Abstract
Immune monitoring of kidney allograft recipients and personalized therapeutics may help reach the aspirational goal of “one transplant for life.” The invasive kidney biopsy procedure, the diagnostic tool of choice, has become safer and the biopsy classification more refined. Nevertheless, biopsy-associated complications, interobserver variability in biopsy specimen scoring, and costs continue to be significant concerns. The dynamics of the immune repertoire make frequent assessments of allograft status necessary, but repeat biopsies of the kidney are neither practical nor safe. To address the existing challenges, we developed urinary cell mRNA profiling and investigated the diagnostic, prognostic, and predictive accuracy of absolute levels of a hypothesis-based panel of mRNAs encoding immunoregulatory proteins. Enabled by our refinements of the PCR assay and by investigating mechanistic hypotheses, our single-center studies identified urinary cell mRNAs associated with T cell–mediated rejection, antibody-mediated rejection, interstitial fibrosis and tubular atrophy, and BK virus nephropathy. In the multicenter National Institutes of Health Clinical Trials in Organ Transplantation-04, we discovered and validated a urinary cell three-gene signature of T-cell CD3 ε chain mRNA, interferon gamma inducible protein 10 (IP-10) mRNA, and 18s ribosomal RNA that is diagnostic of subclinical acute cellular rejection and acute cellular rejection and prognostic of acute cellular rejection and graft function. The trajectory of the signature score remained flat and below the diagnostic threshold for acute cellular rejection in the patients with no rejection biopsy specimens, whereas a sharp rise was observed during the weeks before the biopsy specimen that showed acute cellular rejection. Our RNA sequencing and bioinformatics identified kidney allograft biopsy specimen gene signatures of acute rejection to be enriched in urinary cells matched to acute rejection biopsy specimens. The urinary cellular landscape was more diverse and more enriched for immune cell types compared with kidney allograft biopsy specimens. Urinary cell mRNA profile–guided clinical trials are needed to evaluate their value compared with current standard of care.
Keywords: urinary cell mRNA, kidney transplantation, acute allograft rejection, gene expression, kidney biopsy, mRNA, allografts, kidney transplantation series
Introduction
Although kidney transplantation is the treatment of choice for patients with irreversible kidney failure, the long-term outcome of transplanted kidneys has not improved substantially over the years (1,2). Allograft rejection, nephrotoxic drugs, nonadherence, metabolic factors, and kidney disease recurrence have undermined the aspirational goal of “one transplant for life,” so much so that 12% of the patients waitlisted for a kidney transplant are repeat transplants (1,3).
The kidney allograft biopsy remains an essential diagnostic component. This invasive procedure, however, is not without risks such as bleeding, arteriovenous fistula formation, and even death, albeit in rare cases. Biopsy-associated costs and interobserver variability in biopsy specimen scoring are additional concerns (4,5). Serum creatinine, used to monitor kidney allograft status, is nonspecific and has a low sensitivity to detect acute rejection, as reflected by acute rejection being detected on surveillance biopsy specimens without a concurrent increase in creatinine level (6–9). A compelling need exists for more sensitive and more specific tools, preferably noninvasive, to assess kidney allograft status. The immune response is dynamic, and a noninvasive tool would have the advantage of monitoring its kinetics. We postulate that the much-needed transition toward precision medicine would be accelerated by the development of noninvasive biomarkers of kidney allograft status.
Kidney Allograft: An In Vivo Flow Cytometer?
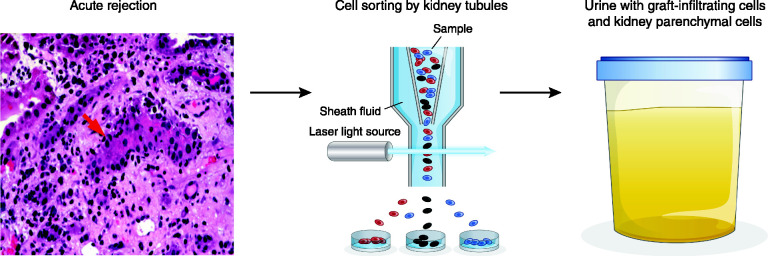
Acute T cell–mediated rejection is characterized by the infiltration of the kidney allograft by T cells, macrophages, and an assortment of other cell types. The concurrent presence of graft-infiltrating cells in the interstitial space and the presence of cells within the tubules (tubulitis) are the histologic hallmarks of T cell–mediated rejection (10). We hypothesized that the kidney allograft undergoing immune rejection functions as an “in vivo flow cytometer,” sorting graft-infiltrating cells and targeted kidney parenchymal cells into urine, and that mRNA phenotyping of urinary cells offers a noninvasive means of diagnosing immune rejection (Figure 1) (11).
Figure 1.
Formulation that a kidney allograft functions as an in vivo flow cytometer. Acute T cell–mediated rejection is characterized by the infiltration of the kidney allograft by T cells, macrophages, and other cell types. The concurrent presence of graft-infiltrating cells in the interstitial space and the presence of cells within the tubules (tubulitis) are the histologic hallmarks of T cell–mediated rejection. In our conceptualization, a kidney allograft undergoing acute T cell–mediated rejection functions as an in vivo flow cytometer and sorts graft-infiltrating cells and targeted graft parenchymal cells into the urinary space; therefore, profiling of urinary cells for their gene expression pattern offers a noninvasive means of diagnosing acute T cell–mediated rejection. Adapted from ref. 11, with permission.
Table 1 lists our urinary cell mRNA studies. Representative biomarker studies from other investigators are also included in Table 1, but the list is not exhaustive. We apologize for any inadvertent omissions. The Banff nomenclature for diagnostic categories has evolved over the years. In this review, the nomenclature in place at the time the mRNA profiling studies were performed has been retained, but—for all practical purposes—acute rejection and acute cellular rejection represent acute T cell–mediated rejection. In our studies, the diagnosis of antibody-mediated rejection required histologic features, intragraft deposition of C4d, and circulating IgG antibodies directed at donor HLA.
Table 1.
Urine profiles informative of human kidney allograft status
| Year | Author | Biomarker | Analyte | Subjects/Samples | Diagnostic Usea | AUROCb | Study Design |
| Acute rejection | |||||||
| 2001 | Li et al. (22) | Granzyme B, perforin | Urinary cell mRNA | 85/151 | Acute rejectionc | Granzyme B: 0.86 Perforin: 0.86 |
Prospective, single center |
| 2003 | Muthukumar et al. (27) | PI-9, granzyme B, perforin | Urinary cell mRNA | 87/95 | Acute rejection | Granzyme B: 0.88 Perforin: 0.90 PI-9: 0.76 |
Cross-sectional, single center |
| 2003 | Dadhania et al. (56) | Granzyme B | Urinary cell mRNA | 99/99 | Acute rejection UTI | Not reported | Cross-sectional, single center |
| 2003 | Ding et al. (30) | CD103 | Urinary cell mRNA | 79/89 | Acute rejection | 0.73 | Cross-sectional, single center |
| 2004 | Tatapudi et al. (31) | IP-10, CXCR3 | Urinary cell mRNA | 82/90 | Acute rejection | IP-10: 0.90 CXCR3: 0.76 |
Cross-sectional, single center |
| 2005 | Muthukumar et al. (45) | FOXP3 | Urinary cell mRNA | 83/83 | Acute rejection | 0.85 | Cross-sectional, single center |
| 2006 | Yannaraki et al. (23) | Perforin, granzyme B, Fas ligand | Urinary cell mRNA | 37/162 | Acute rejection | Not reported | Prospective, single center |
| 2008 | Aquino-Dias et al. (24) | Perforin, granzyme B, Fas ligand, PI-9, FOXP3 | Peripheral blood monocytes and urinary cell mRNA | 65/65 | Acute rejection | Peripheral blood monocytes/urinary cell Perforin: 0.881/0.917 Granzyme B: 0.941/0.851 Fas ligand: 0.935/0.887 PI-9: 0.935/0.875 FOXP3: 0.954/1.000 |
Cross-sectional, single center |
| 2010 | Afaneh et al. (47) | Signature of OX40, OX40L, PD-1, and FOXP3 | Urinary cell mRNA | 46/46 | Acute rejection | 0.98 | Cross-sectional, single center |
| 2011 | Lorenzen et al. (61) | miR-210 | Urinary miRNA | 81/88 | Acute rejection | 0.7±0.07 | Cross-sectional, single center |
| 2012 | Hirt-Minkowski et al. (32) | CXCL10 | Urinary protein in urine cellfree supernatant | 213/442 | Acute rejection and subclinical inflammation | Clinical inflammation: 0.74 Subclinical inflammation: 0.69 |
Cross-sectional, single center |
| 2013 | Suthanthiran et al. (37) | Signature of CD3ε mRNA, IP-10 mRNA, and 18S rRNA | Urinary cell mRNA | 485/4300 | Acute rejection | 0.85 | Prospective, multicenter |
| 2013 | Hricik et al. (35) | CXCL9 | Urinary cell mRNA and urinary protein | 280/2770 | Acute rejection | CXCL9 mRNA: 0.789 CXCL9 protein: 0.856 |
Prospective, multicenter |
| 2016 | Suhre et al. (63) | Signature of CD3ε mRNA, IP-10 mRNA, 18S rRNA, 3-sialyllactose/xanthosine, quinolinate/X-16397 | Urinary cell mRNA and metabolites in urine cellfree supernatant | 241/1516 | Acute rejection | 0.93 | Prospective, multicenter |
| 2017 | Raza et al. (55) | MCP-1/CCL2 | Urinary protein in urine cellfree supernatant | 409/409 | Acute rejection | 0.81±0.03 | Cross-sectional, single center |
| 2020 | Sigdel et al. (64) | 11-Metabolite panel: glycine, glutaric acid, adipic acid, inulobiose, threose, sulfuric acid, taurine, N-methylalanine, asparagine, 5-aminovaleric acid lactam, myo-inositol Four-metabolite panel: arabinose, 2-hydroxy-2-methylbutanoic acid, hypoxanthine, benzyl alcohol, N-acetyl-d-mannosamine |
Urinary metabolite in urine cellfree supernatant | 310/326 | Acute rejection: 11-metabolite panel BKVN versus acute rejection: four-metabolite panel |
Acute rejection: 0.985 BKVN: not reported |
Cross-sectional, single center |
| 2020 | Yang et al. (65) | Urinary Q score of cfDNA, m-cfDNA, clusterin, CXCL10, creatinine, and total protein | Urinary cellfree supernatant: urinary cfDNA, m-cfDNA, CXCL10, clusterin, total protein, and creatinine |
601/601 | Acute rejection | 0.99 | Prospective, multicenter |
| 2020 | Nolan et al. (66) | Urinary Q score of cfDNA, m-cfDNA, clusterin, CXCL10, creatinine, and total protein | Urinary cellfree supernatant: urinary cfDNA, m-cfDNA, CXCL10, clusterin, total protein, and creatinine |
215/223 | Acute rejection | 0.983 | Prospective, multicenter |
| Subclinical inflammation | |||||||
| 2011 | Ho et al. (33) | Ratio of urinary CXCL10 to creatinine | Urinary protein | 91/102 | Borderline, subclinical, and clinical tubulitis | 0.835 | Cross-sectional, single center |
| 2015 | Rabant et al. (36) | CXCL9 and CXCL10 | Urinary cellfree supernatant | 300/1722 | Low levels predictive of immunologic quiescence | At 1 mo: AR: ratio of CXCL9 to Cr, 0.50; ratio of CXCL10 to Cr, 0.72 Subclinical AR: ratio of CXCL10 to Cr, 0.69 Clinical AR: ratio of CXCL10 to Cr, 0.74 |
Prospective, single center |
| Interstitial fibrosis and tubular atrophy | |||||||
| 2010 | Ho et al. (54) | CCL2 | Urinary protein at 6 mo | 111/111 | Allograft fibrosis (urinary protein level at 6 mo predictive of IFTA and graft dysfunction at 24 mo) | Not reported | Prospective, multicenter |
| 2012 | Anglicheau et al. (53) | Four-gene signature of vimentin mRNA, NKCC2 mRNA, E-cadherin mRNA, and 18S rRNA | Urinary cell mRNA and 18S rRNA | 114/114 | Allograft fibrosis | 0.95 | Cross-sectional, single center |
| Urinary tract infection and BKV | |||||||
| 2002 | Ding et al. (57) | BKV VP1 mRNA | Urinary cell mRNA | 180/120 | BKVN | 0.949 | Cross-sectional, single center |
| 2010 | Dadhania et al. (58) | BKV VP1 mRNA, granzyme B, PI-9 | Urinary cell mRNA | 89/89 | Validation of noninvasive diagnosis of BKVN, and prognostication of kidney allograft function after BKVN diagnosis by measurement of transcripts for BKV VP1, granzyme B, and PI-9 | BKV VP1 mRNA: 0.99 | Cross-sectional, single center |
| 2018 | Burnham et al. (68) | Urine cellfree supernatant cfDNA | 82/141 | UTI, BKV |
Escherichia coli: 0.966 Enterococcus Faecalis: 0.968 Klebsiella pneumonia: 1.000 Pseudomonas aeruginosa: 1.000 |
Cross-sectional, single center |
|
| 2019 | Cheng et al. (69) | Urine cellfree supernatant cfDNA | 51/51 | UTI, BKV | 0.64 | Cross-sectional, single center |
|
| Delayed graft function | |||||||
| 2019 | Khalid et al. (62) | MicroRNA panel: miR-9, -10a, -21, -29a, -221, and -429 | Urinary miRNA | 165/33 | Delayed graft function | 0.94 | Prospective, single center |
AUROC, area under the receiver operating characteristic curve; PI-9, proteinase inhibitor-9; UTI, urinary tract infection; CXCR3, C-X-C Motif Chemokine Receptor 3; FOXP3, forkhead box P3; OX40, tumor necrosis factor receptor superfamily, member 4; OX40L, OX40 ligand; PD-1, programmed cell death protein-1; m-cfDNA, microbial cellfree DNA; NKCC2, sodium-potassium-chloride-cotransporter; miRNA, microRNA; CD3ε, CD3 ε chain; rRNA, ribosomal RNA; MCP-1, monocyte chemoattractant protein 1; BKVN, BK virus nephropathy; cfDNA, cellfree DNA; AR, acute rejection; IFTA, interstitial fibrosis and tubular atrophy; BKV VP1, BK virus virion protein 1.
Li et al. (22), Suthanthiran et al. (37), and Suhre et al. (63) report on both diagnostic and anticipatory ability of the measured biomarkers. Suthanthiran et al. (37), Afaneh et al. (47), Anglicheau et al. (53), Dadhania et al.(59), and Suhre et al. (63) report on the diagnostic accuracy of a single biomarker and the parsimonious signatures developed by combining measured biomarkers. Muthukumar et al. (27), Muthukumar et al. (45), Afaneh et al. (47), and Dadhania et al. (59) report both the diagnostic and prognostic utility of measured biomarkers.
A perfect predictor has an AUROC of 1.0, whereas a measure that has no association yields an AUROC of 0.5.
Acute rejection and acute T cell–mediated rejection are used interchangeably in this review, reflecting—in part—the evolution of Banff diagnostic categories over time.
Development of Our Urinary Cell mRNA Profiling Protocol
Isolation of RNA from urinary cells and absolute quantification of mRNAs using the PCR assay are logistically and technically challenging. Urine should be processed within a few hours of collection because of the high abundance of RNA-hydrolyzing enzymes in urine (12). RNA is also inherently unstable. We have largely overcome this challenge by adding an RNA-stabilizing reagent to the urinary cell pellet (13). Our RNA isolation method involves a centrifugation step to sediment urinary cells. A filtration method for capturing urinary cells represents a viable alternative to centrifugation to sediment the urinary cells (14,15). We have processed urine using a filter-based method and have trained kidney allograft recipients to use a commercially available filter (16). We have isolated RNA from the samples prepared using the filtration method and shown that absolute levels of mRNAs are similar to that using the centrifugation protocol (16).
The PCR assay, invented by Mullis et al. (17), has had a transformative effect on biomedicine. We incorporated a preamplification step and a customized amplicon, thereby improving the performance of the PCR assay. This preamplification procedure compensates for the low RNA yield from a urine sample, and the amplicon—by serving as the universal reference standard—obviates the need for gene-specific standard curves and enables absolute quantification of any mRNA.
Noninvasive Diagnosis of T Cell–Mediated Rejection
T cell–mediated rejection involves the infiltration of cytotoxic T cells into the allograft. Perforin mRNA encodes a pore-forming protein, and granzyme-B mRNA encodes a serine peptidase, and these proteins are integral components of the lytic machinery of cytotoxic cells (18–20). We designed and developed gene-specific DNA competitor constructs for the absolute quantification of mRNAs in competitive quantitative PCR assays (21), and identified that absolute levels of mRNA for granzyme B and perforin in urine from kidney allograft recipients are diagnostic of acute rejection; the area under the receiver operating characteristic curve (AUROC) was 0.86 for perforin mRNA and 0.86 for granzyme B mRNA (22). A perfect predictor has an AUROC of 1.0, whereas a measure that has no association yields an AUROC of ≤0.5. Levels of mRNA for perforin and granzyme B in sequential urine samples foretold the development of acute rejection (22). These findings have been confirmed and extended by others (23,24).
Serine proteinase inhibitor-9 (PI-9) is a natural antagonist of granzyme B and is expressed in cytotoxic T cells (25,26). Using real-time quantitative PCR assays, we found that the PI-9 mRNA level is significantly higher in urine matched to acute rejection biopsy specimens than in urine matched to chronic allograft nephropathy biopsy specimens, urine matched to other biopsy specimens, or urine from patients with stable graft function (27). PI-9 levels were significantly higher in patients with Banff acute rejection grade II or higher compared with those with less than grade II, and PI-9 levels predicted future graft function after an episode of acute rejection (27).
CD103 is expressed on CD8 T cells involved in kidney allograft rejection (28), and is essential for the intraepithelial homing of T cells (29). Because tubulitis is an essential feature of T cell–mediated rejection (10), we investigated whether the CD103 mRNA level in urinary cells is associated with acute rejection (30). Our study showed the CD103 mRNA level is significantly higher in urine matched to acute rejection biopsy specimens than in urine matched to biopsy specimens classified as chronic allograft nephropathy or other findings, and in urine from patients with stable graft function (30).
Soluble mediators and their receptors govern cellular traffic into an allograft. mRNA levels of chemokine IP-10, and its receptor C-X-C Motif Chemokine Receptor 3 (CXCR3), were significantly higher in urine matched to acute rejection biopsy specimens compared with urine from patients with biopsy specimens classified as chronic allograft nephropathy or other findings and urine from patients with stable graft function (31). Immunoperoxidase staining of biopsy samples showed prominent staining for IP-10 protein and CXCR3 protein in kidney tubules and graft-infiltrating cells in biopsy specimens classified as acute rejection but not in those from patients with stable graft function. Furthermore, IP-10+ cells and CXCR3+ cells were observed crossing kidney tubular cells. The level of IP-10/C-X-C Motif Chemokin Ligand 10 (CXCL10) in urine, at the mRNA level and at the protein level, has been associated with acute rejection, and levels of C-X-C Motif Chemokin Ligand 9 (CXCL9) and CXCL10 have been associated with subclinical acute rejection and kidney allograft function (32–36).
Clinical Trials in Organ Transplantation
The Clinical Trials in Organ Transplantation-01 (CTOT-01) study evaluated the diagnostic utility of urinary cell mRNAs and proteins in 280 kidney transplant recipients and identified that CXCL9 mRNA level and CXCL9 protein level are significantly higher in urine matched to acute rejection biopsy specimens than in biopsy specimens showing no rejection, and the CXCL9 level at 6 months is lower in those less likely to develop acute rejection (35). The CTOT-04 study investigated the following hypotheses: (1) urinary cell mRNA profiles are diagnostic of acute cellular rejection; and (2) urinary cell mRNA profiles, ascertained in sequentially collected urine specimens, predict future development of acute cellular rejection (37). A total of 4300 urine samples were collected at designated time points in the post-transplantation period and at the time of biopsies from 485 kidney allograft recipients. Absolute levels of mRNA for perforin, granzyme B, PI-9, CD103, CD3 ε chain (CD3ε), IP-10, and CXCR3 were measured using preamplification-enhanced, real-time, quantitative PCR assays developed in our laboratory. Levels of mRNA for TGF-β1 and 18S ribosomal RNA (18S rRNA) served as measures of RNA integrity. Data analyses showed that 18S-normalized levels of mRNA for CD3ε, perforin, granzyme B, and IP-10 were higher in urine matched to acute cellular rejection biopsy specimens compared with urine matched to biopsy specimens without rejection, and in urine from patients with stable graft function (P<0.001 using separate Kruskal–Wallis tests comparing the three groups for each of the four mRNAs).
The CTOT-04 study developed and validated a urinary cell three-gene signature of 18S-normalized CD3ε mRNA, 18S-normalized IP-10 mRNA, and 18S rRNA. This signature distinguished patients with acute cellular rejection biopsy specimens from patients with no rejection biopsy specimens with an AUROC of 0.85 (P<0.001). Bootstrap resampling yielded a crossvalidated AUROC of 0.83. The three-gene signature yielded an AUROC of 0.75 in an external validation set, a value not significantly lower than the AUROC of 0.85. The level of the three-gene signature differed between patients who received anti–IL-2 receptor antibodies and those who received T cell–depleting antibodies, but was diagnostic of acute cellular rejection in both groups.
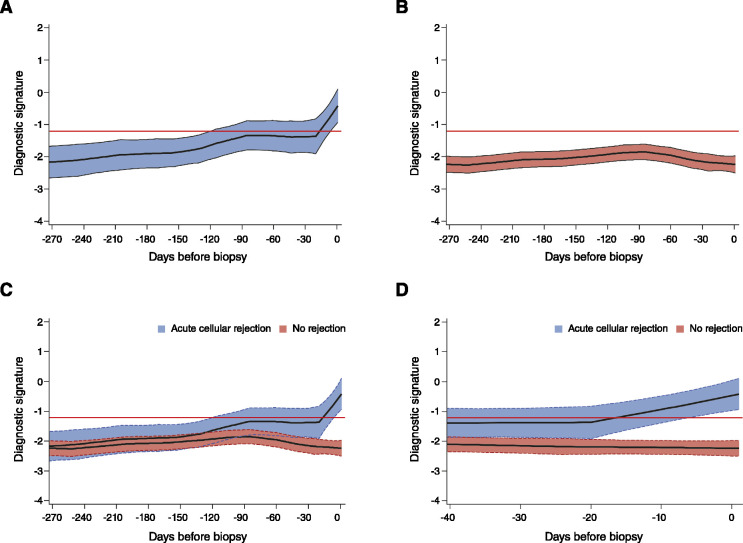
The three-gene diagnostic signature predicted future episodes of acute cellular rejection. Figure 2 shows the LOESS curves with 95% confidence interval bands for the group of patients who developed acute cellular rejection and the group of patients with no rejection biopsy specimens. The signature score trajectory remained flat in the group without rejection biopsy samples, whereas the score progressively increased in the group who developed acute cellular rejection.
Figure 2.
Retrospective trajectory of diagnostic signature in acute cellular rejection and no rejection. The average within-person retrospective trajectory of the diagnostic signature (i.e., the trajectory as a function of the time before biopsy) in urine samples obtained at or before biopsy (which passed quality control) is shown (A) for the group of 38 patients with first biopsy specimens showing acute cellular rejection (201 urine samples) and (B) the group of 113 patients with specimens showing no rejection (833 urine samples). Only specimens obtained during the first 400 days after transplantation were included. (C) The diagnostic signature remained relatively flat and well below the −1.213 threshold that was diagnostic of acute cellular rejection during the 270 days before biopsy in the group of patients with findings showing no rejection. (D) There was a significant difference in the trajectories between the two groups, with a marked increase in the diagnostic signature during the 20-day period before the first specimen showing acute cellular rejection (P<0.001). The y-axis values are diagnostic signature scores without intrinsic units of measurement; they were calculated from the logistic regression equation (−6.1487+0.8534 log10[CD3ε/18S]+0.6376 log10[IP-10/18S]+1.6464 log10[18S]) as follows. Absolute levels of CD3ε mRNA, IP-10 mRNA, and 18S rRNA in the cells from each urine sample were measured by PCR assay, with the units of measurement being copies per microgram of total RNA for each mRNA measure, and copies (×10−6) per microgram of total RNA for 18S rRNA. The mRNA copy numbers were 18S normalized by dividing the mRNA copy number by the 18S rRNA copy number in the same sample, and the ratio was log10 transformed. In all of the panels, the black lines indicate the trajectory, the colored bands the 95% confidence interval, and the red lines the diagnostic threshold. Adapted from ref. 37, with permission.
Acute Rejection and Urinary Cell Transcriptomics
RNA sequencing is a powerful molecular tool for the unbiased characterization of genome-wide transcriptional changes at an unprecedented level of precision, and for deciphering mechanisms and prioritization of biomarkers. Our RNA sequencing of urinary cells and bioinformatics identified, at a false discovery rate <0.01 and log2 fold change ≥2, 180 genes that were differentially expressed in urine matched to T cell–mediated rejection biopsy specimens versus urine matched to specimens showing no rejection, and 544 genes that were differentially expressed in urine matched to antibody-mediated rejection biopsy specimens versus urine matched to biopsy specimens showing no rejection (38). RNA sequencing—in addition to validating the diagnostic accuracy of urinary cell levels of mRNA for CD3ε, IP-10, granzyme B, perforin, CXCR3, CD103, and PI-9—identified several novel biomarkers of T cell–mediated rejection, including CD2, CD8A, CCL5, GZMA, NKG7, CTLA4, ITM2A, SLAM F6, and IKZF3 (38,39).
A large number of genes were shared between T cell–mediated rejection and antibody-mediated rejection. Supervised gene name–based pathway analysis, using the ENRICHR database (40) and the KEGG 2016 human molecular pathways database (https://www.genome.jp/kegg/) (41), showed T cell–receptor signaling pathways, chemokine-signaling pathways, T helper 1 and 2 cell differentiation, necroptosis, natural killer cell–mediated cytotoxicity, cell adhesion molecules, cytokine-to-cytokine receptor interaction, phagosome, and antigen processing and presentation were shared between T cell–mediated rejection and antibody-mediated rejection.
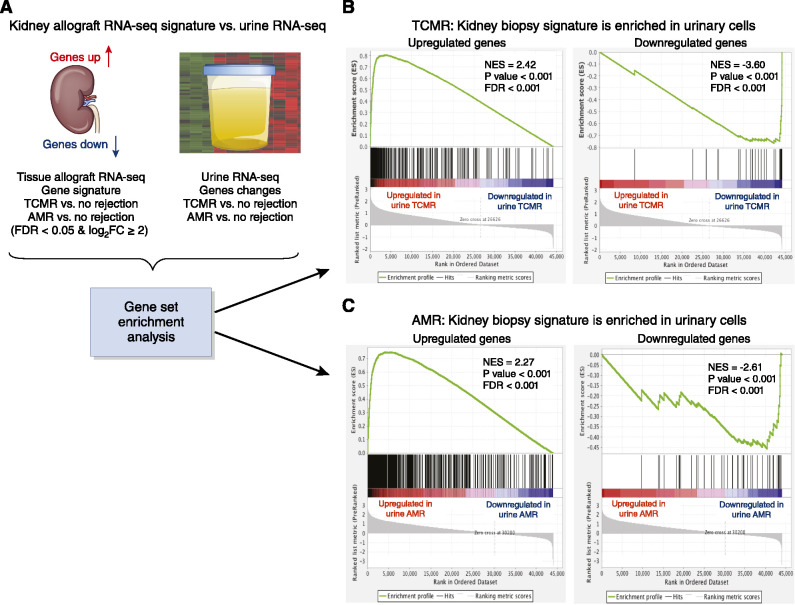
Gene-set enrichment analysis (42) identified that the gene signatures of T cell–mediated rejection and of antibody-mediated rejection are enriched in urine matched to these biopsy specimens (Figure 3). Cell-type enrichment analysis (43) revealed a diverse immune cellular landscape in urine compared with biopsy specimens. Together, RNA sequencing and bioinformatics supported the idea that the kidney allograft may function as an in vivo flow cytometer and sort graft-infiltrating cells into urine.
Figure 3.
Kidney allograft biopsy specimen gene signatures are enriched in urinary cells. Kidney allograft biopsy specimen signatures derived by RNA sequencing (RNA-seq) of 49 biopsy specimens from 49 patients. (A) Gene-set enrichment analysis was performed to compare biopsy specimen gene signatures with urinary cell gene expression patterns. (B) The upregulated gene signature from T cell–mediated rejection (TCMR) biopsy specimens versus specimens showing no rejection is also significantly upregulated in the TCMR urine versus urine showing no rejection: normalized enrichment score (NES)=2.42, P<0.001, false discovery rate (FDR)<0.001. The downregulated gene signature in TCMR biopsy specimens versus specimens showing no rejection was also enriched in TCMR urine versus no-rejection urine: NES=−3.60, P<0.001, FDR<0.001. (C) The upregulated gene signature in antibody-mediated rejection (AMR) biopsy specimens versus specimens showing no rejection was significantly upregulated in AMR urine versus no-rejection urine: NES=2.27, P<0.001, FDR<0.001. The downregulated signature in AMR biopsy specimens versus no-rejection biopsy specimens was enriched in AMR urine versus no-rejection urine: NES=−2.61, P<0.001, FDR <0.001. The ranked list of genes in the biopsy specimen (x axis) and the enrichment score (ES; y axis) are shown. A positive ES indicates the top-ranked genes in the biopsy specimen are enriched in urinary cells. The top portion of the plot shows the ES (green line). The ES for this gene set is the score at the peak of the plot. The middle portion shows where urinary cell genes appear in the ranked list of biopsy specimen genes. The bottom portion shows the value of the ranking metric moving down the list of ranked genes, and goes from positive (correlation with TCMR) to negative (correlation with no rejection). The NES accounts for differences in gene-set size and correlations between the urine and biopsy specimen gene sets. The FDR is the estimated probability that a gene set with a given NES represents a false positive. Adapted from ref. 34, with permission. FC, fold change.
Urinary Cell mRNA Levels Predictive of Reversal of Acute Rejection
Forkhead box P3 (FOXP3) is a specification factor for regulatory T cells (44). Our single-center study found the FOXP3 mRNA level is significantly higher in urine from kidney allograft recipients who responded to antirejection therapy for acute rejection than in urine from those who did not, and the AUROC for predicting reversal was 0.85 (45). We replicated the predictive utility of urinary cell FOXP3 mRNA levels using an external cohort (46). In this validation study, reversal was associated with a reduction in the urinary cell three-gene signature from above the acute rejection diagnostic threshold to a level below the threshold after antirejection treatment, whereas the signature remained above the threshold in those without reversal during the 4 weeks after antirejection therapy. Measurement of urinary cell FOXP3 mRNA levels and the urinary cell three-gene signature at the time of diagnostic biopsy (and weekly thereafter over a 4-week period) and kidney allograft biopsies after antirejection therapy may help discern the relationship among clinical, histologic, and molecular responses to antirejection treatment and decipher the basis for the differential responsiveness of T cell–mediated rejection to antirejection therapy.
OX40 and OX40L are T-cell costimulatory proteins, whereas PD-1 and its ligands PD-L1 and PD-L2 are negative regulators that promote T-cell apoptosis. We found the OX40 mRNA level in urine predicted reversal of acute rejection, with an AUROC of 0.84, and the best linear combination of OX40 mRNA and FOXP3 mRNA predicts reversal with an AUROC of 0.90 (47).
Urinary Cell mRNA Levels Diagnostic of Interstitial Fibrosis and Tubular Atrophy
Development of interstitial fibrosis and tubular atrophy (IFTA) involves both inflammatory and noninflammatory mechanisms (48–52). We discovered and validated a four-gene model of vimentin, NKCC2, E-cadherin, and 18S that is diagnostic of IFTA (53).
CCL2 is a chemoattractant for monocytes, macrophages, T cells, and natural killer cells. In a study of 122 kidney allograft recipients, urinary CCL2 levels were predictive of IFTA after controlling for donor age, delayed graft function, deceased kidney donation, and use of angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker (54). CCL2 levels have also been associated with kidney allograft rejection (55).
Urinary Cell mRNA Profile and Urinary Tract Infection
Bacterial urinary tract infections (UTIs) are common after kidney transplantation. We measured the granzyme-B mRNA level in urine from kidney allograft recipients with UTIs, acute rejection but without UTIs, and patients with neither rejection nor UTIs. We also measured transcript levels in urine from those with or without UTIs who were not transplant recipients. This study revealed bacterial UTI increases the urinary cell granzyme-B mRNA level (56). The finding that bacterial UTI does not confound the diagnosis of acute cellular rejection using urinary cell mRNAs was validated in the CTOT-04 study (37).
Polyomavirus BK has emerged as a significant post-transplant complication. We determined and validated that BK virus VP1 (the polyoma capsid protein of the polyomavirus) mRNA levels in urinary cells are diagnostic of BK virus nephropathy (57). Urinary cell levels of granzyme B and PI-9 were higher in those who developed subsequent graft dysfunction, compared with those who did not, after BK virus nephropathy (58). A two-biomarker model of levels of serum creatinine and plasminogen activator inhibitor-1 mRNA predicted graft failure within 36 months, with an AUROC of 0.92 (59); this finding was recently validated in an independent cohort (60).
Additional Profiling Strategies
MicroRNA (miRNA) profiling, metabolomics, proteomics, and cellfree DNA each hold considerable promise as biomarkers of kidney allograft status. miRNAs are endogenous, small (about 22 nt long), non-coding RNAs that target a vast array of mRNAs, reducing their abundance and/or impairing their translation, and are considered master regulators of immune cell development and function. In the transplantation arena, miRNA-10b and -210 were reported to be downregulated and miRNA-10a upregulated in urine from kidney graft recipients with acute rejection compared with patients without rejection; the miRNA-210 level was also associated with long-term graft function (61). A panel of six miRNAs—miRNA-9, -10a, -21, -29a, -221, and -429—has been reported to be predictive of delayed graft function in kidney allograft recipients (62).
Metabolomics provides biologic readouts for perturbations of biochemical processes. In our study, a composite signature of the ratios of urinary 3-sialyllactose to xanthosine and quinolinate to X-16397 along with the urinary cell three-gene signature outperformed either signature alone in diagnosing acute rejection in kidney allografts (63). Recently, an 11-metabolite panel diagnostic of acute rejection and a four-metabolite panel discriminating acute rejection from BK virus nephropathy have been identified (64).
Multimodal profiling may offer advantages over measurement of a single analyte. A combination of six urinary biomarkers consisting of cellfree DNA, methylated cellfree DNA, clusterin, CXCL10, creatinine, and total protein (measured in urine supernatant using an ELISA-based approach) was reported to discriminate acute rejection from no rejection in kidney allografts, with an AUROC of 0.99 and an accuracy of 96% (65). In a follow-up study, a composite Q score >32 was diagnostic of acute rejection, with an overall sensitivity of 96% and specificity of 99% (66).
Translation to the Clinic
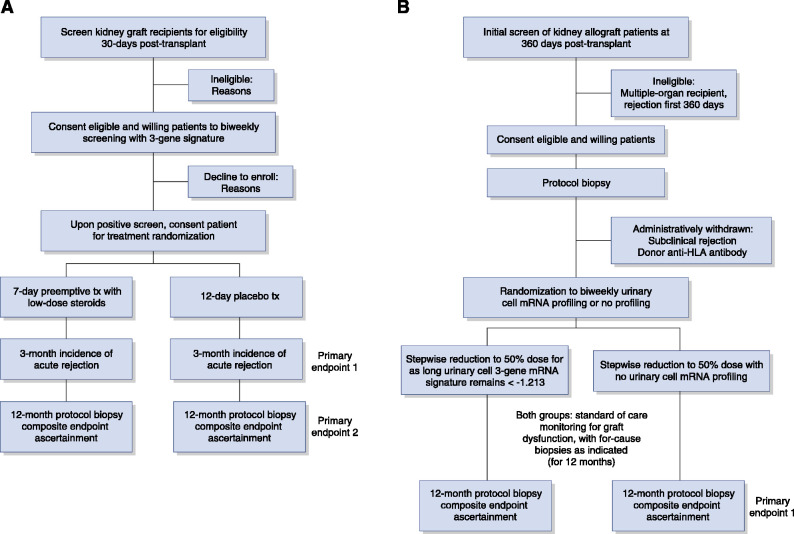
On the basis of data showing that the urinary cell three-gene signature diagnostic of acute cellular rejection tends to cross the diagnostic threshold almost a month before biopsy specimen–confirmed rejection (Figure 2), an interventional trial of preemptive antirejection therapy could test the hypotheses that (1) this signature predicts the development of acute rejection, and (2) preemptive therapy prevents the development of acute rejection. In this trial, urinary cell three-gene signature of CD3ε mRNA, IP-10 mRNA, and 18s rRNA would be measured in serial urine specimens collected from kidney allograft recipients, and study participants would be randomized to a preemptive antirejection-therapy arm or a standard-of-care arm. Figure 4A outlines the envisioned trial.
Figure 4.
Preliminary consort diagrams for two potential randomized clinical trials (RCTs) to evaluate the utility of the Clinical Trials in Organ Transplantation-04 three-gene (18S-normalized CD3ε mRNA, 18S-normalized IP-10 mRNA, and 18S rRNA) signature diagnostic of acute cellular rejection. (A) Single-center RCT to evaluate the efficacy of preemptive antirejection therapy to prevent acute cellular rejection. Starting 30 days post-transplant, eligible patients with kidney allografts would be screened biweekly for elevated levels of the three-gene signature. Inclusion criteria: single kidney transplant, adult recipients (>18 years of age), single kidney graft, and stable graft function. Exclusion criteria: multiorgan recipient, rejection in the preceding 30 days, recipient or donor positive for hepatitis C virus recipient or donor, HIV+ recipient or donor, three-gene signature score greater than −1.213. Upon detection of an elevated signature score postenrollment, the patient would be randomized to 7 days of preemptive treatment with either low-dose steroids (e.g., 60 mg, oral, per day, active arm) or placebo. The primary outcomes would be (1) incident biopsy specimen–confirmed acute rejection during the 3 months post-randomization; and (2) a composite end point of incident biopsy specimen–confirmed acute rejection, the presence of subclinical acute rejection, Banff grade II or more interstitial fibrosis/tubular atrophy (IF/TA), arteriolar hyalinosis, donor-specific anti-HLA antibodies, C4d deposition in the 12-month surveillance biopsy specimen, graft loss, or death. Secondary end points: kidney allograft functional status (eGFR, incidence and degree of albuminuria [measured by albumin-creatinine ratio]), incidence of BK virus replication, incidence of BK virus nephropathy, and incidence of cytomegalovirus disease. (B) Single-center RCT to evaluate the efficacy of urinary cell three-gene signature to facilitate a 50% reduction in tacrolimus dosage. At 12 months post-transplant, eligible, consented patients would undergo a stepwise reduction in tacrolimus dosage to 50% of pre-enrollment dosage. The patients will be randomized to either a biweekly monitoring of three-gene signature arm or no three-gene signature monitoring arm. In those assigned to the urinary cell mRNA monitoring arm, stepwise reduction will stop if the score is greater than −1.213. Both groups would receive standard-of-care monitoring for graft dysfunction, with for-cause biopsies and treatment as indicated for the next 12 months. At 12 months post-randomization, all patients would be evaluated for overt graft dysfunction and have a protocol biopsy for the detection of subclinical rejection and/or graft dysfunction. The primary outcomes would be (1) a composite end point of incident biopsy specimen–confirmed acute rejection, the presence of subclinical acute rejection, Banff grade II or more IF/TA, arteriolar hyalinosis, donor-specific anti-HLA antibodies, C4d deposition in the 12-month surveillance biopsy specimen, graft loss, or death; and (2) cumulative tacrolimus dosage. Secondary end points: kidney allograft functional status (eGFR, incidence and degree of albuminuria [measured by albumin-creatinine ratio]), incidence of BK virus replication, incidence of BK virus nephropathy, and incidence of cytomegalovirus disease. An independent data safety monitoring board will monitor these institutional review board–approved trials. Tx, treatment.
On the basis of data showing that this three-gene signature reflects the potency of immunosuppressive therapy (37), a randomized controlled interventional trial of stepwise reduction in immunosuppressive therapy could test whether urinary cell mRNA profile–guided immunosuppression minimization is safe. In this trial, illustrated in Figure 4B, kidney allograft recipients with stable graft function, normal protocol biopsy specimens, and no donor-specific antibodies would undergo a stepwise reduction in tacrolimus dosage to 50% of the pre-enrollment dosage. The patients randomized to the three-gene signature–guided arm will not undergo the scheduled reduction in dosage if the signature crosses the acute rejection diagnostic threshold, and they will be reverted to their prior dosage. This study design would test the hypothesis that a 50% reduction in tacrolimus dosage is safe in patients monitored using the three-gene signature. Multimodal profiling of study participants using urinary cell BK-VP1 mRNA levels, plasma donor-derived cellfree DNA (57,67,68), urinary cell DNA metagenomic sequencing (69), and antibodies to donor HLA would be performed as safety measures.
These two single-center trials could serve as precursors to a multicenter randomized controlled trial of biomarker-guided management (70). Additional clinical trials incorporating decision analysis may help reduce the need for clinically indicated or surveillance biopsies of the kidney allograft.
Disclosures
M. Lubetzky reports being employed by Division of Nephrology and Hypertension, Weill Cornell Medical Center. T. Salinas reports being employed by New York–Presbyterian Hospital, Weill Cornell Medical Center. J.E. Schwartz reports serving as treasurer of the Academy of Behavioral Medicine Research; receiving honoraria from Atcor; being on the editorial board of Blood Pressure; having consultancy agreements with Duke University, Kaiser Permanente of Southern California, University of Alabama at Birmingham, Weill Cornell Medical College, and Yale University; and being employed by Stony Brook University. M. Suthanthiran reports receiving research funding from CareDx, Inc. and National Institutes of Health; having consultancy agreements with, and receiving honoraria from, CareDx, Inc. and Sparks Therapeutics; and being employed by Weill Cornell Medical College.
Funding
The studies summarized here were supported by National Institute of Allergy and Infectious Diseases awards RO1 AI026932, RO1 AI060706, R01 AI072790, and R37 NIH MERIT AI051652 (to M. Suthanthiran). A Mendez National Institute of Transplantation Foundation award (to M. Suthanthiran) supported, in part, RNA sequencing studies summarized in this review. The CTOT-04 study was supported by National Institute of Allergy and Infectious Diseases award UO1AI63589 (to A. Shaked), with a subaward to M. Suthanthiran.
Acknowledgments
The authors gratefully acknowledge the exceptional contributions of our colleagues Dr. Danny Anglicheau, Dr. Phyllis August, Dr. Darshana Dadhania, Dr. Ruchuang Ding, Dr. Olivier Elemento, Dr. Choli Hartono, Dr. John Lee, Dr. Jun Lee, Dr. Baguoi Li, Dr. Marie Matignon, Dr. Thangamani Muthukumar, Dr. Vijay Sharma, Dr. Surya Seshan, Dr. Karsten Suhre, Dr. Ravi Raju Tatapudi, Dr. Akanksha Verma, and Dr. Hua Yang; and the superb technical expertise of Ms. Christina Chang, Ms. Christine Hoang, Ms. Carol Li, and Ms. Catherine Snopkowski.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Stegall MD, Gaston RS, Cosio FG, Matas A: Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Foutz J, Wainright JL, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2018 annual data report: Kidney. Am J Transplant 20[Supp 1]: 20–130, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Veronese FV, Manfro RC, Roman FR, Edelweiss MI, Rush DN, Dancea S, Goldberg J, Gonçalves LF: Reproducibility of the Banff classification in subclinical kidney transplant rejection. Clin Transplant 19: 518–521, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Furness PN, Taub N; Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project : International variation in the interpretation of renal transplant biopsies: Report of the CERTPAP Project [published correction appears in Kidney Int 60: 2429, 2001]. Kidney Int 60: 1998–2012, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Anglicheau D, Suthanthiran M: Noninvasive prediction of organ graft rejection and outcome using gene expression patterns. Transplantation 86: 192–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 78: 242–249, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Roberts IS, Reddy S, Russell C, Davies DR, Friend PJ, Handa AI, Morris PJ: Subclinical rejection and borderline changes in early protocol biopsy specimens after renal transplantation. Transplantation 77: 1194–1198, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro R, Randhawa P, Jordan ML, Scantlebury VP, Vivas C, Jain A, Corry RJ, McCauley J, Johnston J, Donaldson J, Gray EA, Dvorchik I, Hakala TR, Fung JJ, Starzl TE: An analysis of early renal transplant protocol biopsies--The high incidence of subclinical tubulitis. Am J Transplant 1: 47–50, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Lee JR, Muthukumar T, Dadhania D, Ding R, Sharma VK, Schwartz JE, Suthanthiran M: Urinary cell mRNA profiles predictive of human kidney allograft status. Immunol Rev 258: 218–240, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryzgunova OE, Laktionov PP: Extracellular nucleic acids in urine: Sources, structure, diagnostic potential. Acta Naturae 7: 48–54, 2015 [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros M, Sharma VK, Ding R, Yamaji K, Li B, Muthukumar T, Valderde-Rosas S, Hernandez AM, Muñoz R, Suthanthiran M: Optimization of RNA yield, purity and mRNA copy number by treatment of urine cell pellets with RNAlater. J Immunol Methods 279: 135–142, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Miranda KC, Bond DT, McKee M, Skog J, Păunescu TG, Da Silva N, Brown D, Russo LM: Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 78: 191–199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P, Nandi S, Tan TZ, Ler SG, Chia KS, Lim WY, Bütow Z, Vordos D, De la Taille A, Al-Haddawi M, Raida M, Beyer B, Ricci E, Colombel M, Chong TW, Chiong E, Soo R, Park MK, Ha HK, Gunaratne J, Thiery JP: Highly sensitive and specific novel biomarkers for the diagnosis of transitional bladder carcinoma. Oncotarget 6: 13539–13549, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snopkowski C, Li C, Albakry S, Botticelli B, Cassidy M, Lee J, Lubetzky M, Dadhania D, Yang H, Ding R, Muthukumar T, Suthanthiran M: Efficacious home processing of urine for messenger RNA profiling: A point-of-care approach for molecular monitoring of kidney transplant recipients. 2019
- 17.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H: Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51: 263–273, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Henkart PA: Lymphocyte-mediated cytotoxicity: Two pathways and multiple effector molecules. Immunity 1: 343–346, 1994; [DOI] [PubMed] [Google Scholar]
- 19.Smyth MJ: Dual mechanisms of lymphocyte-mediated cytotoxicity serve to control and deliver the immune response. BioEssays 17: 891–898, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Kägi D, Ledermann B, Bürki K, Zinkernagel RM, Hengartner H: Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol 14: 207–232, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Li B, Sehajpal PK, Khanna A, Vlassara H, Cerami A, Stenzel KH, Suthanthiran M: Differential regulation of transforming growth factor beta and interleukin 2 genes in human T cells: Demonstration by usage of novel competitor DNA constructs in the quantitative polymerase chain reaction. J Exp Med 174: 1259–1262, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, Serur D, Mouradian J, Schwartz JE, Suthanthiran M: Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med 344: 947–954, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Yannaraki M, Rebibou JM, Ducloux D, Saas P, Duperrier A, Felix S, Rifle G, Chalopin JM, Hervé P, Tiberghien P, Ferrand C: Urinary cytotoxic molecular markers for a noninvasive diagnosis in acute renal transplant rejection. Transpl Int 19: 759–768, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Aquino-Dias EC, Joelsons G, da Silva DM, Berdichevski RH, Ribeiro AR, Veronese FJ, Gonçalves LF, Manfro RC: Non-invasive diagnosis of acute rejection in kidney transplants with delayed graft function [published correction appears in Kidney Int 74: 393, 2008]. Kidney Int 73: 877–884, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Bird CH, Sutton V, McDonald L, Coughlin PB, De Jong TA, Trapani JA, Bird PI: A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem 271: 27802–27809, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Medema JP, de Jong J, Peltenburg LT, Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP, Melief CJ, Offringa R: Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci U S A 98: 11515–11520, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthukumar T, Ding R, Dadhania D, Medeiros M, Li B, Sharma VK, Hartono C, Serur D, Seshan SV, Volk HD, Reinke P, Kapur S, Suthanthiran M: Serine proteinase inhibitor-9, an endogenous blocker of granzyme B/perforin lytic pathway, is hyperexpressed during acute rejection of renal allografts. Transplantation 75: 1565–1570, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Hadley G: Role of integrin CD103 in promoting destruction of renal allografts by CD8 T cells. Am J Transplant 4: 1026–1032, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Butcher EC, Picker LJ: Lymphocyte homing and homeostasis. Science 272: 60–66, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Ding R, Li B, Muthukumar T, Dadhania D, Medeiros M, Hartono C, Serur D, Seshan SV, Sharma VK, Kapur S, Suthanthiran M: CD103 mRNA levels in urinary cells predict acute rejection of renal allografts. Transplantation 75: 1307–1312, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Tatapudi RR, Muthukumar T, Dadhania D, Ding R, Li B, Sharma VK, Lozada-Pastorio E, Seetharamu N, Hartono C, Serur D, Seshan SV, Kapur S, Hancock WW, Suthanthiran M: Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int 65: 2390–2397, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Hirt-Minkowski P, Amico P, Ho J, Gao A, Bestland J, Hopfer H, Steiger J, Dickenmann M, Burkhalter F, Rush D, Nickerson P, Schaub S: Detection of clinical and subclinical tubulo-interstitial inflammation by the urinary CXCL10 chemokine in a real-life setting. Am J Transplant 12: 1811–1823, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ho J, Rush DN, Karpinski M, Storsley L, Gibson IW, Bestland J, Gao A, Stefura W, HayGlass KT, Nickerson PW: Validation of urinary CXCL10 as a marker of borderline, subclinical, and clinical tubulitis. Transplantation 92: 878–882, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Schaub S, Nickerson P, Rush D, Mayr M, Hess C, Golian M, Stefura W, Hayglass K: Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant 9: 1347–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Hricik DE, Nickerson P, Formica RN, Poggio ED, Rush D, Newell KA, Goebel J, Gibson IW, Fairchild RL, Riggs M, Spain K, Ikle D, Bridges ND, Heeger PS; CTOT-01 consortium : Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am J Transplant 13: 2634–2644, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabant M, Amrouche L, Morin L, Bonifay R, Lebreton X, Aouni L, Benon A, Sauvaget V, Le Vaillant L, Aulagnon F, Sberro R, Snanoudj R, Mejean A, Legendre C, Terzi F, Anglicheau D: Early low urinary CXCL9 and CXCL10 might predict immunological quiescence in clinically and histologically stable kidney recipients. Am J Transplant 16: 1868–1881, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, Knechtle SJ, Friedewald J, Becker YT, Sharma VK, Williams NM, Chang CS, Hoang C, Muthukumar T, August P, Keslar KS, Fairchild RL, Hricik DE, Heeger PS, Han L, Liu J, Riggs M, Ikle DN, Bridges ND, Shaked A; Clinical Trials in Organ Transplantation 04 (CTOT-04) Study Investigators : Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med 369: 20–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma A, Muthukumar T, Yang H, Lubetzky M, Cassidy MF, Lee JR, Dadhania DM, Snopkowski C, Shankaranarayanan D, Salvatore SP, Sharma VK, Xiang JZ, De Vlaminck I, Seshan SV, Mueller FB, Suhre K, Elemento O, Suthanthiran M: Urinary cell transcriptomics and acute rejection in human kidney allografts. JCI Insight 5: e131552, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dooley BJ, Verma A, Ding R, Yang H, Muthukumar T, Lubetzky M, Shankaranarayanan D, Elemento O, Suthanthiran M: Urinary cell transcriptome profiling and identification of ITM2A, SLAMF6, and IKZF3 as biomarkers of acute rejection in human kidney allografts. Transplant Direct 6: e588, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A: Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44: W90–W97, 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanehisa M: KEGG bioinformatics resource for plant genomics and metabolomics. Methods Mol Biol 1374: 55–70, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP: Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102: 15545–15550, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aran D, Hu Z, Butte AJ: xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 18: 220, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vent-Schmidt J, Han JM, MacDonald KG, Levings MK: The role of FOXP3 in regulating immune responses. Int Rev Immunol 33: 110–128, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan SV, Kapur S, Hancock WW, Schwartz JE, Suthanthiran M: Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med 353: 2342–2351, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Luan D, Dadhania DM, Ding R, Muthukumar T, Lubetzky M, Lee JR, Sharma VK, August P, Mueller FB, Schwartz JE, Suthanthiran M: FOXP3 mRNA profile prognostic of acute T-cell-mediated rejection and human kidney allograft survival [published online ahead of print October 7, 2020]. Transplantation 10.1097/TP.0000000000003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afaneh C, Muthukumar T, Lubetzky M, Ding R, Snopkowski C, Sharma VK, Seshan S, Dadhania D, Schwartz JE, Suthanthiran M: Urinary cell levels of mRNA for OX40, OX40L, PD-1, PD-L1, or PD-L2 and acute rejection of human renal allografts. Transplantation 90: 1381–1387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arias M, Serón D, Moreso F, Bestard O, Praga M: Chronic renal allograft damage: Existing challenges. Transplantation 91[Supp]: S4–S25, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Maluf DG, Mas VR, Archer KJ, Yanek K, Gibney EM, King AL, Cotterell A, Fisher RA, Posner MP: Molecular pathways involved in loss of kidney graft function with tubular atrophy and interstitial fibrosis. Mol Med 14: 276–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strutz F: Pathogenesis of tubulointerstitial fibrosis in chronic allograft dysfunction. Clin Transplant 23[Supp 21]: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Boor P, Ostendorf T, Floege J: Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Anglicheau D, Muthukumar T, Hummel A, Ding R, Sharma VK, Dadhania D, Seshan SV, Schwartz JE, Suthanthiran M: Discovery and validation of a molecular signature for the noninvasive diagnosis of human renal allograft fibrosis. Transplantation 93: 1136–1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho J, Rush DN, Gibson IW, Karpinski M, Storsley L, Bestland J, Stefura W, HayGlass KT, Nickerson PW: Early urinary CCL2 is associated with the later development of interstitial fibrosis and tubular atrophy in renal allografts. Transplantation 90: 394–400, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Raza A, Firasat S, Khaliq S, Khan AR, Mahmood S, Aziz T, Mubarak M, Naqvi SA, Rizvi SA, Abid A: Monocyte chemoattractant protein-1 (MCP-1/CCL2) levels and its association with renal allograft rejection. Immunol Invest 46: 251–262, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Dadhania D, Muthukumar T, Ding R, Li B, Hartono C, Serur D, Seshan SV, Sharma VK, Kapur S, Suthanthiran M: Molecular signatures of urinary cells distinguish acute rejection of renal allografts from urinary tract infection. Transplantation 75: 1752–1754, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Ding R, Medeiros M, Dadhania D, Muthukumar T, Kracker D, Kong JM, Epstein SR, Sharma VK, Seshan SV, Li B, Suthanthiran M: Noninvasive diagnosis of BK virus nephritis by measurement of messenger RNA for BK virus VP1 in urine. Transplantation 74: 987–994, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Dadhania D, Snopkowski C, Ding R, Muthukumar T, Lee J, Bang H, Sharma VK, Seshan S, August P, Kapur S, Suthanthiran M: Validation of noninvasive diagnosis of BK virus nephropathy and identification of prognostic biomarkers. Transplantation 90: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dadhania D, Snopkowski C, Muthukumar T, Lee J, Ding R, Sharma VK, Christos P, Bang H, Kapur S, Seshan SV, Suthanthiran M: Noninvasive prognostication of polyomavirus BK virus-associated nephropathy. Transplantation 96: 131–138, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abuhelaiqa E, Snopkowski C, Li C, Salvatore S, Lee JR, Muthukumar T, Lee JB, Hartono C, Ding R, Seshan SV, Suthanthiran M, Dadhania DM: Validation of a noninvasive prognostic signature for allograft failure following BK virus associated nephropathy [published online ahead of print December 22, 2020]. Clin Transplant 10.1111/ctr.14200 [DOI] [PubMed] [Google Scholar]
- 61.Lorenzen JM, Volkmann I, Fiedler J, Schmidt M, Scheffner I, Haller H, Gwinner W, Thum T: Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am J Transplant 11: 2221–2227, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Khalid U, Newbury LJ, Simpson K, Jenkins RH, Bowen T, Bates L, Sheerin NS, Chavez R, Fraser DJ: A urinary microRNA panel that is an early predictive biomarker of delayed graft function following kidney transplantation. Sci Rep 9: 3584, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suhre K, Schwartz JE, Sharma VK, Chen Q, Lee JR, Muthukumar T, Dadhania DM, Ding R, Ikle DN, Bridges ND, Williams NM, Kastenmüller G, Karoly ED, Mohney RP, Abecassis M, Friedewald J, Knechtle SJ, Becker YT, Samstein B, Shaked A, Gross SS, Suthanthiran M: Urine metabolite profiles predictive of human kidney allograft status. J Am Soc Nephrol 27: 626–636, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigdel TK, Schroeder AW, Yang JYC, Sarwal RD, Liberto JM, Sarwal MM: Targeted urine metabolomics for monitoring renal allograft injury and immunosuppression in pediatric patients. J Clin Med 9: 2341, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang JYC, Sarwal RD, Sigdel TK, Damm I, Rosenbaum B, Liberto JM, Chan-On C, Arreola-Guerra JM, Alberu J, Vincenti F, Sarwal MM: A urine score for noninvasive accurate diagnosis and prediction of kidney transplant rejection. Sci Transl Med 12: eaba2501, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nolan N, Valdivieso K, Mani R, Yang JYC, Sarwal RD, Katzenbach P, Chalasani K, Hongo D, Lugtu G, Mark C, Chen E, Nijor R, Savoca D, Wexler DS, Whitson T, Huang SJ, Lu LH, Zawada RJX, Hytopoulos E, Sarwal MM: Clinical and analytical validation of a novel urine-based test for the detection of allograft rejection in renal transplant patients. J Clin Med 9: 2325, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filippone EJ, Farber JL: The monitoring of donor-derived cell-free DNA (ddcfDNA) in kidney transplantation. Transplantation 105: 509–516, 2021 [DOI] [PubMed] [Google Scholar]
- 68.Burnham P, Dadhania D, Heyang M, Chen F, Westblade LF, Suthanthiran M, Lee JR, De Vlaminck I: Urinary cell-free DNA is a versatile analyte for monitoring infections of the urinary tract. Nat Commun 9: 2412, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng AP, Burnham P, Lee JR, Cheng MP, Suthanthiran M, Dadhania D, De Vlaminck I: A cell-free DNA metagenomic sequencing assay that integrates the host injury response to infection. Proc Natl Acad Sci U S A 116: 18738–18744, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho J, Sharma A, Kroeker K, Carroll R, De Serres S, Gibson IW, Hirt-Minkowski P, Jevnikar A, Kim SJ, Knoll G, Rush DN, Wiebe C, Nickerson P: Multicentre randomised controlled trial protocol of urine CXCL10 monitoring strategy in kidney transplant recipients. BMJ Open 9: e024908, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]