Abstract
Diabetes and its associated complications pose an immediate threat to humankind. Diabetic kidney disease is one of the most devastating complications, increasing the risk of death more than ten-fold over the general population. Until very recently, the only drugs proven and recommended to slow the progression of diabetic kidney disease were angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers, which act by inhibiting the renin-angiotensin system. Despite their efficacy as kidney and cardiovascular protective therapies and as antihypertensive agents, renin-angiotensin system inhibitors have been grossly underutilized. Moreover, even when renin-angiotensin system inhibitors are used, patients still have a high residual risk of diabetic kidney disease progression. Finally, the kidney-protective effect of renin-angiotensin system inhibitors has been categorically demonstrated only in patients with macroalbuminuria included in the Irbesartan Diabetic Nephropathy Trial (IDNT) and Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trials, not in other individuals. The lack of new therapies to treat diabetic kidney disease over the past 2 decades has therefore represented a tremendous challenge for patients and health care providers alike. In recent years, a number of powerful new therapies have emerged that promise to transform care of patients with diabetes and kidney disease. The challenge to the community is to ensure rapid implementation of these treatments. This white paper highlights advances in treatment, opportunities for patients, challenges, and possible solutions to advance kidney health, and introduces the launch of the Diabetic Kidney Disease Collaborative at the American Society of Nephrology, to aid in accomplishing these goals.
Keywords: diabetes, kidney disease, SGLT2 inhibitor, disparity, equity
Introduction
Diabetic kidney disease (DKD) is the leading cause of kidney failure worldwide (1). Currently, in North America, 48 million patients have diabetes, of whom 95% have type 2 diabetes. Because 30%–40% of people with either type 1 diabetes or type 2 diabetes will develop DKD, it is estimated that approximately 15 million patients in North America have DKD (2,3). Despite the urgent need to slow the onset and progression of DKD, until recently, therapy was limited to glycemic control and blood pressure management, combined with renin-angiotensin system (RAS) inhibition (4–7). Clinical trials demonstrate that, together, these approaches can slow the onset and progression of DKD. The first of these clinical trials demonstrating benefit of RAS blockade was presented in 1993, nearly 3 decades ago (8). After >25 years and extensive clinical experience with RAS blockers, recent studies have shown that 25%–40% of patients with CKD, with or without diabetes, who should be given these drugs are receiving them (9,10). Although the reasons for this discordance between evidence-based guidelines and clinical practice are complex and diverse, clearly, this rate of usage of standard-of-care therapies is unacceptably low.
With the recent emergence of new classes of drugs to treat patients with DKD, we are presented with an unparalleled opportunity to preserve life and prevent kidney failure for millions of people around the world. In the face of unequivocal evidence, the challenge to members of the health care community, including health systems, payors, administrators, and providers, is to ensure these powerful new therapies reach the patients who need them, and the history of inadequate implementation of RAS blockade is not repeated. In this brief review, we provide a number of steps and mechanisms needed to achieve this goal.
SGLT2 Inhibitors and Diabetic Kidney Disease
Several recent large, randomized clinical trials have demonstrated that sodium-glucose cotransporter-2 (SGLT2) inhibitors have profound effects on reducing the onset and progression of DKD in the setting of type 2 diabetes (11–13) (trials summarized in Table 1). These kidney disease benefits were first observed as secondary end points in trials designed to test cardiovascular safety and effectiveness on the primary composite cardiovascular (CV) outcomes (14). Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy (CREDENCE) was the first clinical trial designed to address the question of whether SGLT2 inhibitors reduce the risk of kidney disease end points—doubling of serum creatinine, initiation of dialysis, or death from CV or kidney disease—as a primary outcome (15). The answer was a resounding “yes,” with a relative risk reduction of 30% (hazard ratio [HR], 0.70; 95% confidence interval [95% CI], 0.59 to 0.82, P<0.00001) and a number needed to treat of 22 over 2.6 years. In addition, the risk of adverse effects, such as ketoacidosis or worsened peripheral vascular disease requiring amputation, was exceedingly low and no higher in the treatment group than in the placebo group. Importantly, the beneficial effects of canagliflozin were observed in addition to the standard-of-care for DKD, including RAS blockade, because 99% of patients were receiving either an angiotensin-converting enzyme inhibitor or an angiotensin II type 1 receptor blocker. Similar to canagliflozin, other members of this class of drugs have shown overall consistent kidney benefits, particularly when similar definitions of “significant kidney function loss” are used across trials (16,17) (Table 1).
Table 1.
Summary of current and recent clinical trials of new diabetic kidney disease therapies
| Study | Intervention | Inclusion Criteria | Main Kidney Outcomes | Main Cardiovascular Outcomes | Primary Outcome |
| EMPA-REG OUTCOME (NCT01131676) (45) (7) |
Empagliflozin versus placebo | Type 2 diabetes History of atherosclerotic CV disease eGFR ≥30 ml/min per 1.73 m2 |
46% RR reduction of composite of doubling of serum creatinine accompanied by eGFR ≤45 ml/min per 1.73 m2, KRT, or death from kidney cause | 38% RR reduction in CV death; 35% RR reduction of hospitalization for HF 32% RR reduction for death from any cause; no difference in nonfatal stroke or MI |
CV |
| CANVAS (NCG01032629; NCT01989754) (46) |
Canagliflozin versus placebo | Type 2 diabetes with high CV risk eGFR |
40% RR reduction of kidney composite of sustained ≥40% decline in eGFR, KRT, or death from kidney causes | 14% RR reduction in CV death, nonfatal MI or nonfatal stroke; 33% RR reduction for hospitalization for HF (increased amputation risk) |
CV |
| DECLARE-TIMI-58 (NCT01730534) (47) |
Dapagliflozin versus placebo | Type 2 diabetes, high CV risk eGFR >60 ml/min per 1.73 m2 |
47% RR reduction in kidney composite sustained ≥40% reduction in eGFR to <60 ml/min per 1.73 m2, kidney failure, kidney death | 27% RR reduction in hospitalization for HF; 17% RR reduction in CV death or hospitalization for HF | CV |
| VERTIS CV (NCT01986881) (48) |
Ertugliflozin versus placebo | Type 2 diabetes, Atherosclerotic CV disease eGFR ≥30 ml/min per 1.73 m2 |
19% RR reduction (nonsignificant) kidney composite (kidney death, KRT, or doubling serum creatinine) 34% RR reduction (significant) for sustained ≥40% reduction in eGFR, kidney failure, kidney death |
8% RR reduction in CV death; 12% RR reduction in CV death or hospitalization for HF; no difference in MACE (CV death, nonfatal stroke or nonfatal MI) | CV |
| CREDENCE (NCT02065791) (15) |
Canagliflozin versus placebo | Type 2 diabetes eGFR 30–90 ml/min per 1.73 m2 UACR 300–5000 mg/g Stabilized on max tolerated dose of ACEi or ARB |
30% RR in kidney failure, sustained doubling of serum creatinine, kidney or CV death 28% RR reduction in secondary end point of dialysis, kidney transplant or kidney death |
20% RR reduction in CV death, nonfatal stroke or MI 39% RR reduction in hospitalization for HF |
Kidney and CV |
| DAPA-CKD (NCT03036150) (20) |
Dapagliflozin versus placebo | Type 2 diabetes with diabetic kidney disease or nondiabetic kidney disease eGFR 25–75 ml/min per 1.73 m2 UACR 200–5000 mg/g Max tolerated ACEi or ARB |
39% RR reduction in composite of sustained decline of eGFR >50%, kidney failure, or death from kidney or CV causes | 29% RR reduction in CV death or hospitalization for HF | Kidney and CV |
| EMPA-KIDNEY (NCT03594110) |
Empagliflozin versus placebo | Nondiabetic, eGFR between 20–45 ml/min ml/min per 1.73 m2 or CKD-EPI GFR 45–90 with UACR > 200 mg/g | Ongoing trial, primary composite end point kidney failure, sustained drop in eGFR ≥40% from baseline, kidney death or CV death | Ongoing Secondary outcome for CV death or hospitalization for HF |
Kidney and CV |
| EMPEROR-reduced (NCT03057977) (7) |
Empagliflozin versus placebo | HFrEF (Class II - IV, EF <40% and elevated BNP), eGFR > 20 ml/min with or without diabetes | Reduction in decline of eGFR from 2.28 ml/min (placebo) to 0.55 ml/min in treatment group 50% RR reduction in composite end point of KRT, sustained eGFR decline >50% |
25% RR reduction in CV death or hospitalization for HF; 30% RR reduction in hospitalization for HF | HF/CV |
| FIDELIO-DKD (NCT02540993) (30) |
Finerenonea versus placebo | Type 2 diabetes Albuminuria CKD Serum potassium less than or equal to 4.8 mmol/L |
Time to first occurrence of the composite end point of onset of kidney failure, sustained decrease of eGFR ≥40% from baseline over at least 4 weeks or kidney death | 14% RR reduction in CV death, nonfatal stroke, nonfatal MI, or hospitalization for HF | Kidney |
| SONAR (NCT01858532) (33) |
ET1 blockera versus placebo On top of standard of care |
Type 2 diabetes eGFR 25–75 ml/min per 1.73 m2 (cap of 300 for subjects with baseline eGFR >60 ml/min per 1.73 m2), UACR 300–5000 mg/g |
35% RR reduction time to doubling of serum creatinine (confirmed by a 30-day serum creatinine) or kidney failure (<15 ml/min per 1.73 m2, dialysis or transplant) | No significant differences between CV death or hospitalization for HF | Kidney |
| FLOW (NCT03819153) |
Semaglutide versus placebo | Type 2 diabetes eGFR 50–75 with UACR 300–5000 mg/g or eGFR 25–50 ml/min per 1.73 m2 (CKD-EPI) and UACR >100 and <5000 mg/g (on RAASa) |
Ongoing trial; time to persistent eGFR decline of > or = 50%, reaching kidney failure, death from kidney disease or CV disease | Ongoing trial; CV death (primary); MACE (secondary) | Kidney and CV |
EMPA-REG OUTCOME, (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; CV, cardiovascular; RR, relative risk; HF, heart failure; MI, myocardial infarction; CANVAS, CANagliflozin cardioVascular Assessment Study; DECLARE-TIMI-58, Dapagliflozin Effect on CardiovascuLAR Events; VERTIS CV, eValuation of ERTugliflozin effIcacy and Safety Cardiovascular Outcomes Trial; MACE, major adverse cardiac events; CREDENCE, Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy; UACR, urine albumin-to-creatinine ratio; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II type 1 receptor blockers; DAPA-CKD, Dapagliflozin in Patients with Chronic Kidney Disease; EMPA-KIDNEY, Study of Heart and Kidney Protection With Empagliflozin; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; EMPEROR-reduced, EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with Reduced Ejection Fraction; HFrEF, heart failure with reduced ejection fraction; EF, ejection fraction; BNP, NT-proB-type natriuretic peptide; FIDELIO-DKD, FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease; SONAR, Study Of diabetic Nephropathy with AtRasentan; FLOW, Semaglutide renal outcomes trial; RAAS, renin-angiotensin-aldosterone system.
No US Food and Drug Administration approval.
SGLT2 Inhibitors and Nondiabetic Kidney Disease
In April 2020, the Dapagliflozin in Patients with Chronic Kidney Disease study, which tested the benefit of SGLT2 inhibitors for DKD and non-DKD, was stopped prematurely for overwhelming efficacy (6,18,19). The study was published in October 2020 and the results demonstrate protection from adverse kidney disease and CV events in patients with CKD defined as eGFR 25–75 ml/min per 1.73 m2 and a urine albumin-creatinine ratio of 200–5000 mg/g (20). The risk reduction in the dapagliflozin-treated group for the primary kidney disease composite outcome (sustained decline of GFR of >50%, ESKD or death from kidney disease or CV causes) was 44% (HR, 0.56; 95% CI, 0.45 to 0.68, P<0.001), with only 19 patients needed to treat to prevent one primary outcome event over 2.4 years. Death from CV causes or heart failure (HF) hospitalization was also reduced by 29% (HR, 0.71; 95% CI, 0.55 to 0.92, P<0.009), confirming kidney and heart protective effects of these therapies in patients with and without diabetes. Moreover, the highest bar was crossed as the risk of all-cause mortality was reduced by 31% (HR, 0.71; 95% CI, 0.53 to 0.88, P=0.004).
Additional support for benefit of SGLT2 inhibition in patients who were nondiabetic or diabetic was provided in the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with Reduced Ejection Fraction trial, which demonstrated kidney-protective effects of empagliflozin in patients with class II, III, or IV HF and reduced ejection fraction <40% (with or without diabetes) (7). Approximately half of patients did not have diabetes. The risk of primary outcome of CV death or hospitalization for HF was reduced by 25% (HR, 0.75; 95% CI, 0.65 to 0.86, P<0.001) and annual rate of decline of eGFR (main secondary outcome) was reduced to 0.55 ml/min in the empagliflozin-treated group versus 2.28 ml/min in the placebo group (P<0.001). A prespecified analysis for the main secondary outcome—a composite of kidney disease end points including transplantation, KRT, or sustained eGFR decline of >50%—demonstrated a remarkable 50% risk reduction (HR, 0.50; 95% CI, 0.32 to 0.77) in patients treated with the SGLT2 inhibitor in addition to standard-of-care, confirming kidney disease benefits in patients with diabetes, HF, or both.
SGLT2 Inhibitor Therapy Provides an Opportunity to Advance Health Equity
In the United States, minority populations are disproportionately affected by diabetes and kidney disease. The risk of kidney failure is 3.7-fold higher in Black, 2.7-fold higher in Hawaiian/Pacific Islander, and 1.7-fold higher in Latinx and Native American/Alaskan Native people. The prevalence of diabetes is also higher in these populations, at 14%–17% versus 12% in the White/Non-Latinx population (21). Although the number of Black patients was low in all of the SGLT2 inhibitor trials, the effect size for kidney benefits of SGLT2 inhibitors was similar or even greater. These data highlight the very real and ongoing need for increased efforts to ensure equitable enrollment of patient populations including participants who are Black, Indigenous, or People of Color in clinical trials, and the importance of community engagement to build trust and policies to ensure that equitable access to emerging therapies is a priority for all patients who need them.
Heart Disease Kills Patients with CKD
In addition to the dramatic protective effects on the kidney, SGLT2 inhibitors have profound protective effects on the heart, reducing CV mortality and hospital admissions due to HF. Although heart disease is widely recognized as a major health threat, it is important to remember CKD is a major driver of CV death and HF in patients with diabetes. Indeed, most patients with DKD will die from CV causes without progressing to kidney failure. The reasons for the high incidence of heart disease in this patient population are complex; however, kidney disease per se accounts for significant disease burden. The positive effect of a kidney-targeted therapy on CV outcomes underscores the close and intertwined relationship between these two organs.
Breakthrough Status Granted by the US Food and Drug Administration for Dapagliflozin
On October 5, 2020, the US Food and Drug Administration (FDA) gave Farxiga (dapagliflozin) breakthrough therapy designation for the treatment of patients with CKD, irrespective of diabetes status, and there was full FDA approval for this indication on April 30, 2021. Breakthrough status designation is designed to expedite development and approval of particularly promising new treatments. Only 23 drugs received this designation before October 2020, all focused on rare or orphan diseases, infectious diseases, genetic causes of cancer, or other diseases that affect small subgroups of patients. No prior breakthrough designation has been provided for a potential kidney disease therapy. It is striking to consider the potential public health and societal effect of SGLT2 inhibitors in the context of other therapies in breakthrough status. This situation alone sends a “shout from the rooftops” to the community that we have entered a new era for treatment of patients with CKD. It is now up to the nephrology community to embrace the challenge.
Estimates of the Effect of SGLT2 Inhibition in Patients with CKD
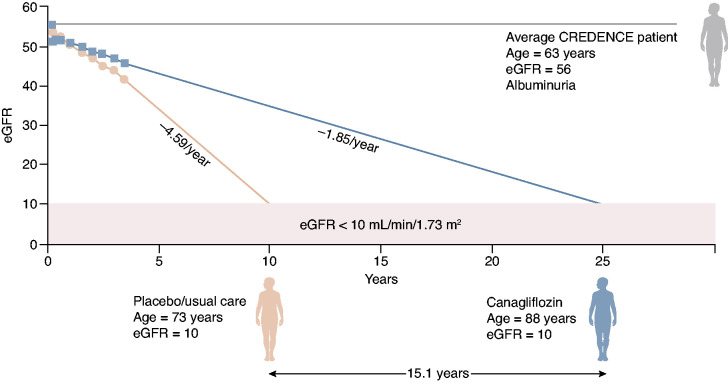
To demonstrate the effect of SGLT2 inhibition treatment in a real-world context, Adeera Levin, former International Society of Nephrology president, and Vlado Perkovic, principal investigator for CREDENCE, extrapolated information from the CREDENCE trial to show how SGLT2 inhibitor therapy could keep patients alive without kidney failure. An adapted figure is shown in Figure 1. In the example provided, it is possible to preserve life and slow the need for kidney replacement therapy by >15 years. Although additional and rigorous modeling studies are needed to confirm this prediction, it is estimated that 35 million Americans have CKD with eGFR of 25–75 ml/min per 1.73 m2, due to diabetes (15 million) or nondiabetic causes (20 million). Of these 35 million, approximately 15 million (10 million with diabetes, 5 million without diabetes) would meet DAPA-CKD entry criteria on the basis of eGFR criteria. Because urinary albumin is not frequently measured in clinical practice and the CV outcome trials demonstrated benefit irrespective of the level of albuminuria, this estimate is on the basis of a practical approach to initiate therapy in the clinic. In addition, SGLT2 inhibitors are prescribed to reduce CV events and death. For these reasons, Kidney Disease Improving Global Outcomes guidelines do not include albuminuria as a criterion for initiation. Although the public health effect alone is sufficient reason to support urgency of implementation, an estimate of health care dollars saved is equally staggering. In the United States, Medicare spends an estimated US$130 billion per year on kidney disease care. The predicted reduction of progression to kidney failure in patients treated with SGLT2 inhibitors points to billions of dollars in savings.
Figure 1.
An average patient in CREDENCE, represented by a male patient with type 2 diabetes, age 63, with an eGFR of 56 ml/min per 1.73 m2, receiving standard care in CREDENCE would need to start dialysis at age 73; the same patient receiving treatment with the SGLT2 inhibitor canagliflozin, in addition to standard care, would not require dialysis until age 88. To delay onset of dialysis treatment by 15.1 years is a profound benefit, especially given the projected 5-year survival of only 35% for such a patient receiving hemodialysis. Graphic courtesy of A. Levin and V. Perkovic. CREDENCE, Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy.
Risk-Benefit Ratio of SGLT2 Inhibitors
SGLT2 inhibitors should be utilized whenever possible by people with type 2 diabetes, or albuminuric/proteinuric nondiabetic CKD, to reduce risks of kidney disease progression and CV events. Although side effects of SGLT2 inhibitors are rare overall, actionable complications include euglycemic ketoacidosis, genital mycotic infections, and volume depletion.
Although it is clear there are many patients with CKD who would benefit from receiving these medications, they are generally not recommended for patients with polycystic kidney disease, recurrent lower genitourinary infections, history of ketoacidosis, or autoimmune diseases requiring immunosuppression. Clinical trials to determine safety and efficacy in patients with kidney allografts are urgently needed and should be supported by funding agencies and industry partners. Importantly, previous concerns about Fournier’s gangrene were never borne out in clinical trials, where the risk of this type of infection was not higher in SGLT2 inhibitor–treated patients. Also, for lower limb amputations, the FDA warning related to concerns arising from the CANagliflozin cardioVascular Assessment Study trial was rescinded in 2020 because amputation risk was not elevated in any of the other SGLT2 inhibitor clinical trials involving >50,000 patients. Importantly for nephrology practice, even in patients with the lowest eGFR at baseline in DAPA-CKD (43 ml/min per 1.73 m2), the risk of significant volume depletion was similar compared with placebo, and in meta-analyses, the risk of AKI was lower in SGLT2 inhibitor–treated patients (11,20,22). Excellent comprehensive patient care should be provided and patients advised to hold their SGLT2 inhibitor on “sick days” (i.e., nausea, vomiting, diarrhea, days without eating, surgery, etc.), especially to avert potentially serious ketoacidosis (11). A thorough history should be taken to identify frequent genitourinary infections and/or lower urinary tract symptoms and physical exam performed to identify peripheral vascular and/or foot issues to ensure ubiquitous good foot care practice. It cannot be emphasized enough that the positive effect of SGLT2 inhibitors (i.e., 15 years without kidney failure and 300,000 lives saved in 2 years) is enormous compared with the risk of adverse effects. It is the strong belief of these authors that the benefits of SGLT2 inhibitors far outweigh the generally manageable risks. Of course, it will be important to follow long-term benefits and/or risks of these therapies. Notably, patients carrying SGLT2 point mutations are not reported to have long-term health consequences. FDA approval of these therapies and additional clinical study results are pending for nondiabetic CKD and nonproteinuric DKD and CKD, respectively.
Looking Beyond SGLT2 Inhibitors
GLP1 Receptor Agonists.
Encouraging signals indicative of kidney protection have been observed in clinical trials of GLP1 receptor agonists in type 2 diabetes for CV or glycemic primary outcomes. CV outcome trials for GLP1 receptor agonists included secondary or exploratory kidney disease outcomes. Three agents in this class (liraglutide, semaglutide, dulaglutide) demonstrated a slowing of eGFR decline by >0.75 ml/min per 1.73 m2 per year compared with placebo or standard-of-care, an effect size highly predictive of a treatment that will prevent progression to kidney disease end points, such as serum creatinine doubling or kidney failure. This treatment effect was especially pronounced in those with eGFR <60 ml/min per 1.73 m2 (23–26). A prespecified exploratory analysis of A Study Comparing Dulaglutide With Insulin Glargine on Glycemic Control in Participants With Type 2 Diabetes (T2D) and Moderate or Severe Chronic Kidney Disease, comparing dulaglutide to insulin glargine as basal glucose-lowering therapy in patients with moderate to severe CKD (mean eGFR 38 ml/min per m2), demonstrated the risk of reaching a composite end point of kidney failure or ≥40% eGFR decline was substantially lower in the high-dose dulaglutide group (1.5 mg weekly) than in the insulin glargine group (5% versus 11%, P=0.04) (26). A time-to-event analysis showed the HR for this composite end point in participants with macroalbuminuria was 0.25 (95% CI, 0.10 to 0.68, P=0.006) (27). Notably, in a post hoc sensitivity analysis of the Researching cardiovascular Events with a Weekly INcretin in Diabetes trial, the CV outcome trial for dulaglutide, HRs for 40% or 50% eGFR decline were 0.70 (95% CI, 0.57 to 0.85; P=0.0004) and 0.56 (95% CI, 0.41 to 0.76; P=0.0002) in the dulaglutide group compared with placebo, respectively (28). Thus, the available evidence regarding GLP1 receptor agonists support their kidney-protective effects across a spectrum from patients without DKD to advanced stages of CKD in type 2 diabetes. These results have led to the FLOW trial, which is evaluating semaglutide (versus placebo) on primary kidney disease outcomes in patients with type 2 diabetes and CKD.
Nonsteroidal Mineralocorticoid Receptor Antagonists.
In addition to SGLT2 inhibitors, other therapies also show promise for patients with diabetes and CKD. The FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease trial, published in November 2020, demonstrated a benefit of a nonsteroidal mineralocorticoid blocker in patients with DKD (29). The proposed mechanism of action of finerenone is reduction of mineralocorticoid receptor (MR) activation that promotes inflammation and fibrosis (30). In patients with DKD characterized by moderately to severely increased albuminuria (urine albumin-creatinine ratio ≥30–299 or ≥300 mg/g, respectively), the number needed to treat was 29 to prevent one primary composite outcome event (kidney failure, sustained reduction in GFR >40%, or kidney death) over 2.6 years with a risk reduction of 18% (HR, 0.82; 95% CI, 0.73 to 0.93, P<0.001). Secondary CV outcomes were also superior in the finerenone-treated group, with the exception of nonfatal stroke. The proposed benefits of nonsteroidal mineralocorticoid antagonists are the result of higher selectivity than steroidal MR antagonists (such as spironolactone) and higher receptor affinity than eplerenone. In preclinical studies, finerenone significantly reduced proteinuria and end-organ damage compared with eplerenone in rats (31). It is also interesting to note the effect of finerenone on blood pressure lowering is generally attenuated compared with steroidal MR antagonists, which is thought to be due to its inability to cross the blood-brain barrier and affect central mechanisms of blood pressure control (32).
Endothelin 1A Receptor Blockade.
The potential therapeutic role for endothelin 1A receptor blockade in DKD was most clearly shown in the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial (33). SONAR used a 6-week enrichment phase in an attempt to reduce the risk of HF and identify patients more likely to respond by excluding those who developed fluid retention and/or had a reduction of urine albumin-to-creatinine ratio <30%. Patients who showed a positive reduction in urine albumin-to-creatinine ratio (>30%) were then randomized to placebo or treatment. Although the trial was stopped prematurely by the sponsor due to a low rate of primary outcome events, there was still a 35% relative risk reduction in the composite kidney end point of doubling of serum creatinine or kidney failure. In SONAR, anemia and fluid retention adverse events were more frequent with atrasentan, but the risk of hospital admission for HF was similar in atrasentan compared with patients treated with placebo (47 of 1325 patients, 4%, in treatment group versus 34 of 1323 patients, 3%, in the placebo group).
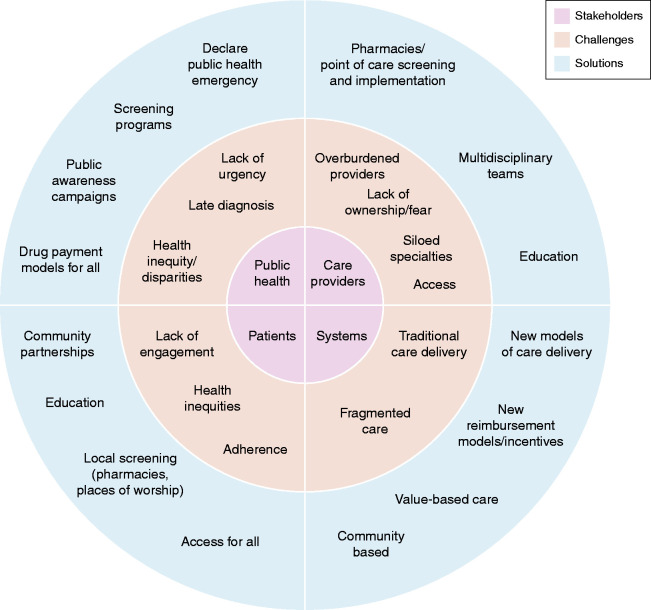
Implementation of New Options
To realize the benefits of these treatments, it is essential to devise strategies to promote rapid and universal adoption for patients (34,35) (Figure 2). Successful implementation involves the whole team and requires a collective effort from all stakeholders, including patients, nephrologists and other members of the kidney care team, primary care physicians (PCPs) and subspecialists, including cardiologists and endocrinologists, the pharmaceutical industry, payors, and government representatives from the US Human Health Services (HHS), the Centers for Medicare & Medicaid Services, the Centers for Disease Control and Prevention, and the FDA. To do this effectively, it is essential that:
-
1.
Nephrologists take the lead in developing and embracing goal-directed medical therapy for patients with DKD and CKD. This will require DKD and CKD physician treatment champions in health systems, academic institutions, and private practices, and in government, societal, research, and pharmaceutical settings, who will use their positions and perspectives to argue for and advance goal-directed therapy in our patients. Leadership should take a multifaceted approach that includes education of the health care team and patients and their families. It must also encompass advocacy to stimulate appropriate prescribing, additional research, and public policy changes that help make the most effective treatments available for all those who will benefit.
-
2.
Nephrologists, PCPs, and subspecialists, including endocrinologists and cardiologists, develop effective partnerships to encourage a team-based approach to patient care. It is time to replace the traditional care models with multidisciplinary approaches that better serve our patients with DKD and CKD.
-
3.
Effective educational resources (for trainees, practitioners, and patients) are developed and accessible to effect implementation of best practices and rapid adoption of new therapies.
-
4.
Policy and regulatory agencies, and payors and health care systems, are actively engaged to enhance access and make receiving these medications available for all patients.
Figure 2.
Current stakeholders, challenges, and possible solutions for implementation of new therapies.
Defining Barriers and Providing Solutions
Although it is clear we must adopt these innovative therapies to save lives and prevent loss of kidney function, history provides a stark reminder that many challenges can undermine rapid implementation of effective therapies, even in the face of overwhelming data. To overcome these challenges, it is important to define the barriers and provide solutions. In January 2020, the American Society of Nephrology (ASN) Diabetic Kidney Disease Collaborative convened a meeting of stakeholders in Washington, DC. Below is a list of barriers and potential solutions proposed by patients, nephrologists, government officials, health care system administrators, and pharmaceutical representatives.
-
1.
Lack of awareness and a sense of urgency: numerous sources cite a general lack of awareness among members of the public and patients with diabetes that kidney disease is a common complication (36). It is estimated that nine out of ten people with CKD are unaware of their diagnosis, and one out of two people with very low GFR do not know they have kidney disease (Centers for Disease Control and Prevention, Chronic Kidney Disease Initiative, cdc.gov). Indeed, the global burden and risk of kidney failure are often underestimated. Approximately 90% of patients with DKD die from accelerated CV disease, infections, and other causes before reaching kidney failure. Currently, the National Kidney Foundation, US HHS, and ASN are participating in a public campaign to raise awareness about kidney disease, as mandated in the Executive Order to Advance American Kidney Health (https://aspe.hhs.gov/system/files/pdf/262046/AdvancingAmericanKidneyHealth.pdf).
-
2.
Lack of patient and community engagement: to communicate to patients at risk and to ensure equitable health care access, community engagement is key. Involvement of patient and other stakeholder organizations and leadership is essential for success. Patients should be armed with questions to ask health care providers so they are empowered for better treatment and to use their voices to persuade governmental agencies to make policy changes.
-
3.
Inequitable health care access: a major barrier in kidney care remains the disparities that exist across the country. Disproportionate burden of diabetic and nondiabetic CKD exists in Black, Latinx, American Indian/Alaskan Native, and Native Hawaiian/Pacific Islander communities in the United States. Concerted focus and efforts on removing inequities to kidney health care are essential for success and must focus on recognizing and capturing social determinants of health, advancing health policy changes, dismantling structural racism, and investing in communities. The opportunity to prevent kidney disease has the potential to make the greatest effect in communities most affected by CKD. Effective and equitable implementation of SGLT2 inhibition may abolish some of the disparities that exist and could be promoted as an exemplar for health care justice.
-
4.
Fear of prescribing new therapies: the benefits to patients who will receive these therapies, extending longevity and prevention of kidney failure, far outweigh the relatively minor side effects of these agents. Developing strategies to communicate this message is key. It is also important to recognize that SGLT2 inhibitors should be utilized primarily as life-saving and kidney-protective agents, independent of their role as glucose-lowering drugs—analogous to the way in which angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers are recommended as kidney-protective agents when blood pressure is well controlled. A practical prescribing guide for SGLT2 inhibitors has been published and is publicly available as an open access publication (37–39).
-
5.
Prohibitive cost of therapies: current out-of-pocket costs for SGLT2 inhibitors and GLP-1 receptor agonists are significant. Although coverage for SGLT2 inhibitors and GLP-1 receptor agonists was often provided in 2019 Part D plans, they were variable for specific drugs. Out-of-pocket costs that are often hundreds of dollars per month are common for Medicare and Medicaid beneficiaries who are prescribed these agents either because of lack of coverage or because of high copayments (40). Health care organizations and the Centers for Medicare & Medicaid Services will be influenced by demonstration of cost effectiveness and quality metrics, which need to be developed and by champions in the federal branches of government (e.g., HHS).
-
6.
Complexity of multiple payors and insurance forms: this is a long-term issue requiring strategies to demonstrate benefit to multiple payors. In the short term, state-specific information on insurance and payor reimbursement could be provided to nephrologists and other clinicians to ease the burden of delivering care successfully.
-
7.
Absence of early DKD identification: increased early diagnosis and referral to promote early and optimal treatment will be key. Widespread national initiatives for screening patients who are high risk (patients with diabetes and hypertension), in communities and pharmacies, could help identify eligible patients. The RxEACH trial performed in Canada demonstrated the ability of community pharmacists to accurately identify a large number of patients with CKD that had previously been undiagnosed. Patients were identified to be at high risk on the basis of presence of one or more risk factors. The screening protocol was defined by the CKD Clinical Pathway (ckdpathway.ca) (41). Institutions and practices can identify nephrologists as local “DKD champions” to enhance uptake and function as local experts. Electronic medical record tools across the health care system should also be designed to identify patients who have no regular PCP but are seen in other settings and have persistently low eGFR and/or albuminuria.
-
8.
Culture change needed in nephrology: for the universal adoption of “preventive nephrology” to take place, new models of care must be promoted for providers and health care systems alike, allowing appropriate reimbursement for providing optimal care. Policy changes such as development of a Merit-based Incentive Payment Value Pathway that includes quality metrics supporting the appropriate use of SGLT2 inhibitors and the addition of quality metrics that support appropriate use of SGLT2 inhibitors to Center for Medicare & Medicaid Innovation care models, such as the Kidney Care Choices model and primary care–focused models, could help encourage appropriate uptake of these therapies. It is clear that appropriate uptake of SGLT2 inhibitors will support the first objective of the Advancing American Kidney Health Executive Order: to reduce the number of Americans developing kidney failure by 25% by 2030.
-
9.
Suboptimal education: current education about DKD and CKD for patients with diabetes or hypertension is not uniform and reaches a minority of individuals. Simplified education should be provided in multiple languages, and innovative delivery approaches—including patient-to-patient and school- or work-based programs—should be developed.
-
10.
Adherence to the best treatments: although innovative therapies are exciting, adherence to a treatment plan must occur once the drug is prescribed. Several strategies to enhance adherence including understanding the communities we serve, developing better education and adherence tools, empowering patients, and ensuring that equitable partnerships and care plans are developed with patient input.
-
11.
Saturation of the system: concern about a huge number of patients with early-stage CKD, who would overwhelm current nephrology services, can be avoided by development of multidisciplinary team care, telehealth models, e-consults, and new models of community-based initiation, and may help ease this perceived barrier to care. Importantly, nephrologists need not be the only physicians prescribing kidney-protective therapies; PCPs and other internal medicine specialists should also prescribe them for kidney and heart protection.
-
12.
Poor crosscollaboration and fragmented health care: development of interdisciplinary teams with champions for care of patients at high risk of CKD, with or without diabetes, will help address this challenge. Several models are being piloted or implemented in health care systems, which can serve as models across the United States (42).
Deliverables
Although it is relatively straightforward to identify challenges and develop exciting, innovative, and possible solutions, next steps and success require focused action. A number of implementation strategies have been described (reviewed in references 43 and 44) and can be grouped into six categories: planning, education, financing, restructuring, quality management, and attention to policy contexts. Several organizations have launched collaborative and independent work groups and committees to catalyze implementation. It is clear that any solution will need to address all six categories and stakeholders. A list of available resources for the kidney community on the Diabetic Kidney Disease Collaborative website, Kidney Disease Improving Global Outcomes guidelines, and American Diabetes Association Standards of Care for DKD are available through the links provided in Box 1.
Box 1.
List of resources and weblinks for diabetic kidney disease
Link to ASN webinars and resources: https://www.asn-online.org/dkd-c/
Link to Kidney Disease Improving Global Outcomes guidelines: https://www.kidney-international.org/article/S0085-2538(20)30718-3/fulltext/
American Diabetes Association Standards of Care for diabetic kidney disease: https://care.diabetesjournals.org/content/43/Supplement_1/S135/
Emerging new therapies show great promise for substantially reducing the burden of kidney disease, which suggests nephrologists will soon have a menu of potent new management tools to benefit patients. The clear benefits of SGLT2 inhibitors to improve outcomes in patients with CKD, with or without diabetes, provides added urgency. Every day we delay will result in more deaths and patients with kidney failure. We have hit a tipping point in nephrology. We need to embrace these opportunities, transform kidney medicine, and change history.
Disclosures
A.S. Kliger reports employment with Metabolism Associates, New Haven; reports having consultancy agreements with ASN; reports receiving honoraria from several universities and medical schools, professional organizations for lectures, seminars, and webinars; and reports other interests/relationships with ASN and Renal Physicians Association. D. Cherney reports consultancy agreements with Abbvie, AstraZeneca, Boehringer Ingelheim-Lilly, Janssen, Merck, Mitsubishi-Tanabe, NovoNordisk, Prometic, and Sanofi; reports receiving research funding from AstraZeneca, Boehringer Ingelheim-Lilly, Janssen, Merck, Novo Nordisk, and Sanofi; reports receiving honoraria from Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, JNJ, Lilly, Merck, Novo Nordisk, Otsuka, Prometic, and Sanofi; and reports serving as a scientific advisor or member of AstraZeneca, Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, and Sanofi. F.C. Brosius reports receiving honoraria from various universities, serving as an Associate Editor of Diabetes, and serving on the Editorial Boards of American Journal of Physiology and Journal of Clinical Investigation. Gilead Sciences, Inc. has contracted with the University of Michigan for F. Brosius's consultative services regarding diabetic nephropathy, and all funds go to the university. K.R. Tuttle reports consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, and Novo Nordisk; receiving research funding from Bayer and Goldfinch Bio; receiving honoraria from Bayer, Gilead, and Goldfinch Bio; and serving as a scientific advisor or member of CJASN, Lancet Diabetes Endocrinology, Nature Reviews Nephrology, National Institute of Diabetes and Digestive and Kidney Diseases, and Kidney Health Initiative. P.O. Gee is the founder of iAdvocate, Inc., a nonprofit organization; reports receiving honoraria from APOLLO APOL1 Long-term Kidney Transplantation Outcomes Consortium Community Advisory Council, Bayer International, CareDX, Center for Disease Innovation Patient Advisory Board/Kidney Research Institute Patient Advisory Committee, Otsuka Pharmaceutical Advisory Board, Patient Family Advisors Network, and Vertex International; reports serving as a scientific advisor or member of AAKP BOD, APOLLO Steering Committee's Community Advisory Council, ESRD Network 5 MRB, Otsuka Pharmaceuticals Advisory Board, and University of Washington Center for Dialysis Innovation Patient Advisory Board and the Human Factors Working Group Member and PFCC partners Advisory Board; other interests/relationships include AAKP Ambassador, AKF Ambassador and Kidney Health Coach, ASN Diabetic Kidney Disease-Collaborative Task Force, KHI PFPC Member, KPAC Member, NKF KAC, NCC PFE-LAN SME, PCORI Ambassador, PFA Network Advisors Diversity, Equity, and Inclusion Workgroup, Quality Insights Renal Network 5 PAC Chair, and UNOS Ambassador. R.C. Harris reports consultancy agreements with, and receiving research funding from, Bayer; reports receiving honoraria from University of California Los Angeles; reports patents and inventions with eNOS db/db mouse; and reports serving as a scientific advisor or member of Bayer Scientific Advisory Board. S.E. Quaggin reports consultancy agreements with AstraZeneca, Genentech, Goldfinch, Janssen, Lowy Medical Research Foundation, Novartis, and Roche; reports having an ownership interest in Mannin Research; reports receiving research funding from AstraZeneca and Mannin Research; reports receiving honoraria from Korean Scientific plenary lecture; and reports serving as a scientific advisor or member of AstraZeneca, Genentech/Roche, JCI, Karolinska CVRM Institute, Lowy Medical Research Institute, Mannin, and Novartis, and being Chief Scientific Officer and founder of Mannin Research.
Funding
The work is supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases P30DK114857 (to S.E. Quaggin).
Acknowledgments
We gratefully acknowledge unrestricted education grants provided by Bayer, Boehringer-Ingelheim, and Eli Lilly to support the DKD-C.
The DKD-C gratefully acknowledges the support of ASN staff Susie Stark, Darlene Rodgers, Meaghan (Allain) Malley, Bonnie Freshley, Kerry Leigh, and Tod Ibrahim. Editorial support was provided by Alison Harkin.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Koye DN, Magliano DJ, Nelson RG, Pavkov ME: The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 25: 121–132, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV: Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF Trial. Circulation 141: 90–99, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators : Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL: Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol 6: 2444–2451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano-Maneiro L, Puente-García A: Renin-angiotensin-aldosterone system blockade in diabetic nephropathy. Present evidences. J Clin Med 4: 1908–1937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnier M, Zanchi A: Blockade of the renin-angiotensin-aldosterone system: A key therapeutic strategy to reduce renal and cardiovascular events in patients with diabetes. J Hypertens 24: 11–25, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Lefton CK: ACE inhibitor slows onset of renal disease in diabetics by 50%. Nephrol News Issues 7: 8–11, 1993 [PubMed] [Google Scholar]
- 9.Tuttle KR, Alicic RZ, Duru OK, Jones CR, Daratha KB, Nicholas SB, McPherson SM, Neumiller JJ, Bell DS, Mangione CM, Norris KC: Clinical characteristics of and risk factors for chronic kidney disease among adults and children: An analysis of the CURE-CKD Registry. JAMA Netw Open 2: e1918169, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DP, Drawz PE, Foley RN: Trends in angiotensin-converting enzyme inhibitor and angiotensin ii receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol 30: 1314–1321, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ: SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, Heerspink HL, Wong MG, Ninomiya T, Wada T, Perkovic V: Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab 21: 1237–1250, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS: SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393: 31–39, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Kluger AY, Tecson KM, Lee AY, Lerma EV, Rangaswami J, Lepor NE, Cobble ME, McCullough PA: Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol 18: 99, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Cherney DZI, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, Shih WJ, Frederich R, Maldonado M, Pong A, Cannon CP; VERTIS CV Investigators : Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: An analysis from the randomised VERTIS CV trial. Diabetologia 64: 1256–1267, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Raalte DH, Bjornstad P, Heerspink HJL, Persson F, Cherney DZI: Importance of standardizing renal outcomes in clinical trials: Illustration by recent sodium glucose cotransporter 2 inhibitor studies. Kidney Int 99: 768–770, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt DL, Verma S, Braunwald E: The DAPA-HF Trial: A momentous victory in the war against heart failure. Cell Metab 30: 847–849, 2019 [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators : Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention : Prevalence of Both Diagnosed and Undiagnosed Diabetes. 2020; Available from: https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html. Accessed March 22, 2021
- 22.Iskander C, McArthur E, Nash DM, Gandhi-Banga S, Weir MA, Muanda FT, Garg AX: Identifying Ontario geographic regions to assess adults who present to hospital with laboratory-defined conditions: A descriptive study. CMAJ Open 7: E624–E629, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and Investigators : Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377: 839–848, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Mann JFE, Hansen T, Idorn T, Leiter LA, Marso SP, Rossing P, Seufert J, Tadayon S, Vilsbøll T: Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: A post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol 8: 880–893, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, Botros FT: Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 6: 605–617, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, Simon AL, Ying J, Beck GJ, Wanner C, Floege J, Li PK, Perkovic V, Vonesh EF, Greene T: GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol 30: 1735–1745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuttle KR, Rayner B, Lakshmanan MC, Kwan AYM, Konig M, Shurzinske L, Botros FT; Clinical outcomes by albuminuria status with dulaglutide versus insulin glargine in participants with diabetes and CKD: AWARD-7 exploratory analysis. Kidney360 2: 254–262, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators : Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 394: 121–130, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama A: Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res 42: 293–300, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, Eitner F, Albrecht-Küpper B, Schäfer S: Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 64: 69–78, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim S-Y, Zannad F; Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur Heart J 34: 2453–2463, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators : Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 393: 1937–1947, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Kliger AS, Brosius FC; Diabetic Kidney Disease Task Force of the American Society of Nephrology : Preserving kidney function instead of replacing it. Clin J Am Soc Nephrol 15: 129–131, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuttle KR, Cherney DZI: Therapeutic transformation for diabetic kidney disease. Kidney Int 99: 301–303, 2021 [DOI] [PubMed] [Google Scholar]
- 36.Chu CD, McCulloch CE, Banerjee T, Pavkov ME, Burrows NR, Gillespie BW, Saran R, Shlipak MG, Powe NR, Tuot DS; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis 76: 174–183, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR: Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes 68: 248–257, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Dubrofsky L, Srivastava A, Cherney DZ: Sodium-glucose cotransporter-2 inhibitors in nephrology practice: A narrative review. Can J Kidney Health Dis 7: 2054358120935701, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoungas S, de Boer IH: SGLT2 inhibitors in diabetic kidney disease. Clin J Am Soc Nephrol 16: 631–633, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF: Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the Medicare Part D Program. JAMA Netw Open 3: e2020969, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Hamarneh YN, Hemmelgarn BR, Hassan I, Jones CA, Tsuyuki RT: The effectiveness of pharmacist interventions on cardiovascular risk in adult patients with type 2 diabetes: The multicentre randomized controlled RxEACH Trial. Can J Diabetes 41: 580–586, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Rangaswami J, Bhalla V, de Boer IH, Staruschenko A, Sharp JA, Singh RR, Lo KB, Tuttle K, Vaduganathan M, Ventura H, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Lifestyle and Cardiometabolic Health : Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: A scientific statement from the American Heart Association. Circulation 142: e265–e286, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, Bunger AC, Glass JE, York JL: A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev 69: 123–157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers DA, Feero WG, Khoury MJ: Convergence of implementation science, precision medicine, and the learning health care system: A new model for biomedical research. JAMA 315: 1941–1942, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators : Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
- 48.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK; VERTIS CV Investigators : Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 383: 1425–1435, 2020 [DOI] [PubMed] [Google Scholar]