.5 reductions on survival. By exploiting changes in economic activity or regulatory action, a small number of studies have examined the relation between actual declines in PM2.5 and mortality.8 9 10 These studies compared monthly or yearly rates of mortality between regions rather than between individuals.8 9 10 Nonetheless, the studies provided important empirical evidence about improved mortality outcomes with lower PM2.5 levels. Evaluation of the effect of decreases in exposure to PM2.5 on mortality is challenging because of difficulties in controlling for background trends in air quality and other confounding factors, few available natural experiments amenable to yield discernible health effects, and lack of statistical power.7
Because residential mobility has been shown to generate tangible and sustained changes in exposure to air pollution,11 12 we focused on individuals who relocated from one address to another within Canada. We used a national cohort comprising >10 million Canadian adults, known as the Canadian Census Health and Environment Cohort (CanCHEC), which was created through data linkage of the Canadian census surveys with vital statistics, hospital admission records, and family tax files, thereby providing detailed information on residential history, personal and socioeconomic characteristics, and health status.13 Leveraging residential relocation as an exogenous source of variation to exposure, we conducted a natural experiment to estimate the associations between mortality and the changes in exposure to PM2.5 after residential mobility. Canada represents an ideal setting in which to conduct this study because of recent advances in the electronic capture of a wide array of information across the country, allowing for the creation of this large population based cohort.13 In addition, the PM2.5 levels in Canada were relatively low (8.13 µg/m3 in 2012),14 most of which arose from anthropogenic sources.15
Methods
Study design and population
In this quasi-experimental study, we identified people who relocated from one address to another within Canada from participants in the CanCHEC, a large national cohort in Canada. Details of CanCHEC are presented elsewhere.13 Briefly, CanCHEC comprises respondents to the Canadian long form census questionnaire, which since 1991 has collected data on personal and socioeconomic characteristics from one in five randomly selected Canadian households, through record linkage with the national vital statistics database, the hospital discharge abstract database, and Canadian family tax files (containing households’ economic characteristics and residential histories).13 A combination of standard deterministic and probabilistic record linkage techniques were used for all of the databases, with the exception of vital statistics, which was linked deterministically (see supplementary file: Study population). CanCHEC has been regularly used to quantify PM2.5 related health effects.3 16
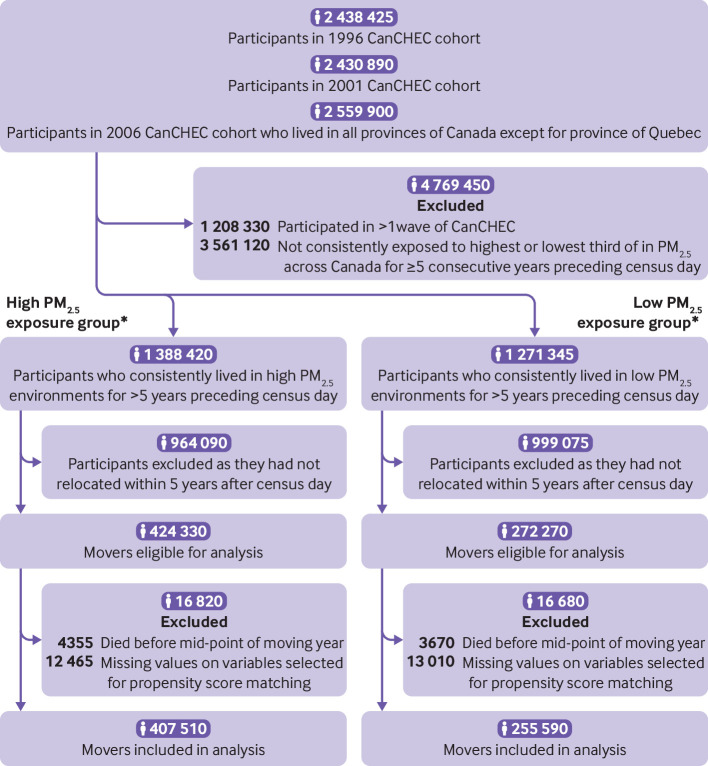
To temporally align with PM2.5 measurements in the CanCHEC (available 1998-2016) and to better represent contemporary exposures to PM2.5, we included participants in the 1996, 2001, and 2006 waves of CanCHEC. For each wave, additional inclusion criteria were that on census day: participants were aged 25-89 years and lived in any province other than Quebec because hospital admission data were unavailable; participants had consistently lived in the highest third of PM2.5 exposure for each year over five years preceding the census day; participants had moved within five years after the census day; and participants were alive before the midpoint of the moving year, which was used as the moving date because the specific date of moving was unavailable (fig 1). For example, in the 1996 wave of CanCHEC, study participants’ time window of exposure was assessed over 1991-96 and their residential mobility was ascertained over 1996-2001. Furthermore, we excluded individuals captured by multiple censuses (n=630 760) by retaining their first participation, and we excluded a small number (12 465) with missing information on covariates used in the analysis, yielding a cohort of 407 510 movers. Once included in the cohort, a participant was assessed for exposure to PM2.5 yearly throughout the remaining study period. To maintain the variability of PM2.5 exposure and sufficient sample size, we further classified the cohort into three exposure groups based on thirds of annual mean PM2.5: those moving within the highest third of PM2.5, those moving to the intermediate third, and those moving to the lowest third.
Fig 1.
Flowchart of cohort creation. *Based on thirds of annual mean fine particulate matter (PM2.5) in cohort. CanCHEC=Canadian Census Health and Environment Cohort
To strengthen inference about the attributions of changes in PM2.5 on mortality, we constructed a second cohort by including individuals who had consistently lived in the lowest third of PM2.5 over five years preceding the census day, yielding a cohort of 255 590 movers (fig 1). As with the first cohort, we classified this cohort into three exposure groups: those moving within the lowest third of PM2.5, those moving to the intermediate third, and those moving to the highest third.
For both cohorts, we followed movers from the midpoint of the moving year (baseline) until their date of death or the end of follow-up (ie, five years after the initial move). Mean follow-up was 4.8 years. All data linkages were performed in the Social Data Linkage Environment and are governed by the directive on microdata linkage of Statistics Canada.
Outcomes
The primary outcome was deaths from natural causes (see supplementary file: Outcomes). We also considered three secondary mortality outcomes: deaths from any cardiometabolic cause,17 18 19 deaths from any respiratory cause, and deaths from any cancer cause. Because of the relatively long latency period of cancer mortality (and incidence), we considered cancer mortality as a negative control. All four outcomes were obtained from the national vital statistics database.
Exposure to PM2.5
We obtained long term measurements of PM2.5 for all cohort members based on postal code addresses between 1991 (five years before the earliest census date) and 2016 (end of follow-up). This was done by using satellite observations of PM2.5 in combination with outputs from a global atmospheric chemistry transport model (GEOS-Chem CTM).20 The PM2.5 estimates were then calibrated using the information on land cover, elevation, and aerosol composition using geographically weighted regression, producing an annual mean concentration of PM2.5 (1×1 km) for each year between 1998 and 2016.20 These annual estimates of PM2.5 cover all of north America and closely agree with ground level measurements at fixed site monitors across the area (R2=0.82, n=1440).20 Using GEOS-Chem CTM outputs and the ground measurements of PM2.5 at fixed site monitors, we further conducted yearly calibration of these surfaces to years before this period, thus creating annual exposure surfaces of PM2.5 for each year between 1991 and 2016.
Covariates
From cohort members’ responses to the census questionnaire, we obtained data on age, sex, race or ethnicity, indigenous identity, immigrant status, marital status, educational attainment, occupation, and employment status. Educational attainment was defined as less than high school, high school, post-secondary non-university, or university. Occupational class was categorised as management, professional, skilled, semi-skilled, unskilled, or not applicable (usually designating those not in the labour force). Using family income tax files, we also derived adequacy of annual household income (classified into 10ths), which accounted for household income, family size, region, and year. In addition, to characterise individuals’ baseline health status, we derived the Charlson comorbidity index score, an index commonly used to categorise an individual’s comorbidity and measure prognosis for mortality, using hospital admission data over three years preceding the baseline.21 These variables are predictors for mortality and are known to influence residential mobility.22
To account for regional differences in mortality that might not be attributable to air pollution, we created four neighbourhood level deprivation variables representing residential instability, material deprivation, dependency, and ethnic concentration (see supplementary file: Covariates).3 Using census tract data, we also created an urban form variable to characterise active commuting and transit use.23 Additionally, we obtained the satellite derived normalised difference vegetation index, an objective measure of vegetative greenness, for a 500 m buffer area around each individual’s postal code (resolved at the centroid).24 25 To characterise access to health services, we measured the distance from each postal code to the nearest healthcare facility (eg, family doctor’s office and hospital) and created a second variable indicating whether or not the move led to a change in the administrative health region.26 All area level variables were defined according to the destination area at baseline.
Lastly, to test the specificity of associations between PM2.5 and mortality, we obtained annual exposures to nitrogen dioxide and ozone using a national land use regression model and an optimal interpolation technique, respectively (see supplementary file: Exposure to NO2 and O3).
Statistical analysis
To emulate a hypothetical randomised experiment in which eligible people can be randomly assigned to either a high or a low (or intermediate) PM2.5 exposure group, followed by comparing their mean mortality rates over five years, we conducted a propensity score matching analysis (see supplementary file: Propensity score).27 The propensity score for the probability of moving from a high to a low or intermediate PM2.5 postal code was estimated for each individual in the high PM2.5 cohort using a logistic regression model with all personal, socioeconomic, health, and environment related covariates, including attained age (in five year age groups), sex, race or ethnicity, indigenous identity, immigrant status, marital status, education, occupation, employment status, household income adequacy, Charlson comorbidity index score, residential proximity to healthcare services, an indicator for changing health region or not, residential greenness, nitrogen dioxide level, ozone level, urban form characteristics, and four neighbourhood level variables about dependency, material deprivation, residential instability, and ethnic concentration. In addition, we included participant’s previous exposure to PM2.5 over the five years preceding baseline, airshed at baseline (lived in east central airshed or not), and the year of moving owing to a concern that the likelihood of moving and mortality risk might vary over time.3 16 These variables were selected a priori for inclusion because they might potentially confound the association between PM2.5 and mortality according to the literature.21 22 28 We estimated a similar propensity score for the probability of moving from a low to high (or intermediate) PM2.5 postal code for each individual in the low PM2.5 cohort.
We matched each individual who moved to a different PM2.5 area with to up to three people who moved within the same PM2.5 area.29 For example, each participant who moved from a high to low PM2.5 was matched with up to three people who moved from a high to high PM2.5 area. A nearest neighbour matching without replacement was applied to match individuals based on the logit of their propensity score, with a caliper of 0.2.30 We assessed the balance in the distribution of covariates before and after matching using standardised differences, with a difference of <0.1 after matching considered a good balance.31 The propensity score estimation and matching were done for each cohort separately.
To estimate the association between changes in PM2.5 and mortality, we used Cox proportional hazards models with matching weights applied and time on study (in days) as the time scale. Following previous studies,32 exposure was represented by an indicator for exposure groups (eg, high to low v high to high). As a secondary measure of changes in PM2.5, we fitted an interaction between the group indicator and the difference in annual mean PM2.5 between an individual’s origin and destination (see supplementary file: Propensity score). In the Cox models, we adjusted for all the covariates used in propensity score matching.33 To further account for the paired nature of the matched cohort, we used robust sandwich-type variance estimators to construct valid 95% confidence intervals of the hazard ratio, which was expressed as the mean percentage change in mortality.34
To maintain balance of covariates in the matched cohorts, we conducted our primary analysis according to the exposure group that an individual was initially assigned at baseline, regardless of any departure from that exposure during the five year follow-up. This is a close analogy to the intention-to-treat principle used extensively in randomised experiments, which provides unbiased effect estimates when there is non-compliance with the initial assignment.35 36 As a supportive analysis, we also conducted a per protocol analysis in which we censored individuals at the time when they moved to a different exposure group during follow-up (about 12%).
We performed a series of sensitivity analyses, including fitting a marginal Cox model that contained the exposure variable and index year only and further adjusting for the number of subsequent relocations and an indicator of whether the participants moved out of the province. To further evaluate the influence of possible non-adherence to the initial exposure, we implemented the inverse probability of censoring weighting in the survival analysis.37 The censoring weights were estimated using a logistic regression model with all available covariates. Because about 5% of the cohorts were excluded owing to missing information on covariates, we also applied multiple imputation and repeated all the analyses to assess the impact. To do this, we implemented multiple imputation by chained equations (with five iterations) and pooled the results following Rubin38 (see supplementary file: Multiple imputation). In addition, we repeated the analyses by retaining all people who had moved regardless of their involvement in multiple censuses, because the exclusion (about 16% of the cohorts) might reduce the representation of the Canadian population. Additionally, using the unmatched sample, we performed a standard Cox model adjusting for the same set of available covariates in propensity score matching model to assess whether our conclusions remain unaltered. Furthermore, we conducted a stratified analysis according to two age groups (25-64 years and ≥65 years), further adjusted for an indicator for whether the destination area had a retirement home, and considered age (in months) as an alternative time scale in the survival analysis (see supplementary file).
Lastly, we explored alternative follow-up periods, including the first year and first three years, and we repeated the analysis for cause specific mortality outcomes. All analyses were done using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.6.2 (R Core Team, Vienna, Austria).
Patient and public involvement
As this study used secondary data from existing databases, it was not feasible to involve study participants in the design, conduct, reporting, or dissemination plans of our research. This study was, however, partially inspired by previous informal conversations with members of the public about air pollution effects. Because of resource and time constraints, we did not involve members of the public in the design, conduct, reporting, or dissemination plans of the current study.
Results
Of 7.4 million respondents to the 1996, 2001, 2006 waves of CanCHEC, a total of 663 100 people who had relocated met the inclusion criteria (fig 1). Among them, 407 510 (61%) had been consistently exposed to high PM2.5 levels for ≥5 years before the census day (the high exposure cohort) and 255 590 (39%) had been exposed to low PM2.5 levels (the low exposure cohort).
Within five years after the censuses, 311 795 (77%) people in the high exposure cohort moved to another high PM2.5 area, 73 580 (18%) moved to an intermediate PM2.5 area, and 22 130 (5%) moved to a low PM2.5 area (table 1 and table 2). People who moved from a high to low PM2.5 area were on average four years older (about 51 years), more likely to have the lowest household income (17%), and less likely to be in an ethnic minority group (14%), compared with the other two exposure groups. People who moved tended to relocate outside larger metropolitan areas that were farther away from health services and were less ethnoculturally diverse. The other two exposure groups were similar for many characteristics but differed in neighbourhood socioeconomic status and urban form (active commuting and transit use) characteristics (table 2).
Table 1.
Baseline personal and clinical characteristics of study population who relocated within Canada, 1997-2006, by changes in exposure to fine particulate matter (PM2.5) arising from residential mobility
| Characteristics | History of high PM2.5 exposure* | History of low PM2.5 exposure* | |||||
|---|---|---|---|---|---|---|---|
| Moved within high PM2.5 area† (n=311 795) | Moved to moderate PM2.5 area† (n=73 580) | Moved to low PM2.5 area† (n=22 130) | Moved within low PM2.5 area† (n=190 535) | Moved to moderate PM2.5 area† (n=54 840) | Moved to high PM2.5† (n=10 215) | ||
| Personal characteristics | |||||||
| Mean (SD) age (years) | 47.3 (16.0) | 46.7 (15.4) | 51.0 (15.6) | 48.7 (15.5) | 50.3 (16.5) | 51.5 (17.1) | |
| Men | 165 250 (53) | 38 260 (52) | 11 510 (52) | 97 175 (51) | 28 515 (52) | 5210 (51) | |
| Race or ethnicity: | |||||||
| White or indigenous | 230 730 (74) | 52 980 (72) | 19 030 (86) | 184 820 (97) | 52 100 (95) | 9600 (94) | |
| Ethnic minority | 81 065 (26) | 20 600 (28) | 3100 (14) | 5715 (3) | 2740 (5) | 615 (6) | |
| Indigenous identity: | |||||||
| Not indigenous | 308 675 (99) | 72 845 (99) | 21 910 (99) | 177 200 (93) | 51 000 (93) | 9705 (95) | |
| Aboriginal | 3120 (1) | 735 (1) | 220 (1) | 13 335 (7) | 3840 (7) | 510 (5) | |
| Landed immigrant | 130 955 (42) | 33 110 (45) | 6860 (31) | 13 335 (7) | 6580 (12) | 1635 (16) | |
| Marital status: | |||||||
| Single | 62 360 (20) | 11 775 (16) | 3100 (14) | 20 960 (11) | 7680 (14) | 1635 (16) | |
| Common law | 21 825 (7) | 4415 (6) | 1550 (7) | 19 055 (10) | 4385 (8) | 715 (7) | |
| Married | 168 370 (54) | 47 090 (64) | 13 940 (63) | 125 755 (66) | 34 550 (63) | 6230 (61) | |
| Separated | 15 590 (5) | 2205 (3) | 665 (3) | 5715 (3) | 1645 (3) | 305 (3) | |
| Divorced | 24 945 (8) | 3680 (5) | 1330 (6) | 9525 (5) | 2740 (5) | 510 (5) | |
| Widowed | 18 710 (6) | 3680 (5) | 1550 (7) | 11 430 (6) | 3840 (7) | 815 (8) | |
| Education: | |||||||
| Less than high school | 74 830 (24) | 16 190 (22) | 4870 (22) | 60 970 (32) | 15 355 (28) | 2655 (26) | |
| High school | 102 890 (33) | 24 280 (33) | 7525 (34) | 68 595 (36) | 19 740 (36) | 3475 (34) | |
| Post-secondary non-university | 65 475 (21) | 16 925 (23) | 4870 (22) | 38 105 (20) | 11 515 (21) | 2145 (21) | |
| University | 68 595 (22) | 16 190 (22) | 4870 (22) | 20 960 (11) | 7680 (14) | 1940 (19) | |
| Employment: | |||||||
| Employed | 208 905 (67) | 52 980 (72) | 15 490 (70) | 120 035 (63) | 34 550 (63) | 5925 (58) | |
| Unemployed | 15 590 (5) | 2945 (4) | 665 (3) | 13 335 (7) | 2740 (5) | 615 (6) | |
| Not in labour force | 87 305 (28) | 17 660 (24) | 5975 (27) | 57 160 (30) | 17 550 (32) | 3675 (36) | |
| Occupation: | |||||||
| Management | 24 945 (8) | 6620 (9) | 2435 (11) | 13 335 (7) | 4385 (8) | 815 (8) | |
| Professional | 40 535 (13) | 10 300 (14) | 3320 (15) | 19 055 (10) | 6030 (11) | 1225 (12) | |
| Skilled | 59 240 (19) | 16 190 (22) | 4870 (22) | 47 635 (25) | 12 615 (23) | 2045 (20) | |
| Semi-skilled | 81 065 (26) | 19 865 (27) | 5090 (23) | 43 825 (23) | 12 065 (22) | 2145 (21) | |
| Unskilled | 28 060 (9) | 5150 (7) | 1330 (6) | 17 150 (9) | 3840 (7) | 715 (7) | |
| Not applicable | 77 950 (25) | 15 450 (21) | 5090 (23) | 49 540 (26) | 15 905 (29) | 3270 (32) | |
| Household income adequacy (10ths): | |||||||
| 10th (lowest) | 31 180 (10) | 8095 (11) | 3760 (17) | 20 960 (11) | 5485 (10) | 1020 (10) | |
| 9th | 28 060 (9) | 8830 (12) | 2655 (12) | 20 960 (11) | 5485 (10) | 920 (9) | |
| 8th | 28 060 (9) | 8830 (12) | 2435 (11) | 19 055 (10) | 5485 (10) | 1020 (10) | |
| 7th | 28 060 (9) | 8095 (11) | 2215 (10) | 19 055 (10) | 5485 (10) | 920 (9) | |
| 6th | 31 180 (10) | 8095 (11) | 2215 (10) | 19 055 (10) | 5485 (10) | 920 (9) | |
| 5th | 31 180 (10) | 7360 (10) | 1990 (9) | 19 055 (10) | 5485 (10) | 1020 (10) | |
| 4th | 34 295 (11) | 7360 (10) | 1990 (9) | 19 055 (10) | 5485 (10) | 1125 (11) | |
| 3rd | 34 295 (11) | 6620 (9) | 1770 (8) | 19 055 (10) | 6030 (11) | 1125 (11) | |
| 2nd | 34 295 (11) | 5885 (8) | 1770 (8) | 17 150 (9) | 5485 (10) | 1125 (11) | |
| 1st (highest) | 31 180 (10) | 4415 (6) | 1330 (6) | 15 245 (8) | 4935 (9) | 1020 (10) | |
| Clinical characteristics | |||||||
| Charlson comorbidity index over past three years: | |||||||
| 0 | 289 970 (93) | 69 165 (94) | 20 580 (93) | 177 200 (93) | 49 905 (91) | 9195 (90) | |
| 1 | 9355 (3) | 1470 (2) | 665 (3) | 5715 (3) | 2195 (4) | 410 (4) | |
| 2 | 6235 (2) | 1470 (2) | 445 (2) | 3810 (2) | 1645 (3) | 305 (3) | |
| ≥3 | 6235 (2) | 1470 (2) | 445 (2) | 3810 (2) | 1095 (2) | 305 (3) | |
Exposed to either highest or lowest third of PM2.5 consistently over five years preceding census day, based on annual concentrations of PM2.5 in cohort. All counts were rounded up to the nearest five in compliance with privacy requirements by Statistics Canada.
Defined by thirds of annual mean PM2.5 in cohort.
Table 2.
Baseline environmental and healthcare and socioeconomic characteristics of study population who relocated within Canada, 1997-2006, by changes in exposure to fine particulate matter (PM2.5) arising from residential mobility. Values are number (percentage) unless specified otherwise
| Characteristics | History of high PM2.5 exposure* | History of low PM2.5 exposure* | |||||
|---|---|---|---|---|---|---|---|
| Moved within high PM2.5† (n=311 795) | Moved to moderate PM2.5† (n=73 580) | Moved to low PM2.5† (n=22 130) | Moved within low PM2.5† (n=190 535) | Moved to moderate PM2.5† (n=54 840) | Moved to high PM2.5† (n=10 215) | ||
| Environmental characteristics | |||||||
| Mean (SD) annual PM2.5 averaged over five years previously (µg/m3) | 12.1 (2.1) | 11.6 (2.0) | 11.3 (2.0) | 4.6 (0.8) | 4.8 (0.8) | 4.7 (0.8) | |
| Mean (SD) baseline annual mean nitrogen dioxide (ppb) | 13.7 (3.6) | 11.8 (3.8) | 6.6 (3.6) | 4.3 (3.5) | 8.9 (4.2) | 11.5 (4.1) | |
| Mean (SD) baseline annual mean ozone (ppb) | 45.2 (6.0) | 45.2 (6.8) | 42.2 (7.1) | 33.0 (6.4) | 34.2 (7.4) | 40.5 (8.2) | |
| Mean (SD) baseline greenness within a 500 m buffer around home | 0.5 (0.1) | 0.5 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.4 (0.1) | 0.4 (0.1) | |
| Urban form characteristics: | |||||||
| Active urban core | 43 650 (14) | 2205 (3) | 220 (1) | 0 (0) | 6030 (11) | 1840 (18) | |
| Transit reliant suburb | 49 885 (16) | 2205 (3) | 0 (0) | 0 (0) | 2740 (5) | 1125 (11) | |
| Car reliant suburb | 183 960 (59) | 45 620 (62) | 6195 (28) | 24 770 (13) | 19 195 (35) | 4700 (46) | |
| Exurban | 6235 (2) | 10 300 (14) | 3760 (17) | 17 150 (9) | 1095 (2) | 205 (2) | |
| Non-CMA/CA | 28 060 (9) | 13 245 (18) | 11 950 (54) | 148 615 (78) | 25 775 (47) | 2350 (23) | |
| Lived in east central airshed | 308 675 (99) | 66 220 (90) | 17 485 (79) | 22 865 (12) | 12 065 (22) | 6025 (59) | |
| Healthcare and socioeconomic characteristics | |||||||
| Moved to another health region | 81 065 (26) | 30 905 (42) | 15 935 (72) | 41 920 (22) | 21 935 (40) | 6845 (67) | |
| Proximity to healthcare services ≤3 km (fifths): | |||||||
| 1st (lowest) | 43 650 (14) | 19 130 (26) | 8630 (39) | 26 675 (14) | 2195 (4) | 205 (2) | |
| 2nd | 49 885 (16) | 16 190 (22) | 2875 (13) | 22 865 (12) | 4935 (9) | 510 (5) | |
| 3rd | 53 005 (17) | 14 715 (20) | 1770 (8) | 20 960 (11) | 8225 (15) | 1225 (12) | |
| 4th | 59 240 (19) | 8095 (11) | 885 (4) | 15 245 (8) | 12 065 (22) | 2045 (20) | |
| 5th (highest) | 65 475 (21) | 3680 (5) | 220 (1) | 9525 (5) | 15 355 (28) | 4495 (44) | |
| >3 km | 40 535 (13) | 11 775 (16) | 7745 (35) | 95 270 (50) | 11 515 (21) | 1735 (17) | |
| Dependency (fifths): | |||||||
| 1st (lowest) | 53 005 (17) | 27 960 (38) | 4870 (22) | 26 675 (14) | 10 970 (20) | 2145 (21) | |
| 2nd | 56 125 (18) | 18 395 (25) | 4205 (19) | 24 770 (13) | 8225 (15) | 1635 (16) | |
| 3rd | 65 475 (21) | 11 035 (15) | 3540 (16) | 26 675 (14) | 8225 (15) | 1735 (17) | |
| 4th | 68 595 (22) | 8830 (12) | 3540 (16) | 38 105 (20) | 11 515 (21) | 2245 (22) | |
| 5th (highest) | 68 595 (22) | 7360 (10) | 5975 (27) | 74 310 (39) | 15 905 (29) | 2450 (24) | |
| Material deprivation (fifths): | |||||||
| 1st (lowest) | 56 125 (18) | 34 585 (47) | 9735 (44) | 45 730 (24) | 9870 (18) | 1735 (17) | |
| 2nd | 62 360 (20) | 16 925 (23) | 5310 (24) | 38 105 (20) | 12 065 (22) | 2145 (21) | |
| 3rd | 62 360 (20) | 11 035 (15) | 3320 (15) | 28 580 (15) | 13 160 (24) | 2245 (22) | |
| 4th | 62 360 (20) | 5885 (8) | 1550 (7) | 26 675 (14) | 10 420 (19) | 2245 (22) | |
| 5th (highest) | 68 595 (22) | 5150 (7) | 2215 (10) | 51 445 (27) | 9325 (17) | 1840 (18) | |
| Residential instability (fifths): | |||||||
| 1st (lowest) | 53 005 (17) | 38 260 (52) | 9295 (42) | 60 970 (32) | 6580 (12) | 1125 (11) | |
| 2nd | 62 360 (20) | 19 865 (27) | 8630 (39) | 60 970 (32) | 10 970 (20) | 1840 (18) | |
| 3rd | 71 715 (23) | 6620 (9) | 2875 (13) | 40 010 (21) | 13 160 (24) | 2045 (20) | |
| 4th | 71 715 (23) | 5150 (7) | 885 (4) | 20 960 (11) | 14 260 (26) | 2655 (26) | |
| 5th (highest) | 53 005 (17) | 3680 (5) | 445 (2) | 7620 (4) | 9870 (18) | 2555 (25) | |
| Ethnic concentration (fifths): | |||||||
| 1st (lowest) | 40 535 (13) | 13 245 (18) | 8190 (37) | 78 120 (41) | 12 615 (23) | 1635 (16) | |
| 2nd | 56 125 (18) | 12 510 (17) | 5090 (23) | 49 540 (26) | 15 355 (28) | 2245 (22) | |
| 3rd | 53 005 (17) | 11 775 (16) | 2655 (12) | 30 485 (16) | 12 615 (23) | 2245 (22) | |
| 4th | 65 475 (21) | 12 510 (17) | 2435 (11) | 19 055 (10) | 8225 (15) | 2045 (20) | |
| 5th (highest) | 96 655 (31) | 23 545 (32) | 3760 (17) | 13 335 (7) | 6030 (11) | 2045 (20) | |
CMA=census metropolitan area; CA=census agglomeration area.
Exposed to either highest or lowest third of PM2.5 consistently over five years preceding census day, based on annual concentrations of PM2.5 in cohort. All counts were rounded up to the nearest five in compliance with privacy requirements by Statistics Canada.
Defined by thirds of annual mean PM2.5 in cohort.
In the low exposure cohort, 190 535 (75%) people moved to another low PM2.5 area, 54 840 (21%) moved to an intermediate PM2.5 area, and 10 215 (4%) moved to a high PM2.5 area (table 1 and table 2). Those who moved from a low to high PM2.5 area were more likely than the other two exposure groups to be immigrants (16%) and to have a university degree (18%). They also had more comorbidities and were more likely to move to metropolitan areas in a different health region but closer to health services. Household income was similar among all three groups.
After propensity score matching, four comparisons were formed related to movements between PM2.5 areas: high to low (or high to intermediate) versus high to high group, and low to high (or low to intermediate) versus low to low group (see supplementary file: Table S1). Matching substantially increased the similarity between all the comparison groups, with standardised differences <0.1 for all covariates examined (see supplementary file: Figure S1). This was further supported by the similar distribution of all the variables between these comparisons after matching (see supplementary file: Figures S2-S5).
Changes in exposure to air pollution
In the matched groups with previous high exposure to PM2.5, the annual mean PM2.5 level was 10.6 µg/m3 in the year before moving. After moving, exposure to PM2.5 remained about 10.0 µg/m3 in the high to high exposure group, but exposure was noticeably reduced to 7.4 µg/m3 and 5.0 µg/m3 in the high to intermediate exposure and high to low exposure groups, respectively (see supplementary file: Figure S6). Conversely, in the matched groups with previous low exposure, the annual mean PM2.5 level was 4.5 µg/m3 in the year before moving, compared with 4.6 µg/m3 in the low to low exposure group, 6.7 µg/m3 in the low to intermediate exposure group, and 9.2 µg/m3 in the low to high exposure group after moving. As expected, the annual mean levels of nitrogen dioxide and ozone were similar between the matched groups (see supplementary file: Figures S2-S5).
Changes in mortality
Within five years of moving, a total of 8965 people died in the matched groups with previous high exposure to PM2.5 and 8035 in the matched group with previous low exposure. In intention-to-treat analysis, compared with those who moved from a high PM2.5 area to another high PM2.5 area, those who moved to low PM2.5 areas had fewer disease related deaths after a mean reduction of 5.3 µg/m3 in PM2.5 (mean percentage change in mortality −12.8%, 95% confidence interval −23.0% to −1.3%; table 3). This corresponded to a −2.1% (95% confidence interval −4.3% to 0.1%) change in mortality per µg/m3 reduction in PM2.5. Additionally, among those who moved from high PM2.5 to intermediate PM2.5 areas, mean percentage change in mortality was −6.8% (−11.7% to −1.7%) after a mean reduction of 3.3 µg/m3 in PM2.5. This corresponded to a −1.4% (−2.8% to 0.1%) change in mortality per µg/m3 reduction in PM2.5. In contrast, mean percentage change in mortality in those who moved from low PM2.5 to intermediate or high PM2.5 areas was 1.8% (−4.1% to 6.9%) and 13.2% (−1.5% to 30.2%), respectively. These findings corresponded to a 0.2% and 1.6% increase in mortality per µg/m3 increase in PM2.5.
Table 3.
Estimated associations between changes in exposure to fine particulate matter (PM2.5) as a result of relocating (defined by thirds) and risk of disease related mortality in Canada, 1997 to 2016, by intention-to-treat analysis and per protocol analysis, respectively. Values are percentage change (95% confidence intervals) unless stated otherwise
| Change in exposure | No of participants | Intention-to-treat analysis | Per protocol analysis | |||||
|---|---|---|---|---|---|---|---|---|
| No of events | Exposure groups compared | Per µg/m3 change in PM2.5 | No of events | Exposure groups compared | Per µg/m3 change in PM2.5 | |||
| High to low PM2.5 area* | ||||||||
| Change in mortality | 25 310 | 1530 | −12.8 (−23.0 to −1.3) | −2.1 (−4.3 to 0.1) | 1415 | −14.4 (−24.9 to −2.4) | −2.4 (−4.7 to 0) | |
| High to intermediate PM2.5 area* | ||||||||
| Change in mortality | 157 985 | 7435 | −6.8 (−11.7 to −1.7) | −1.4 (−2.8 to 0.1) | 6770 | −10.4 (−15.4 to −5.1) | −2.6 (−4.1 to −1.0) | |
| Low to intermediate PM2.5 area† | ||||||||
| Change in mortality | 112 650 | 6960 | 1.2 (−4.1 to 6.9) | 0.2 (−2.0 to 2.4) | 6290 | 0.2 (−5.5 to 6.2) | 0 (−2.4 to 2.4) | |
| Low to high PM2.5 area† | ||||||||
| Change in mortality | 15 940 | 1075 | 13.2 (−1.5 to 30.2) | 1.6 (−1.2 to 4.5) | 955 | 10.4 (−5.1 to 28.5) | 1.0 (−2.0 to 4.2) | |
Reference level is moving from high to high PM2.5 environments (defined by upper third). All counts were rounded up to nearest five in compliance with privacy requirements by Statistics Canada.
Reference level is moving from low to low PM2.5 environments (defined by lower third). All counts were rounded up to nearest five in compliance with privacy requirements by Statistics Canada.
In the per protocol analysis, where individuals were censored for not adhering to the initial exposure, the associations were strengthened for those with reduced exposure to PM2.5 and were somewhat attenuated for those with increased exposure to PM2.5, but the overall patterns of associations remained broadly consistent (table 3). Across all analyses, greater changes in mortality were found in individuals with larger changes in exposure to PM2.5.
In sensitivity analyses, reductions in mortality in association with decreases in PM2.5 remained quantitatively consistent (fig 2). For example, the association was unaltered when considering a marginal model, adjusting for residential mobility, and performing multiple imputation (see supplementary file: Table S2). Restricting to the first one year and three years in follow-up yielded similar results as that using the entire follow-up (five years). When the inverse probability censoring weighting approach was applied, noticeable reductions in mortality associated with decreases in PM2.5 remained (fig 2). Analogously, the associations between higher PM2.5 and increased mortality did not change appreciably in the sensitivity analyses, although the association appeared somewhat strengthened when the analysis was restricted to the first year of follow-up.
Fig 2.
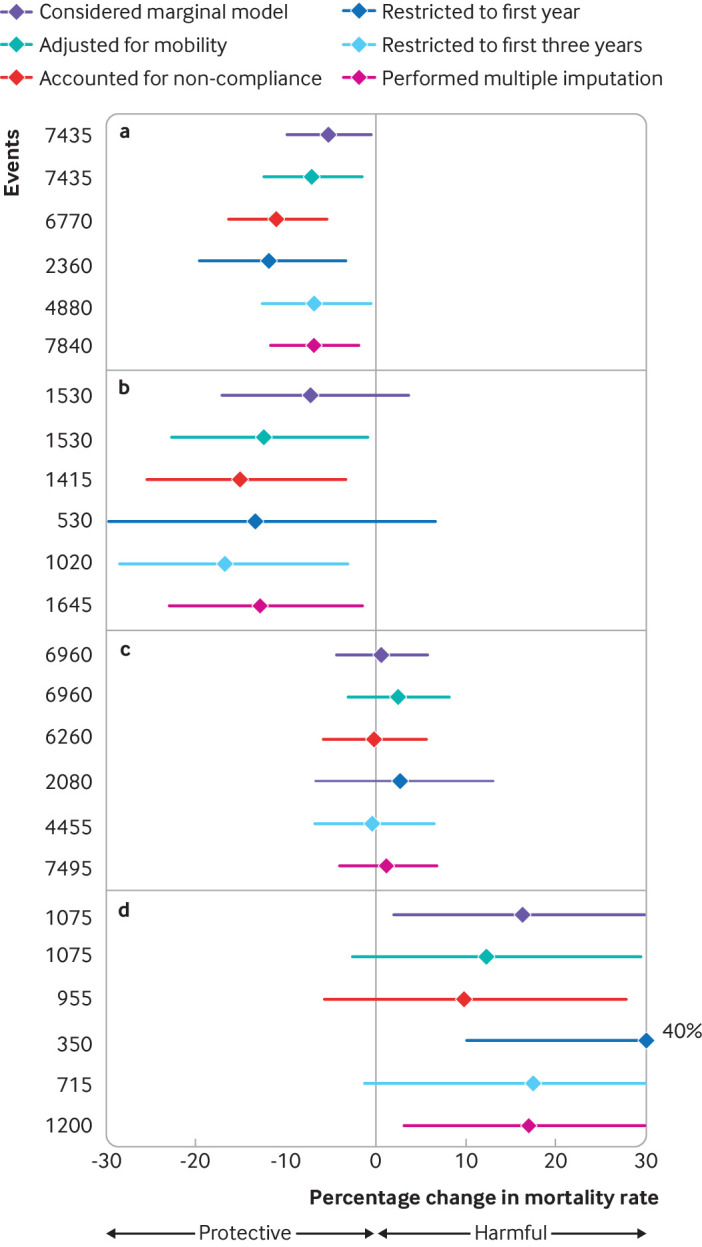
Sensitivity analysis of associations between changes in residential fine particulate matter (PM2.5) and risk of disease related mortality when moving from (a) high to intermediate, (b) high to low, (c) low to intermediate, and (d) low to high PM2.5 areas in Canada, 1997 to 2016
Similar results to the main analysis were obtained from a sensitivity analysis in which a standard Cox model with the unmatched cohorts was considered. Despite the unmatched cohorts being more heterogeneous than the matched cohorts, the associations between PM2.5 and mortality remained evident (eg, mean percentage change in mortality when moving from a high to low PM2.5 area was −14.3% and from a low to high PM2.5 area was 9.1%; see supplementary file: Table S3). In addition, similar results were obtained when all individuals were included irrespective of whether they were captured by multiple censuses (see supplementary file: Table S4). Furthermore, the risk estimates were observed to remain quantitatively similar between the two age groups and after further adjustment for the indicator for whether moving to the destination area with a retirement home (see supplementary file: Table S5).
Lastly, the decreases in PM2.5 levels were found most strongly associated with reductions in cardiometabolic deaths, whereas the increases in PM2.5 levels were mostly related to respiratory deaths (fig 3). No strong evidence was found with cancer related deaths.
Fig 3.
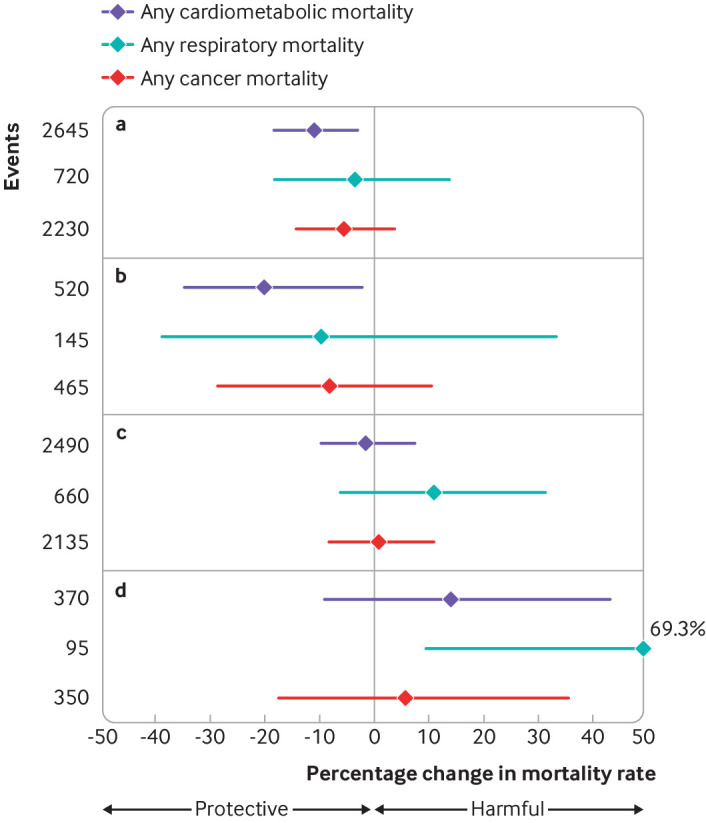
Estimated associations between changes in residential fine particulate matter (PM2.5) and risk of cause specific mortality when moving from (a) high to intermediate, (b) high to low, (c) low to intermediate, and (d) low to high PM2.5 areas in Canada, 1997 to 2016
Discussion
With progressive declines in PM2.5 and other common air pollutants in many regions worldwide over the past few decades, interest in the extent to which these decreases might reduce long term adverse effects of air pollution has increased. Understanding the health benefits of reductions in air pollutants, especially at relatively low concentrations, would have broad implications for future risk assessment and regulations. In a quasi-experimental study in Canada, we observed that reductions in ambient PM2.5 arising from residential mobility were associated with decreases in disease related mortality. For example, among individuals who moved from a high to intermediate PM2.5 area that corresponded to a mean reduction of 3.3 μg/m3 in PM2.5, a 6.8% reduction in mortality was observed over the five years after the move. A greater decline in mortality was found in those who experienced a larger reduction in PM2.5 levels after moving. These PM2.5 related effects on mortality were consistent in various sensitivity analyses and persisted over the five years after moving. Furthermore, we found that the decline was the greatest for cardiometabolic deaths in relation to the decreases in PM2.5 levels. Coincided with these observations, we also observed noticeable increases in mortality among individuals who moved from a low to a high PM2.5 area, and to a lesser degree, from a low to an intermediate PM2.5 area.
Limitations and strengths of this study
Our findings should be interpreted in light of some limitations. Firstly, causal inference tools such as propensity score matching rely on a key assumption of no unmeasured confounding. Although we derived numerous covariates, we lacked information on individual level behavioural risk factors such as smoking and physical activity. To tackle this problem, we included various covariates for personal and socioeconomic characteristics, comorbidities, access to health services, and neighbourhood deprivation. Because income, education, ethnicity, and other personal and socioeconomic factors are strongly associated with personal behavioural factors,39 40 and comorbidities and mortality share some common behavioural factors, adjusting for these covariates should reduce the influence of the unmeasured covariates on our results. In addition, people living in less populous or rural areas in Canada are known to have higher prevalence of smoking and obesity and are more likely to die prematurely than their urban counterparts.41 42 These are also the areas with relatively low PM2.5 levels. If those individuals who moved to a low PM2.5 area tended to be affected by these factors, this might potentially bias the associations downwards. Secondly, although we considered common environmental factors, including ozone, nitrogen dioxide, and greenspaces, as well as neighbourhood level deprivation, we could not further account for environmental noise because this information was not available. However, previous studies from the United States43 and Canada44 have shown that noise levels are weakly associated with PM2.5 levels. Thirdly, information on household membership was unavailable, although it is unclear whether potential clustering at the household level would influence the results after adjustment for available variables. Given the nature of observational data, we acknowledge that our inability to completely rule out the possibility of unmeasured confounding is a shortcoming. Fourthly, our exposure assessment was based on postal codes, which do not completely reflect personal exposure. Additionally, our study considered a relatively short exposure time window over several years, as opposed to decades or longer. Given the inherent imprecision of the spatially derived exposure, our exposure assessment was likely subject to non-differential misclassification that might have attenuated our results. Lastly, people who moved home were likely to be different from the general population. Although our quasi-experimental design to construct a tightly matched study of people who moved improved the comparability of different comparison groups in this study, it came at the expense of a reduction in statistical power and generalisability.
A major strength of this study was the quasi-experimental population based design using modern techniques for causal inference and adjustment of confounders. This allowed us to disentangle more direct evidence about the changes in exposure to PM2.5 on mortality, which is of major public health importance. Through emulating a hypothetical randomised experiment, this study compared mortality risk in individuals who experienced changes in PM2.5 to what would have been expected in the absence of changes in PM2.5 over the same periods. By design, this eliminated any temporal confounding by chronic trends in outcomes that might affect previous studies using a before-and-after design.8 9 10 In addition, the large nationally represented cohort with detailed information on residential histories offered a unique opportunity to construct meaningful changes in PM2.5 and evaluate the effect on mortality, especially at the relatively low levels of PM2.5. As well, we obtained extensive individual level information on income, education, race or ethnicity, health status, access to health services, environmental factors, and many other characteristics, which allowed for good control for known risk factors. Notably, we were able to directly adjust for exact income obtained from the revenue office, as opposed to self-reported income in broad categories as done in many previous studies. Aspects of our analytical approach also reduced concerns about confounding, such as adjustment for these factors using propensity score matching and outcome regression. This allowed us to minimise confounding as a result of any residual imbalance in covariates. Furthermore, our use of multiple cohorts (for characterising an array of changes in PM2.5), two complementary risk measures (representing both relative and absolute changes in PM2.5), and a negative control allowed us to further validate our findings. Lastly, the use of satellite based estimates of PM2.5 ensured virtually complete spatial coverage of exposure to PM2.5 for the cohort.
Comparison with other studies and discussion of potential mechanism
This study adds to a small but emerging body of literature that has shown a direct impact of reductions in PM2.5 on improving survival.8 9 10 45 A study on a ban of coal sales in Dublin and adjacent cities in Ireland, examined the relation between the ensuing reduction in black smoke concentrations and mortality.8 When the study compared weekly mortality rates before and after the coal ban, a strong association was found between reduced black smoke levels and decreases in respiratory mortality.8 A study in Tokyo, Japan similarly linked reductions in PM2.5 resulting from regulatory action on diesel emissions to the decreases in daily rates of cardiorespiratory and lung cancer deaths.10 More recently, a cohort study of 10 million Medicare beneficiaries in the US compared all cause mortality and other health outcomes between regions with and without non-attainment designations for the national ambient air quality standards before and after the designations were introduced.9 The study found that reduced PM2.5 levels from the non-attainment designations were associated with statistically significant improvements in these outcomes. Furthermore, using data on PM2.5 and life expectancy for 211 US counties between 1978 and 2001, a study assessed the impact of the natural course of declining PM2.5 and found that for every 10 µg/m3 reduction in PM2.5, there was an associated increase of about 0.61 years in life expectancy.45 The average reduction in PM2.5 across the 211 US counties was 6.5 µg/m3, which is similar to that among the participants in the present study who moved from a high to low PM2.5 area (5.0 µg/m3). This underscores an appealing aspect of utilising residential mobility as a natural experiment to construct meaningful changes in exposure that allowed more direct observations on the health effects of air pollutants.
A similar approach was used in two previous studies, where changes in residential proximity to roadways were found to be associated with cardiovascular mortality in Vancouver, Canada11 and with all cause mortality and incidence of acute myocardial infarction in the Nurses’ Health Study in the US.12 Despite heterogeneity in study designs, exposure measurements, health outcomes, and analytical methods across studies, our study supports these previous results. Notably, the trend in mortality reduction with lower PM2.5 levels found in the present study remains clearly evident, even at PM2.5 concentrations considerably lower than in many cities worldwide (eg, annual mean PM2.5 in Los Angeles, CA was 11 μg/m3 in 2014, in London, UK was 15 μg/m3 in 2013, in Mumbai, India was 63 μg/m3 in 2013, and in Beijing, China was 85 μg/m3 in 2013),14 indicating the potential benefits from continuously mitigating air pollution. In addition, the PM2.5 levels in Canada were largely attributable to anthropogenic sources, as in many other developed and low-to-middle income countries. This further highlights the potential achievability of ongoing air quality improvements.
This study also linked increased PM2.5 concentrations arising from residential mobility to increased mortality, which is in line with mounting evidence showing the impact of PM2.5 on increasing mortality in diverse locations, including Europe and north America. For example, in a large cohort study comprising eight European cohorts, a positive association was observed between PM2.5 level and deaths from natural causes.46 In a US national cohort of about 1.6 million respondents to the national health interview surveys, higher PM2.5 levels were associated with all cause mortality and mortality from cardiopulmonary causes and lung cancer.47 Similar associations were reported in a Canadian study using the 2001 CanCHEC.16 More recently, using the 2001 and other waves of CanCHEC, a study estimated a 5.3% increase in disease related mortality for every 10 µg/m3 increase in PM2.5. 3 That study also reported a supralinear shape in the relation between PM2.5 concentration and mortality, with a steeper increase in the mortality risk at the lower levels of PM2.5.3 Conversely, we observed a greater increase in mortality in individuals exposed to increased PM2.5 from low to high levels than from low to intermediate levels (1.6% v 0.2% increase in mortality per μg/m3 increase in PM2.5). This could be explained by the relatively few deaths in our matched cohorts. In addition, the differences in study designs and methodologies are likely to be important. In the present study, we constructed a tightly matched study of people who moved using the quasi-experimental design in conjunction with propensity score matching. By design, this study explicitly excluded those who were inherently different from each other in the comparison groups, and thus avoided using parametric extrapolations as done using more traditional approaches. Other factors that could also contribute to these differences include optimising covariate balance before running Cox models and defining the changes in PM2.5 arising from a specific real world action that is independent from treatment status (rather than relying on the variation presented by nature). Thus, the risk estimates obtained here are not necessarily equal to those published previously.
In the present study, we also observed a reduction in cardiometabolic deaths associated with lower PM2.5 levels and increased respiratory deaths associated with higher PM2.5 levels. Cardiorespiratory deaths have been frequently linked to short term fluctuations in PM2.5 levels.48 In line with this, we observed a trend for a stronger association of mortality with increases in PM2.5 levels for the first year than for longer follow-up (see fig 2). This pattern was less clear for the association with decreases in PM2.5 levels, which persisted over the entire follow-up. Although this could be attributed to chance alone, it might also suggest that peaks in air pollution can trigger an immediately increased risk of mortality, but the converse might not be true. Given the importance of exposure to PM2.5 on public health, understanding the timing of exposure on mortality, especially for different directions in the changes of PM2.5 levels, merits further investigation. As well, with progressive declines in other pollutants (eg, nitrogen dioxide and components of PM2.5), it would be invaluable to elucidate the joint impacts of reducing exposures to PM2.5 and these other pollutants on population health.
Conclusions
The findings from this quasi-experimental study strengthen the evidence that a decrease in PM2.5 levels, even at the relatively low levels seen in Canada, was associated with improvements in mortality outcomes. The study also shows the adverse effect of increased PM2.5 levels on worsening mortality. These results provide support for continuously improving air quality.
What is already known on this topic
Ambient air pollution, especially from fine particulate matter (PM2.5), is a global public health concern
With progressive declines in PM2.5 and other common air pollutants in many developed as well as low-to-middle income countries over the past few decades, interest in the extent to which these decreases might improve public health is mounting
Understanding the benefits of declines in PM2.5 on human health, especially at relatively low levels, would have broad implications for future risk assessment and regulations
What this study adds
An association was observed between decreases in long term exposure to PM2.5 and lower mortality, whereas increases in long term exposure to PM2.5 were associated with an increase in mortality
These results were obtained at relatively low levels of PM2.5, underscoring the need for further improvements in air quality
Acknowledgments
RTB had retired from Health Canada by the end of this study.
Web extra.
Extra material supplied by authors
Supplementary material: additional information, tables, and figures
Contributors: HC conceived the study. HC, TB, and JSK contributed to the study design. LP and TO prepared and cleaned the data. AvD, RVM, and PH contributed to exposure assessment. HC, LC, TB, JSK, RTB, TO, and LP contributed to data analyses. HC took the lead in drafting the manuscript. All authors contributed to the interpretation of data, provided critical revisions to the manuscript, and approved the final draft. HC is the guarantor. HC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by Health Canada (#810630). The funders had no role in considering the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from funded by Health Canada for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (HC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Findings will be disseminated via media outreach to the general public—for example, press releases by the media departments of the authors’ research institutes, and plain language publications in social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the Research Ethics Board of Health Canada and Public Health Agency of Canada.
Data availability statement
Statistics Canada’s policy on data privacy and confidentiality prohibits the analytical cohorts used (1996, 2001, and 2006 CanCHEC) to be freely available in the manuscript, supplementary files, or in a public repository. However, access can be granted through Statistics Canada’s Research Data Centre program. The programs used to assign environmental exposures (PCCF+ and postal code imputation) are also available to researchers through subscription or request. Environmental exposures are available on request to the original authors of the data. The analytical code used was all standard R and SAS code (eg, matchit, mice, glm, coxph, data steps).
References
- 1. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223-49. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Q, Wang Y, Zanobetti A, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med 2017;376:2513-22. 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pappin AJ, Christidis T, Pinault LL, et al. Examining the Shape of the Association between Low Levels of Fine Particulate Matter and Mortality across Three Cycles of the Canadian Census Health and Environment Cohort. Environ Health Perspect 2019;127:107008. 10.1289/EHP5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tony Cox LA, Jr. Rethinking the Meaning of Concentration-Response Functions and the Estimated Burden of Adverse Health Effects Attributed to Exposure Concentrations. Risk Anal 2016;36:1770-9. 10.1111/risa.12670 [DOI] [PubMed] [Google Scholar]
- 5. Zigler CM, Dominici F. Point: clarifying policy evidence with potential-outcomes thinking--beyond exposure-response estimation in air pollution epidemiology. Am J Epidemiol 2014;180:1133-40. 10.1093/aje/kwu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dominici F, Greenstone M, Sunstein CR. Science and regulation. Particulate matter matters. Science 2014;344:257-9. 10.1126/science.1247348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boogaard H, van Erp AM, Walker KD, Shaikh R. Accountability Studies on Air Pollution and Health: the HEI Experience. Curr Environ Health Rep 2017;4:514-22. 10.1007/s40572-017-0161-0 [DOI] [PubMed] [Google Scholar]
- 8. Dockery DW, Rich DQ, Goodman PG, et al. HEI Health Review Committee . Effect of air pollution control on mortality and hospital admissions in Ireland. Res Rep Health Eff Inst 2013;(176):3-109. [PubMed] [Google Scholar]
- 9. Zigler CM, Choirat C, Dominici F. Impact of National Ambient Air Quality Standards Nonattainment Designations on Particulate Pollution and Health. Epidemiology 2018;29:165-74. 10.1097/EDE.0000000000000777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yorifuji T, Kashima S, Doi H. Fine-particulate Air Pollution from Diesel Emission Control and Mortality Rates in Tokyo: A Quasi-experimental Study. Epidemiology 2016;27:769-78. 10.1097/EDE.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 11. Gan WQ, Tamburic L, Davies HW, Demers PA, Koehoorn M, Brauer M. Changes in residential proximity to road traffic and the risk of death from coronary heart disease. Epidemiology 2010;21:642-9. 10.1097/EDE.0b013e3181e89f19 [DOI] [PubMed] [Google Scholar]
- 12. Hart JE, Rimm EB, Rexrode KM, Laden F. Changes in traffic exposure and the risk of incident myocardial infarction and all-cause mortality. Epidemiology 2013;24:734-42. 10.1097/EDE.0b013e31829d5dae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tjepkema M, Christidis T, Bushnik T, Pinault L. Cohort profile: The Canadian Census Health and Environment Cohorts (CanCHECs). Health Rep 2019;30:18-26. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO Global Urban Ambient Air Pollution Database (update 2016). https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ambient-air-pollution, Access date: May 1, 2021.
- 15. Meng J, Martin RV, Li C, et al. Source Contributions to Ambient Fine Particulate Matter for Canada. Environ Sci Technol 2019;53:10269-78. 10.1021/acs.est.9b02461 [DOI] [PubMed] [Google Scholar]
- 16. Pinault LL, Weichenthal S, Crouse DL, et al. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environ Res 2017;159:406-15. 10.1016/j.envres.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 17. Pope CA, 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004;109:71-7. 10.1161/01.CIR.0000108927.80044.7F [DOI] [PubMed] [Google Scholar]
- 18. Pope CA, 3rd, Turner MC, Burnett RT, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 2015;116:108-15. 10.1161/CIRCRESAHA.116.305060 [DOI] [PubMed] [Google Scholar]
- 19. Brook RD, Cakmak S, Turner MC, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013;36:3313-20. 10.2337/dc12-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Donkelaar A, Martin RV, Brauer M, Boys BL. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect 2015;123:135-43. 10.1289/ehp.1408646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 22. Roy N, Dubé R, Després C, Freitas A, Légaré F. Choosing between staying at home or moving: A systematic review of factors influencing housing decisions among frail older adults. PLoS One 2018;13:e0189266. 10.1371/journal.pone.0189266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Celis-Morales CA, Lyall DM, Welsh P, et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. BMJ 2017;357:j1456. 10.1136/bmj.j1456 [DOI] [PubMed] [Google Scholar]
- 24. Fong KC, Hart JE, James P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017. Curr Environ Health Rep 2018;5:77-87. 10.1007/s40572-018-0179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crouse DL, Pinault L, Balram A, et al. Urban greenness and mortality in Canada’s largest cities: a national cohort study. Lancet Planet Health 2017;1:e289-97. 10.1016/S2542-5196(17)30118-3 [DOI] [PubMed] [Google Scholar]
- 26.Statistics Canada. Proximity Measures Database. 2020. https://www150.statcan.gc.ca/n1/pub/17-26-0002/172600022020001-eng.htm, Access date: Oct 1, 2020.
- 27. Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat 2002;84:151-61 10.1162/003465302317331982. [DOI] [Google Scholar]
- 28. Brauer M, Brook JR, Christidis T, et al. Mortality-Air Pollution Associations in Low-Exposure Environments (MAPLE): Phase 1. Res Rep Health Eff Inst 2019;(203):1-87. [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenbaum PR. Modern algorithms for matching in observational studies. Annu Rev Stat Appl 2020;7:143-76 10.1146/annurev-statistics-031219-041058. [DOI] [Google Scholar]
- 30. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ouldali N, Toubiana J, Antona D, et al. French Covid-19 Paediatric Inflammation Consortium . Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA 2021;325:855-64. 10.1001/jama.2021.0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17:78. 10.1186/s12874-017-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837-49. 10.1002/sim.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342:d40. 10.1136/bmj.d40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ 2001;165:1339-41. [PMC free article] [PubMed] [Google Scholar]
- 37. Cain LE, Cole SR. Inverse probability-of-censoring weights for the correction of time-varying noncompliance in the effect of randomized highly active antiretroviral therapy on incident AIDS or death. Stat Med 2009;28:1725-38. 10.1002/sim.3585 [DOI] [PubMed] [Google Scholar]
- 38. Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 1987. 10.1002/9780470316696. [DOI] [Google Scholar]
- 39. Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99-106. 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 40. Janssen I, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am J Clin Nutr 2006;83:139-45. 10.1093/ajcn/83.1.139 [DOI] [PubMed] [Google Scholar]
- 41. DesMeules M, Pong R, Lagacé C, et al. How healthy are rural Canadians? An assessment of their health status and health determinants. Canadian Institute for Health Information, 2006. [Google Scholar]
- 42. Pampalon R, Martinez J, Hamel D. Does living in rural areas make a difference for health in Québec? Health Place 2006;12:421-35. 10.1016/j.healthplace.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 43. Ross Z, Kheirbek I, Clougherty JE, et al. Noise, air pollutants and traffic: continuous measurement and correlation at a high-traffic location in New York City. Environ Res 2011;111:1054-63. 10.1016/j.envres.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 44. Gan WQ, McLean K, Brauer M, Chiarello SA, Davies HW. Modeling population exposure to community noise and air pollution in a large metropolitan area. Environ Res 2012;116:11-6. 10.1016/j.envres.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 45. Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 2009;360:376-86. 10.1056/NEJMsa0805646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strak M, Weinmayr G, Rodopoulou S, et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ 2021;374:n1904. 10.1136/bmj.n1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pope CA, 3rd, Lefler JS, Ezzati M, et al. Mortality Risk and Fine Particulate Air Pollution in a Large, Representative Cohort of U.S. Adults. Environ Health Perspect 2019;127:77007. 10.1289/EHP4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: update in relation to the use of generalized additive models. J Air Waste Manag Assoc 2003;53:258-61. 10.1080/10473289.2003.10466149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: additional information, tables, and figures
Data Availability Statement
Statistics Canada’s policy on data privacy and confidentiality prohibits the analytical cohorts used (1996, 2001, and 2006 CanCHEC) to be freely available in the manuscript, supplementary files, or in a public repository. However, access can be granted through Statistics Canada’s Research Data Centre program. The programs used to assign environmental exposures (PCCF+ and postal code imputation) are also available to researchers through subscription or request. Environmental exposures are available on request to the original authors of the data. The analytical code used was all standard R and SAS code (eg, matchit, mice, glm, coxph, data steps).