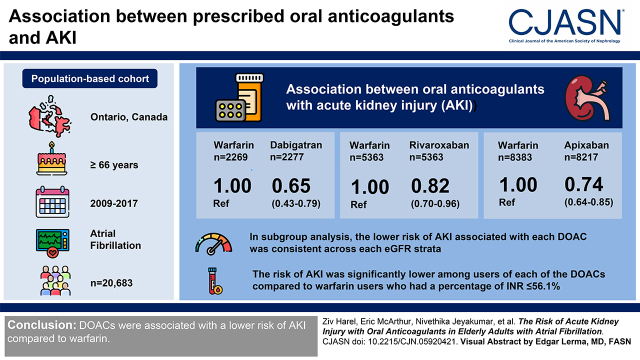
Visual Abstract
Keywords: acute kidney injury, atrial fibrillation, anticoagulants
Abstract
Background and objectives
Anticoagulation with either a vitamin K antagonist or a direct oral anticoagulant may be associated with AKI. Our objective was to assess the risk of AKI among elderly individuals with atrial fibrillation newly prescribed a direct oral anticoagulant (dabigatran, rivaroxaban, or apixaban) versus warfarin.
Design, setting, participants, & measurements
Our population-based cohort study included 20,683 outpatients in Ontario, Canada, ≥66 years with atrial fibrillation who were prescribed warfarin, dabigatran, rivaroxaban, or apixaban between 2009 and 2017. Inverse probability of treatment weighting on the basis of derived propensity scores for the treatment with each direct oral anticoagulant was used to balance baseline characteristics among patients receiving each of the three direct oral anticoagulants compared with warfarin. Cox proportional hazards regression was performed in the weighted population to compare the association between the prescribed anticoagulant and the outcomes of interest. The exposure was an outpatient prescription of warfarin or one of the direct oral anticoagulants. The primary outcome was a hospital encounter with AKI, defined using Kidney Disease Improving Global Outcomes thresholds. Prespecified subgroup analyses were conducted by eGFR category and by the percentage of international normalized ratio measurements in range, a validated marker of anticoagulation control.
Results
Each direct oral anticoagulant was associated with a significantly lower risk of AKI compared with warfarin (weighted hazard ratio, 0.65; 95% confidence interval, 0.53 to 0.80 for dabigatran; weighted hazard ratio, 0.85; 95% confidence interval, 0.73 to 0.98 for rivaroxaban; and weighted hazard ratio, 0.81; 95% confidence interval, 0.72 to 0.93 for apixaban). In the subgroup analysis, the lower risk of AKI associated with each direct oral anticoagulant was consistent across each eGFR strata. The risk of AKI was significantly lower among users of each of the direct oral anticoagulants compared with warfarin users who had a percentage of international normalized ratio measurements ≤56%.
Conclusions
Direct oral anticoagulants were associated with a lower risk of AKI compared with warfarin.
Introduction
Oral anticoagulants are commonly prescribed to prevent stroke and systemic thromboembolism in patients with atrial fibrillation. AKI attributed to oral anticoagulants may occur through a number of potential mechanisms: systemic bleeding leading to hypotension, acute interstitial nephritis, and anticoagulant-related nephropathy. Anticoagulant-related nephropathy was first described in warfarin users and manifests by glomerular hemorrhage and tubular obstruction (1, 2). Subsequent reports of anticoagulant-related nephropathy have documented similar pathologic findings among direct oral anticoagulant (DOAC) users, despite a putatively lower risk of bleeding compared with warfarin (3–6).
Although some observational studies have demonstrated a diminished risk of AKI among DOAC users compared with warfarin users, these studies were conducted within restricted geographic regions (7–10), used insensitive administrative codes to define AKI (7, 8, 10–12), and did not account for key confounders (7, 8, 10, 11). They also failed to include individuals with a higher baseline risk of AKI, such as those with advanced CKD.
We evaluated the association between prescribed oral anticoagulant (dabigatran, rivaroxaban, apixaban, or warfarin) initiated in patients with atrial fibrillation and AKI. We hypothesized that DOACs would be associated with a lower risk of AKI.
Materials and Methods
Study Design and Population
We conducted a population-based cohort study of older adults using linked health care databases in Ontario, Canada (14.5 million residents, 17% of whom are aged 65 years or older) (13). Residents have universal access to hospital care and physician services, and those over 65 years of age (approximately 2.2 million residents) have universal prescription drug coverage.
We included older adults (≥66 years) in Ontario, Canada, who were newly dispensed one of three DOACs (dabigatran, rivaroxaban, or apixaban) or warfarin between January 1, 2009 and March 31, 2017 and who received a diagnosis of atrial fibrillation or atrial flutter within 5 years prior to the date of the newly dispensed medication (the index date). The 5-year look back ensured that all eligible older adults with atrial fibrillation were included in the cohort, as prior to 2014, the Canadian guidelines did not universally recommend the use of oral anticoagulation for all patients with atrial fibrillation who were >65 years. Patients with atrial fibrillation were identified through hospitalizations using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada diagnostic code I48. This code has been previously validated and found to have a positive predictive value of 93% (95% confidence interval [95% CI], 91.6 to 94.2) (14).
We excluded patients in their first year of eligibility for prescription drug coverage (age 65 years) and those who had invalid identifying information, missing data, were non-Ontario residents, or died on or before index date, as well as those with a history of a kidney transplant, the receipt of maintenance dialysis, or valvular heart surgery within 3 years of the index date. We also excluded patients with no outpatient serum creatinine measurement (which was to serve as the baseline value) during the 365 days prior to the index date or those who did not reside within a hospital catchment area with linked laboratory data to ensure accurate ascertainment of AKI. Finally, we also excluded patients who received a prescription for more than one of the anticoagulants of interest on the index date, as well as those who were prescribed any of the anticoagulants within 180 days prior to the index date or prior to a diagnosis of atrial fibrillation in order to ensure patients were new users of these drugs in order to prevent selection bias.
All patients were identified in the Canadian Institute for Health Information Discharge Abstract Database and linked to the Ontario Laboratories Information System (OLIS; https://ehealthontario.on.ca/en/health-care-professionals/lab-results), which captures nearly all outpatient laboratory tests in Ontario (15). Serum creatinine values from OLIS were used to calculate eGFRs using the Chronic Kidney Disease Epidemiology Collaboration equation standardized to isotope dilution mass spectrometry, the current reference standard (16). Study patients were assigned to geographic hospital catchment areas with corresponding linked laboratory data using previously published methods (15). We included only Ontarians who resided within these catchment areas to ensure accurate outcome ascertainment, as not all hospital-based laboratories started contributing to OLIS at the same time. All datasets were housed at ICES and linked using unique encoded identifiers. Details about the databases and administrative codes used for this study are shown in Supplemental Tables 1 and 2. Each database has been used extensively to research health outcomes and health services (17–21). The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. We have reported this study according to guidelines for the Reporting of Studies Conducted Using Observational Routinely Collected Health Data for Pharmacoepidemiology (22).
Outcomes
The primary outcome was a hospital encounter (hospitalization or emergency department presentation) with AKI, defined by thresholds from the Kidney Disease Improving Global Outcomes (KDIGO) working group: a ≥50% increase in serum creatinine concentration from baseline, an absolute increase of ≥0.3 mg/dl, or the receipt of acute dialysis (23). This definition has been utilized in many observational studies to define AKI (24–26). The baseline value was the most recent outpatient serum creatinine value in the 365 days prior to the index date. We compared this baseline serum creatinine value with the highest serum creatinine during the first 7 days after a subsequent hospital encounter. The need for acute dialysis for AKI was determined using a validated approach on the basis of diagnosis and procedural administrative codes (27). The secondary outcome was all-cause mortality.
Statistical Analyses
Continuous variables were described using means (SD) or medians (interquartile range [IQR]) as appropriate. Categorical variables were described as proportions. Incidence rates for each outcome were calculated on the basis of the frequency of the outcome and the person-years of follow-up.
For each DOAC, logistic regression was used to estimate the propensity of treatment with that specific DOAC compared with warfarin conditional on patient covariates measured at the time of anticoagulation initiation. In total, >50 covariates were chosen a priori as potential confounders and included in each of the propensity score models (Table 1, Supplemental Table 3). An inverse probability of treatment weighting approach was then used to construct, for each DOAC, a weighted cohort of patients receiving either warfarin or that DOAC, with each warfarin-treated patient weighted by his or her estimated odds of receiving the DOAC comparator from the corresponding propensity model. This “average treatment effect among the treated” weighting rebalances the warfarin subcohort so that its average measured baseline characteristics better resemble those in the DOAC-treated subcohort. The “average treatment effect among the treated” weighted cohorts for each DOAC are then used in subsequent analyses to describe and compare observed AKI incidence and mortality with each DOAC with those of the propensity-weighted similarly constituted group of patients who received warfarin and, thus, estimate the causal effect of the DOAC among those who received it. Balance in baseline covariates between DOAC and warfarin treatment groups before and after inverse probability of treatment weighting was assessed using the standardized difference, where a standardized difference >10% was considered significant (28).
Table 1.
Baseline characteristics after inverse probability of treatment weighting
| Variable | Warfarin, n=2269 | Dabigatran, n=2277 | Standardized Difference, %a | Warfarin, n=5363 | Rivaroxaban, n=5263 | Standardized Difference, %a | Warfarin, n=8383 | Apixaban, n=8217 | Standardized Difference, %a |
| Demographics | |||||||||
| Age, yr, mean (SD) | 78 (5) | 78 (7) | 0 | 78 (8) | 78 (8) | 0 | 80 (10) | 80 (8) | 0 |
| Women, n (%) | 1011 (50) | 1003 (44) | 1 | 2787 (52) | 2652 (50) | 3 | 4598 (55) | 4408 (54) | 2 |
| Comorbidities, b n (%) | |||||||||
| ATRIA bleeding score, median (IQR) | 3 (1–3) | 3 (1–3) | 3 (1–4) | 3 (1–4) | 3 (2–4) | 3 (2–4) | |||
| CHADS2VaSc, median (IQR) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 4 (3–5) | |||
| Charlson index, mean (SD) | 0.9 (1.0) | 0.9 (1.5) | 2 | 1.0 (1.6) | 1.00 (1.5) | 2 | 1.3 (2.2) | 1.2 (1.7) | 3 |
| Hypertension | 1725 (76) | 1728 (76) | 0 | 3980 (74) | 3867 (74) | 2 | 6420 (77) | 6285 (77) | 0 |
| Prior stroke/TIA | 140 (6) | 139 (6) | 0 | 374 (7) | 342 (7) | 2 | 1040 (12) | 912 (11) | 4 |
| Heart failure | 695 (31) | 695 (31) | 0 | 1472 (280) | 1452 (28) | 0 | 2919 (35) | 2873 (35) | 0 |
| Myocardial infarction | 108 (5) | 99 (4) | 2 | 349 (7) | 293 (6) | 4 | 626 (8) | 526 (6) | 4 |
| Diabetes | 754 (33) | 751 (33) | 0 | 1714 (32) | 1606 (31) | 3 | 2774 (33) | 2641 (32) | 2 |
| Peptic ulcer disease | 25 (1) | 25 (1) | 0 | 113 (2) | 105 (2) | 1 | 185 (2) | 174 (2) | 1 |
| Alcoholism | 45 (2) | 44 (2) | 1 | 88 (2) | 85 (2) | 0 | 181 (2) | 150 (2) | 3 |
| Moderate/severe liver disease | 86 (4) | 84 (4) | 1 | 180 (3) | 190 (4) | 1 | 377 (5) | 376 (5) | 0 |
| Venous thromboembolism | 64 (3) | 57 (3) | 2 | 331 (6) | 281 (5) | 4 | 273 (3) | 249 (3) | 2 |
| Prior major hemorrhage | 142 (6) | 141 (6) | 0 | 369 (7) | 344 (7) | 2 | 668 (8) | 606 (7) | 2 |
| Major cancer | 351 (16) | 344 (15) | 1 | 857 (16) | 835 (16) | 0 | 1349 (16) | 1360 (17) | 1 |
| Prior AKI | 94 (4) | 97 (4) | 0 | 329 (6) | 318 (6) | 0 | 804 (10) | 737 (9) | 2 |
| Laboratory data c | |||||||||
| Baseline eGFR, ml/min, mean (SD) | 69 (12) | 69 (16) | 3 | 70 (18) | 70 (16) | 4 | 65 (24) | 65 (18) | 1 |
| eGFR category, ml/min per 1.73 m2 | |||||||||
| <30 | 15 (1) | 15 (1) | 0 | 47 (1) | 47 (1) | 0 | 189 (2) | 185 (2) | 0 |
| 30 to <60 | 712 (31) | 727 (32) | 1 | 1418 (26) | 1439 (27) | 2 | 3065 (37) | 3025 (37) | 0 |
| ≥60 | 1543 (68) | 1535 (67) | 1 | 3898 (73) | 3777 (72) | 2 | 5128 (61) | 5007 (61) | 1 |
| Albuminuria, n (%) | |||||||||
| Undetectable/normal, <30 mg/g | 441 (19) | 443 (20) | 1 | 835 (16) | 836 (16) | 2 | 1250 (15) | 1222 (15) | 1 |
| Mild, 30–300 mg/g | 172 (8) | 179 (8) | 1 | 375 (7) | 359 (7) | 1 | 654 (8) | 635 (8) | 0 |
| Heavy, >300 mg/g | 34 (2) | 34 (2) | 0 | 85 (2) | 90 (2) | 1 | 171 (2) | 187 (2) | 2 |
| Not measured | 1623 (72) | 1621 (71) | 1 | 4068 (76) | 3978 (76) | 0 | 6308 (75) | 6173 (75) | 0 |
| Concomitant drug use, d n (%) | |||||||||
| Antiplatelet agents (non-ASA) | 152 (7) | 147 (7) | 1 | 522 (10) | 447 (9) | 4 | 1082 (13) | 970 (12) | 3 |
| Anti-inflammatories (i.e., NSAIDs) | 217 (10) | 206 (9) | 2 | 588 (11) | 543 (10) | 2 | 774 (9) | 721 (9) | 1 |
| Antibiotics | 633 (28) | 617 (22) | 2 | 1629 (30) | 1520 (29) | 3 | 2667 (32) | 2526 (31) | 2 |
| Antifungals | 22 (1) | 21 (1) | 1 | 67 (1) | 59 (1) | 1 | 116 (1) | 87 (1) | 3 |
| Anticonvulsants | 132 (6) | 132 (6) | 0 | 450 (8) | 418 (8) | 2 | 681 (8) | 633 (8) | 1 |
| Corticosteroids | 548 (24) | 552 (24) | 0 | 1331 (25) | 1319 (25) | 1 | 2260 (27) | 2235 (27) | 0 |
| Gastroprotective medications | 706 (31) | 712 (31) | 0 | 1875 (35) | 1782 (34) | 2 | 3351 (40) | 3227 (39) | 1 |
ATRIA, anticoagulation and risk factors in atrial fibrillation; IQR, interquartile range; TIA, transient ischemic attack; ASA, acetylsalicylic acid; NSAID, nonsteroidal anti-inflammatory drug.
Standardized differences were used to compare warfarin users with direct oral anticoagulant users. Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of difference between groups with respect to a pooled SD. A standardized difference >10% is considered a meaningful difference between groups. In this study, standardized differences were calculated using warfarin users as the referent.
Comorbidities in the 5 years prior to the index prescription were considered.
Laboratory measurements in the 365 days prior to the index prescription were considered. eGFR was standardized to 1.73 m2.
Medications prescribed during the 180 days prior to the index prescription for warfarin or a direct oral anticoagulant.
Cox proportional hazards regression models were used to estimate the weighted hazard ratios (HRs) between each type of DOAC and the study outcome compared with warfarin. We used a bootstrap estimator to determine the accompanying 95% CIs for each DOAC-warfarin comparator and a bootstrapped permutation test to determine the interaction P values within each stratum of eGFR (29). Patients were followed until the development of AKI (outcome of interest), death, discontinuation of a given anticoagulant, switching to another anticoagulant, or the end of the study period (December 31, 2017).
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Additional Analyses
The quality and safety of anticoagulation with warfarin can be quantified by the percentage of international normalized ratio (INR) measurements in range. The percentage of INR measurements in range has been shown to be strongly correlated with improved safety of warfarin (30). Specifically, a percentage of INR measurements in range >56% is highly correlated with excellent warfarin safety and efficacy (31). We developed two subcohorts, one in which warfarin users had a percentage of INR measurements in range >56% and one with a percentage of INR measurements in range ≤56%, and reran the primary analysis.
Because CKD is the most potent risk factor for AKI, the risk of AKI may differ by baseline kidney function (32). Accordingly, we performed a subgroup analysis by baseline eGFR category (≥60, 30 to <60, and <30 ml/min per 1.73 m2).
We also assessed the severity of the first AKI episode by repeating the primary analysis for AKI on the basis of the KDIGO AKI staging criteria (23) (KDIGO AKI staging criteria thresholds are listed in Supplemental Table 4).
For patients receiving warfarin, an INR>3.0 has been associated with a greater risk of developing AKI (33). Accordingly, in additional analysis 4, we determined the number of patients receiving warfarin who had an INR>3.0 at the time of, or one day prior to, their AKI event.
The DOACs were introduced at various time periods; hence, there may have been secular changes in drug selection, which may have affected the incidence of AKI. To account for this, we repeated our primary analysis adjusting for year. Specifically, study year was included as a covariate in the Cox regression model as a single linear time effect.
To test the robustness of our main results, we performed three post hoc sensitivity analyses. First, we performed an E-value analysis to assess the extent of unmeasured confounding that would be required to negate the observed results (34, 35). Second, we repeated the primary analysis using an intention-to-treat approach. This analysis ignored continuous usage and ascertained outcomes during the entire follow-up period, not censoring at medication stop or switch. Both the propensity score models and weights were unchanged. Finally, to further assess for confounding, we repeated our primary analysis using pneumonia as a negative control outcome.
Results
We identified 210,474 adults ≥66 years with a prescription for a DOAC or warfarin and a prior diagnosis of atrial fibrillation/flutter. Of these, we excluded 189,791 individuals, of which 54,897 had filled a prescription for a study drug within 180 days prior to the index date, 17,325 not having a baseline serum creatinine, and 100,391 with OLIS catchment area ineligibility. This left 20,683 adults in the final weighted cohort, of whom 2277 (11%) received dabigatran, 5263 (25%) received rivaroxaban, 8217 (40%) received apixaban, and 4926 (24%) received warfarin (Supplemental Figure 1).
Table 1 summarizes selected demographic and clinical characteristics of the study population after propensity score weighting by treatment group. Postweighting, good balance was achieved for all covariates. Supplemental Table 5 includes the additional baseline characteristics included in the propensity score model for the weighted cohort but not shown in Table 1. The baseline characteristics of the unweighted cohort are shown in Supplemental Table 6.
The median (IQR) follow-up time was 308 (IQR, 61–682) days. Warfarin users had an almost four-fold shorter duration of follow-up compared with those prescribed each of the DOACs (Supplemental Table 7).
AKI
The weighted incidence of AKI was lower among recipients of any DOAC compared with warfarin (6.4 versus 10.2 per 100 patient-years [py] for dabigatran versus warfarin, 9.1 versus 11.1 per 100 py for rivaroxaban versus warfarin, and 11.1 versus 14.2 per 100 py for apixaban versus warfarin). Each of the DOACs was also associated with a lower risk of AKI compared with warfarin (weighted HR, 0.65; 95% CI, 0.53 to 0.80 for dabigatran; weighted HR, 0.85; 95% CI, 0.73 to 0.98 for rivaroxaban; and weighted HR, 0.81; 95% CI, 0.72 to 0.93 for apixaban) (Table 2).
Table 2.
Association between oral anticoagulants and a hospital encounter with AKI
| Exposure | Unweighted | Weighted | E Value for Point Estimate | E Value for 95% Confidence Interval | |||||
| No. | No. (%) with AKI | Incidence Rate (per 100 patient-yr) | No. | No. (%) with AKI | Incidence Rate (per 100 patient-yr) | Hazard Ratio (95% Confidence Interval) | |||
| Warfarin | 4926 | 551 (11) | 15.6 | 8383 | 874 (10) | 14.2 | 1.00 (reference) | ||
| Apixaban | 8217 | 1017 (12) | 11.1 | 8217 | 1017 (12) | 11.1 | 0.81 (0.72 to 0.93) | 1.77 | 1.36 |
| Warfarin | 4926 | 551 (11) | 15.6 | 5363 | 453 (8) | 11.1 | 1.00 (reference) | ||
| Rivaroxaban | 5263 | 525 (10) | 9.1 | 5263 | 525 (10) | 9.1 | 0.85 (0.73 to 0.98) | 1.63 | 1.16 |
| Warfarin | 4926 | 551 (11) | 15.6 | 2269 | 174 (8) | 10.2 | 1.00 (reference) | ||
| Dabigatran | 2277 | 178 (8) | 6.4 | 2277 | 178 (8) | 6.4 | 0.65 (0.53 to 0.80) | 2.45 | 1.81 |
Mortality
Similar to AKI, the weighted incidence of mortality was generally lower among each DOAC compared with warfarin. Moreover, the risk of all-cause mortality was lower among all eGFR strata for dabigatran compared with warfarin (Supplemental Table 8).
Additional Analyses
There was no significant difference in the risk of AKI associated with each DOAC compared with warfarin users who attained a percentage of INR measurements in range >56%. However, the risk of AKI was significantly lower among users of each of the DOACs compared with warfarin users who had a percentage of INR measurements in range ≤56% (Tables 3 and 4).
Table 3.
Association between oral anticoagulants and AKI among a subcohort of patients on warfarin with a percentage of international normalized ratio (INR) measurements in range >56% (weighted cohort)
| Cohort | Exposure | N | No. (%) with AKI | Incidence Rate per 100 person-yr | Hazard Ratio (95% Confidence Interval) |
| Apixaban versus warfarin | Warfarin | 1091 | 89 (8) | 10.2 | 1.00 (reference) |
| Apixaban | 8217 | 1017 (12) | 11.1 | 1.07 (0.78 to 1.47) | |
| Rivaroxaban versus warfarin | Warfarin | 730 | 52 (7) | 8.2 | 1.00 (reference) |
| Rivaroxaban | 5263 | 525 (10) | 9.1 | 1.09 (0.75 to 1.59) | |
| Dabigatran versus warfarin | Warfarin | 357 | 20 (6) | 6.2 | 1.00 (reference) |
| Dabigatran | 2277 | 178 (8) | 6.4 | 1.01 (0.68 to 1.51) |
Table 4.
Association between oral anticoagulants and AKI among a subcohort of patients on warfarin with a percentage of international normalized ratio (INR) measurements in range ≤56% (weighted cohort)
| Cohort | Exposure | N | No. (%) with AKI | Incidence Rate per 100 person-yr | Hazard Ratio (95% Confidence Interval) |
| Apixaban versus warfarin | Warfarin | 3757 | 435 (12) | 14.3 | 1.00 (reference) |
| Apixaban | 8217 | 1017 (12) | 11.1 | 0.79 (0.66 to 0.94) | |
| Rivaroxaban versus warfarin | Warfarin | 2335 | 231 (10) | 12.0 | 1.00 (reference) |
| Rivaroxaban | 5263 | 525 (10) | 9.1 | 0.77 (0.63 to 0.93) | |
| Dabigatran versus warfarin | Warfarin | 895 | 86 (10) | 12.0 | 1.00 (reference) |
| Dabigatran | 2277 | 178 (8) | 6.4 | 0.54 (0.43 to 0.69) |
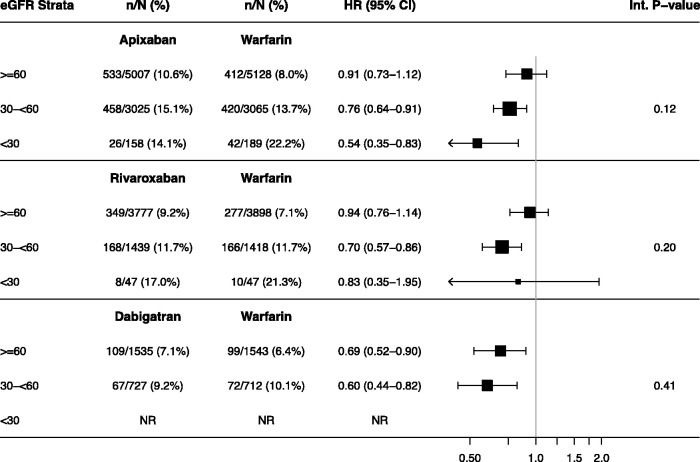
The absolute risk of AKI was higher as eGFR declined among all oral anticoagulants. There was a lower risk of AKI among most strata of kidney function for each of the DOACs relative to warfarin, particularly as the eGFR declined below 60 ml/min per 1.73 m2. Most notably, among those with an eGFR<30 ml/min per 1.73 m2, apixaban users had a significantly lower risk of AKI relative to warfarin (weighted HR, 0.54; 95% CI, 0.35 to 0.83) (Table 5). However, tests for interaction targeting modification of the effects of each of the DOACs by eGFR did not reach statistical significance (Table 5).
Table 5.
Subgroup analysis for risk of AKI by eGFR category (weighted cohort)
|
All data are weighted. NR, not reported due to small cell size (fewer than five patients).
All DOACs were associated with a significantly lower risk of KDIGO stage 1 AKI compared with warfarin. The weighted HRs for AKI among patients with stages 2 and 3 for each DOAC were generally comparable with those for patients with stage 1 AKI receiving that DOAC, although only statistically significant for patients with stage 2 AKI receiving rivaroxaban and only so when uncontrolled for the multiple tests (Supplemental Table 9).
Of the 551 warfarin patients who experienced AKI, 192 of them had an INR on the day of or the day prior to AKI. Of these 192 patients, median INR (IQR) was 2.6 (IQR, 1.9–4.4), with 75 (39%) having a peak value >3.0.
Adjusting for year did not change the results of our primary analysis (Supplemental Table 10).
Our sensitivity analyses confirmed the robustness of our main results (Table 1, Supplemental Tables 11 and 12). Notably, the E values demonstrated for our study indicate that unmeasured confounders would have been unlikely to sufficiently attenuate our main findings and negate a causal relationship between oral anticoagulant choice and AKI. There was also no significant difference in the risk for the negative control outcome pneumonia for both apixaban and rivaroxaban compared with warfarin. However, there was a lower risk for pneumonia for dabigatran compared with warfarin, although this association was much weaker than with AKI (Supplemental Table 12).
Discussion
In this population-wide study cohort of older adults with atrial fibrillation, treatment with DOACs was associated with a lower risk of AKI as compared with warfarin. This was evident for all three widely prescribed DOACs and across different levels of baseline kidney function.
This study is the first to compare the relationship between oral anticoagulant choice and AKI defined by the reference standard of laboratory criteria. Prior studies had defined AKI using administrative codes, which are known to lack sensitivity and therefore may have underestimated the true incidence of AKI as suggested by the lower incidence of AKI in those studies (7, 8, 10–12). In keeping with our findings, prior cohort studies from the United States (8, 11) and Taiwan (10) demonstrated an approximately 30% lower risk of AKI for dabigatran compared with warfarin. However, the American studies (8, 11) failed to demonstrate a significant decline in the risk of AKI for apixaban, which contrasts with our results. This discrepancy may have resulted from methodological differences between studies and differential attention for important confounders, including medications, that may affect DOAC levels.
There are a number of postulated mechanisms for anticoagulant-induced AKI: prerenal azotemia or ischemic acute tubular necrosis resulting from systemic blood loss usually in the context of major bleeding events; acute interstitial nephritis associated with some DOACs; and anticoagulant-associated nephropathy, a condition mediated by glomerular hemorrhage leading to the formation of obstructive red cell casts in distal tubules and free radical injury from lysed red blood cells particularly at supratherapeutic level of anticoagulation (4, 36, 37). Indeed, in our study, almost 40% of warfarin users had an INR>3 on the day of or 1 day prior to their AKI event (33). The lower risk of AKI associated with DOACs aligns well with the lower risk of major bleeding seen in some of the randomized controlled trials of DOACs versus vitamin K antagonists, likely due to the greater stability of anticoagulation conferred by DOACs (5, 6, 38). This is further supported by our findings that the advantage of DOACs seems to be most pronounced in patients with poor INR control (as demonstrated by a percentage of INR measurements in the range <56%) who are more susceptible to bleeding events, which could in turn mediate AKI (31). This provides further rationale for favoring DOACs over warfarin in patients who are unlikely to maintain INR stability.
Our findings provide a degree of reassurance regarding the safety of DOACs in patients with CKD. Patients with CKD have a higher risk of AKI, but our analysis did not detect significant differences in the risks of AKI across eGFR strata for any of the DOACs. Because Canadian authorities have not approved DOACs for use in patients with more impaired kidney function until recently, there were relatively few patients with eGFR<30 ml/min per 1.73 m2 in receipt of DOACs, with the most available experience being with apixaban. Indeed, among patients with baseline eGFR<30 ml/min per 1.73 m2, a group that has the highest risk of AKI and potential bleeding, apixaban was associated with a 50% reduction in the risk of AKI compared with warfarin. Although DOACs are all dependent on kidney metabolism and there is a justified concern about bioaccumulation, our findings align with emerging data on the relative safety of DOACs relative to warfarin in patients with more advanced CKD (39, 40). It is also noteworthy that in the seminal trials comparing DOACs and warfarin for atrial fibrillation, only apixaban was demonstrated to have a lower risk of major or clinically relevant nonmajor bleeding compared with warfarin (4% for apixaban versus 6% for warfarin) (41).
Our findings have important implications for practice. When anticoagulation is indicated in the setting of atrial fibrillation, clinicians should consider the risk of AKI when choosing the best anticoagulant for their patients. This is especially relevant for patients with CKD who are at a higher risk of AKI. Although definitive data from large randomized controlled trials are lacking, DOACs in general and apixaban in particular may confer a lower risk of AKI and therefore provide a viable alternative to warfarin, which has been the traditional oral anticoagulant of choice in patients with CKD. This is especially salient for patients unable to maintain adequate anticoagulation control on warfarin.
The strengths of the study include its large size that encompassed a broad and diverse population in a jurisdiction with universal health care coverage. In addition, we were able to accurately ascertain AKI and stage its severity using widely accepted reference standards (42, 43). In order to mitigate confounding inherent to observational studies, we used a negative control outcome, as well as inverse probability of treatment weighting, and incorporated a broad array of confounders, including interacting medications, into our propensity score.
The study also has limitations. First, Ontarians are eligible for drug coverage only at 65 years of age; thus, our results cannot be directly applied to younger individuals. However, almost 70% of individuals with atrial fibrillation are between the ages of 65 and 85; therefore, our results are generalizable to the vast majority of patients with atrial fibrillation (44). Second, the number of participants with a GFR<30 ml/min per 1.73 m2 was small, which may limit the applicability of our findings to individuals with advanced CKD. Third, despite the presence of multiple plausible mechanisms for the development of anticoagulant-associated AKI, we were unable to identify the specific causes of the AKI episodes that were ascertained in this study. Fourth, although our definition of AKI was on the basis of KDIGO thresholds, it did not include the timing criteria due to insufficient data, which may have led to misclassification. Nevertheless, our findings have biologic plausibility and are in line with those of other studies that used International Statistical Classification of Diseases and Related Health Problems, 10th Revision codes to define AKI (8, 10, 11). Fifth, because most AKI events were stage 1, we are unable to comment on the true effect of DOAC use on more substantial AKI. However, even stage 1 AKI is associated with a higher risk of mortality, hospital length of stay, and cost to the health care system (45, 46). Sixth, we cannot rule out residual confounding as an explanation for the observed relationship between anticoagulant choice and AKI, as highlighted by our negative control analysis. However, our E-value analysis should offset this concern as the E values for the point estimates and 95% CIs for all DOACs were higher than our actual estimates. This indicates that substantial unmeasured confounding would be needed to reduce the observed associations to the null. Although only randomized controlled trials can effectively address residual confounding, the landmark randomized controlled trials establishing the noninferiority of DOACs versus warfarin did not specifically ascertain AKI as a safety outcome. Nevertheless, given the numerous advantages of DOACs over vitamin K antagonists (e.g., no monitoring required, fewer drug-drug interactions, no association with vascular calcification and calciphylaxis, etc.) and their deep entrenchment in clinical practice, it is unlikely that such trials will be repeated. Seventh, follow-up times were limited by continuous usage, particularly in the warfarin group. Eighth, as we did not possess the ability to determine the daily dose of each DOAC, it is unclear if they were inappropriately prescribed at times and whether underdosing may have contributed to the lower risk of AKI among DOAC users relative to warfarin users. Finally, our use of valvular surgery as a proxy for valvular atrial fibrillation may have led to the exclusion and misclassification of some patients.
AKI is common among patients with atrial fibrillation treated with oral anticoagulants. Recognition of this complication should mandate greater attention to acute decrements in kidney function among patients receiving all forms of chronic anticoagulation. DOACs were associated with a lower risk of AKI compared with warfarin, a finding that was present even in those with underlying CKD. These findings suggest that some DOACs may have an acceptable safety profile in patients with advanced CKD and may become a suitable alternative to warfarin in this population.
Disclosures
S.V. Badve reports receiving in kind support from Bayer AG; receiving speakers honorarium from Amgen, speakers honorarium from Bayer, and honoraria from Pfizer, with fees paid to his institution; and serving on advisory boards for AstraZeneca, Bayer, and Vifor, with fees paid to his institution. D. Blum reports receiving honoraria from AstraZeneca and Otsuka. P. Dorian reports receiving research funding and honoraria from Bayer, BMS, Pfizer, and Servier. A.X. Garg reports receiving research funding from Astellas and Baxter; serving on the editorial boards of American Journal of Kidney Disease and Kidney International; serving on the data safety and monitoring board for an anemia trial program, which is funded by GlaxoSmithKline; and serving as Medical Lead Role to Improve Access to Kidney Transplantation and Living Kidney Donation for the Ontario Renal Network (a government-funded agency located within Ontario Health). M. Jun has previously received unrestricted grant support from VentureWise (a wholly owned subsidiary of NPS MedicineWise) funded by AstraZeneca. M. Jun reports receiving meeting sitting fees from NPS MedicineWise and serving as a research advisory group member for a project conducted by NPS MedicineWise Australia (an independent not-for-profit organization focused on improving the quality use of medicines) and funded by an independent educational grant from Gilead Sciences. E. McArthur reports employment with ICES. S.A. Silver reports receiving honoraria from Baxter and Sanofi and serving on the editorial board of Canadian Journal of Kidney Health and Disease. M.M. Sood reports receiving honoraria from AstraZeneca; serving on the editorial boards of American Journal of Kidney Disease, Canadian Journal of Cardiology, and CJASN; serving as an editor of Canadian Journal of Kidney Disease and Health; and serving as a member of the American Society of Nephrology Highlights ESRD Team. R. Wald reports receiving research funding from Baxter; serving on the editorial boards of CJASN, Kidney360, and Kidney Medicine; and serving as a contributor of UpToDate. A.T. Yan reports receiving research funding from AstraZeneca, receiving honoraria from AstraZeneca and Boehringer Ingelheim, and serving on the Journal of Cardiovascular Medicine editorial board and the European Heart Journal: Quality of Care and Clinical Outcomes editorial board. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors thank IMS Brogan Inc. for use of their Drug Information Database.
Parts of this material are on the basis of data and/or information compiled and provided by Canadian Institute for Health Information.
The study design and conduct, opinions, results, and conclusions reported in this paper are those of the authors and are independent of the funding sources. No endorsement by ICES, the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, the Lawson Health Research Institute, Canadian Institutes of Health Research, or the Ministry of Health and Long-Term Care is intended or should be inferred. The analyses, conclusions, opinions, and statements expressed in the material are those of the authors and not necessarily those of Canadian Institute for Health Information.
Dr. Z. Harel, N. Jeyakumar, E. McArthur, and Dr. R. Wald were responsible for concept and design; Dr. A.X. Garg, Dr. Z. Harel, N. Jeyakumar, E. McArthur, and Dr. R. Wald were responsible for acquisition, analysis, or interpretation of data; Dr. Z. Harel, N. Jeyakumar, and Dr. R. Wald were responsible for drafting of the manuscript; Dr. S.V. Badve, Dr. W. Beaubien-Souligny, Dr. D. Blum, Dr. P. Dorian, Dr. A.X. Garg, Dr. Z. Harel, R. Jandoc, N. Jeyakumar, Dr. M. Jun, Dr. A. Kitchlu, E. McArthur, Dr. S.A. Silver, B. Smyth, M.M. Sood, Dr. R. Wald, and Dr. A.T. Yan were responsible for critical revision of the manuscript for important intellectual content; E. McArthur was responsible for statistical analysis; Dr. Z. Harel obtained funding; Dr. A.X. Garg, N. Jeyakumar, and Dr. R. Wald were responsible for administrative, technical, or material support; and Dr. Z. Harel and Dr. R. Wald had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Data Sharing Statement
The analysis was conducted by members of the ICES Kidney, Dialysis and Transplantation team at the ICES Western facility (London, ON, Canada). Z. Harel and E. McArthur are responsible for the data analysis. The protocol can be obtained by emailing Z. Harel at ziv.harel@unityhealth.to.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05920421/-/DCSupplemental.
Supplemental Table 1. Databases used to derive study variables.
Supplemental Table 2. Codes used to identify baseline conditions.
Supplemental Table 3. Variables included in the propensity score model.
Supplemental Table 4. Kidney Disease Improving Global Outcomes AKI staging criteria thresholds.
Supplemental Table 5. Baseline characteristics included in propensity scores, but not shown in Table 1, after inverse probability of treatment weighting.
Supplemental Table 6. Baseline characteristics of the cohort (unweighted).
Supplemental Table 7. Reasons for end of follow-up by oral anticoagulant.
Supplemental Table 8. Association between oral anticoagulant and all-cause mortality stratified by eGFR.
Supplemental Table 9. Association between oral anticoagulants and Kidney Disease Improving Global Outcomes AKI stage thresholds.
Supplemental Table 10. Association between oral anticoagulants and a hospital encounter with AKI, adjusted for study year.
Supplemental Table 11. Association between oral anticoagulants and a hospital encounter with AKI (intention-to-treat analysis).
Supplemental Table 12. Association between oral anticoagulants and a pneumonia (intention-to-treat analysis).
Supplemental Figure 1. Study flow diagram for creation of the main cohort of patients with atrial fibrillation and a new prescription for warfarin, apixaban, rivaroxaban, or dabigatran.
References
- 1.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T: Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: A report of 9 cases. Am J Kidney Dis 54: 1121–1126, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Kabir A, Nadasdy T, Nadasdy G, Hebert LA: An unusual cause of gross hematuria and transient ARF in an SLE patient with warfarin coagulopathy. Am J Kidney Dis 43: 757–760, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ikeda M, Tanaka M, Shimoda S, Saita H, Nishikawa S, Shimada H, Taniguchi K, Hagihara K, Iwanari S, Takeoka H: Dabigatran-induced anticoagulant-related nephropathy with undiagnosed IgA nephropathy in a patient with normal baseline renal function. CEN Case Rep 8: 292–296, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodsky SV, Mhaskar NS, Thiruveedi S, Dhingra R, Reuben SC, Calomeni E, Ivanov I, Satoskar A, Hemminger J, Nadasdy G, Hebert L, Rovin B, Nadasdy T: Acute kidney injury aggravated by treatment initiation with apixaban: Another twist of anticoagulant-related nephropathy. Kidney Res Clin Pract 36: 387–392, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldeira D, Rodrigues FB, Barra M, Santos AT, de Abreu D, Gonçalves N, Pinto FJ, Ferreira JJ, Costa J: Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: A systematic review and meta-analysis. Heart 101: 1204–1211, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, Jardine MJ, Sood MM, Garg AX, Palmer SC, Mark PB, Wheeler DC, Jha V, Freedman B, Johnson DW, Perkovic V, Badve SV: Benefits and harms of oral anticoagulant therapy in chronic kidney disease: A systematic review and meta-analysis. Ann Intern Med 171: 181–189, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Chan YH, Yeh YH, Hsieh MY, Chang CY, Tu HT, Chang SH, See LC, Kuo CF, Kuo CT: The risk of acute kidney injury in Asians treated with apixaban, rivaroxaban, dabigatran, or warfarin for non-valvular atrial fibrillation: A nationwide cohort study in Taiwan. Int J Cardiol 265: 83–89, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Shin JI, Luo S, Alexander GC, Inker LA, Coresh J, Chang AR, Grams ME: Direct oral anticoagulants and risk of acute kidney injury in patients with atrial fibrillation. J Am Coll Cardiol 71: 251–252, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA: Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 80: 181–189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, Wu LS, Tu HT, Kuo CT: Acute kidney injury in Asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol 68: 2272–2283, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, Noseworthy PA: Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 70: 2621–2632, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Hernandez AV, Bradley G, Khan M, Fratoni A, Gasparini A, Roman YM, Bunz TJ, Eriksson D, Meinecke A-K, Coleman CI: Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes 6: 301–307, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Statistics-Canada : Table 17-10-0005-01. Population estimates on July 1st, by age and sex, 2019. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. Accessed April 15, 2020
- 14.Atzema CL, Austin PC, Miller E, Chong AS, Yun L, Dorian P: A population-based description of atrial fibrillation in the emergency department, 2002 to 2010. Ann Emerg Med 62: 570–577.e7, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Iskander C, McArthur E, Nash DM, Gandhi-Banga S, Weir MA, Muanda FT, Garg AX: Identifying Ontario geographic regions to assess adults who present to hospital with laboratory-defined conditions: A descriptive study. CMAJ Open 7: E624–E629, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA: Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Harel Z, Bell CM, Dixon SN, McArthur E, James MT, Garg AX, Harel S, Silver S, Wald R: Predictors of progression to chronic dialysis in survivors of severe acute kidney injury: A competing risk study. BMC Nephrol 15: 114, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harel Z, Wald R, McArthur E, Chertow GM, Harel S, Gruneir A, Fischer HD, Garg AX, Perl J, Nash DM, Silver S, Bell CM: Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol 26: 3141–3150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Harel Z, McArthur E, Hladunewich M, Dirk JS, Wald R, Garg AX, Ray JG: Serum creatinine levels before, during, and after pregnancy. JAMA 321: 205–207, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, Klungel O, Petersen I, Sorensen HT, Dixon WG, Guttmann A, Harron K, Hemkens LG, Moher D, Schneeweiss S, Smeeth L, Sturkenboom M, von Elm E, Wang SV, Benchimol EI: The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 363: k3532, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group : Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash DM, Markle-Reid M, Brimble KS, McArthur E, Roshanov PS, Fink JC, Weir MA, Garg AX: Nonsteroidal anti-inflammatory drug use and risk of acute kidney injury and hyperkalemia in older adults: A population-based study. Nephrol Dial Transplant 34: 1145–1154, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iskander C, Cherney DZ, Clemens KK, Dixon SN, Harel Z, Jeyakumar N, McArthur E, Muanda FT, Parikh CR, Paterson JM, Tangri N, Udell JA, Wald R, Garg AX: Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: A population-based cohort study. CMAJ 192: E351–E360, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh RV, Nash DM, Brimble KS, Markle-Reid M, Tan TC, McArthur E, Khoshniat-Rad F, Sood MM, Zheng S, Pravoverov L, Nesrallah GE, Garg AX, Go AS: Kidney function and potassium monitoring after initiation of renin-angiotensin-aldosterone system blockade therapy and outcomes in 2 North American populations. Circ Cardiovasc Qual Outcomes 13: e006415, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Austin PC: Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiol Drug Saf 17: 1218–1225, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Austin PC: Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 35: 5642–5655, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pokorney SD, Holmes DN, Thomas L, Fonarow GC, Kowey PR, Reiffel JA, Singer DE, Freeman JV, Gersh BJ, Mahaffey KW, Hylek EM, Naccarelli GV, Ezekowitz MD, Piccini JP, Peterson ED; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators : Association between warfarin control metrics and atrial fibrillation outcomes in the outcomes registry for better informed treatment of atrial fibrillation. JAMA Cardiol 4: 756–764, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan PH, Li WH, Hai JJ, Chan EW, Wong ICK, Tse HF, Lip GYH, Siu CW, et al. : Time in therapeutic range and percentage of international normalized ratio in the therapeutic range as a measure of quality of anticoagulation control in patients with atrial fibrillation. Can J Cardiol 32: 1247.e23–1247.e28, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, Wu H, Bhatt U, Nadasdy T, Hebert LA: Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract 115: c142–c146, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele TJ, Ding P, Haneuse S, VanderWeele TJ, Arterburn D: Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med 167: 268–274, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Haneuse S, VanderWeele TJ, Arterburn D: Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321: 602–603, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Brodsky S, Eikelboom J, Hebert LA: Anticoagulant-related nephropathy. J Am Soc Nephrol 29: 2787–2793, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiMaria C, Hanna W, Murone J, Reichart J: Direct oral anticoagulant and AKI: Apixaban-induced acute interstitial nephritis. BMJ Case Rep 12: e230371, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harel Z, Sholzberg M, Shah PS, Pavenski K, Harel S, Wald R, Bell CM, Perl J: Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol 25: 431–442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, Noseworthy PA, Shah ND, Saran R, Nallamothu BK: Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States [published correction appears in Circulation 138: e425, 2018 10.1161/CIR.0000000000000620]. Circulation 138: 1519–1529, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vriese AS, Caluwe R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, De Surgeloose D, Van Hoenacker P, Van Vlem B, Verbeke F: Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: The Valkyrie Study. J Am Soc Nephrol 31: 186–196, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators : Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981–992, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX: Validity of administrative database coding for kidney disease: A systematic review. Am J Kidney Dis 57: 29–43, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Hwang YJ, Shariff SZ, Gandhi S, Wald R, Clark E, Fleet JL, Garg AX: Validity of the International Classification of Diseases, Tenth Revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open 2: e001821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG: Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 155: 469–473, 1995 [PubMed] [Google Scholar]
- 45.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Sparrow HG, Swan JT, Moore LW, Gaber AO, Suki WN: Disparate outcomes observed within Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury stage 1. Kidney Int 95: 905–913, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.