Abstract
AKI is a common complication in hospitalized and critically ill patients. Its incidence has steadily increased over the past decade. Whether transient or prolonged, AKI is an independent risk factor associated with poor short- and long-term outcomes, even if patients do not require KRT. Most patients with early AKI improve with conservative management; however, some will require dialysis for a few days, a few weeks, or even months. Approximately 10%–30% of AKI survivors may still need dialysis after hospital discharge. These patients have a higher associated risk of death, rehospitalization, recurrent AKI, and CKD, and a lower quality of life. Survivors of critical illness may also suffer from cognitive dysfunction, muscle weakness, prolonged ventilator dependence, malnutrition, infections, chronic pain, and poor wound healing. Collaboration and communication among nephrologists, primary care physicians, rehabilitation providers, physical therapists, nutritionists, nurses, pharmacists, and other members of the health care team are essential to create a holistic and patient-centric care plan for overall recovery. Integration of the patient and family members in health care decisions, and ongoing education throughout the process, are vital to improve patient well-being. From the nephrologist standpoint, assessing and promoting recovery of kidney function, and providing appropriate short- and long-term follow-up, are crucial to prevent rehospitalizations and to reduce complications. Return to baseline functional status is the ultimate goal for most patients, and dialysis independence is an important part of that goal. In this review, we seek to highlight the varying aspects and stages of recovery from AKI complicating critical illness, and propose viable strategies to promote recovery of kidney function and dialysis independence. We also emphasize the need for ongoing research and multidisciplinary collaboration to improve outcomes in this vulnerable population.
Keywords: critical illness, acute kidney injury
Introduction
AKI is a major complication among hospitalized patients, especially in intensive care units (ICUs). Approximately 50% of critically ill patients may develop AKI (1). AKI is associated with significant morbidity and mortality, and the severity of kidney injury is a determinant of short-term and long-term outcomes (2,3). On discharge from the hospital, survivors of critical illness and AKI still face significant challenges as they slowly recover mental, emotional, and physical well-being. Long-term consequences of AKI include persistent kidney dysfunction and higher likelihood for CKD and kidney failure (4–6). Even mild AKI is associated with significant risk for persistent deterioration in kidney function at 90 days (7). Patients with AKI requiring dialysis at the time of discharge are a particularly vulnerable group (8). In addition to their multiple comorbidities, dialysis-dependent patients with AKI must cope with the challenges of undergoing KRT in outpatient dialysis facilities that typically care for patients with kidney failure. Such facilities may not yet be well set up to care for patients with a good chance of functional recovery (9).
Recovery from Critical Illness
Patients with AKI are often recovering from a prolonged stay in the ICU, complicated by multiorgan failure, surgical complications, and sepsis. The post–intensive care syndrome is a well-recognized entity that afflicts a significant percentage of the survivors of critical illness (10). The post-ICU syndrome can be a result of the underlying medical condition, ICU-associated delirium, and ICU-acquired myopathy and weakness (11). A prolonged stay in the ICU is associated with significant debilitation after hospitalization, and has a negative effect on long-term outcomes. One study that evaluated 109 survivors of acute respiratory distress syndrome revealed that these patients have significant functional limitation even at 1 year posthospitalization, mostly from musculoskeletal damage and pulmonary complications (12). Early mobilization in the ICU can significantly decrease the severity of muscle weakness and may help to reduce length of ICU stay (13,14). On discharge, survivors of critical illness benefit from family engagement and patient-centered care whether they are discharged to home or a rehabilitation facility (15). Active measures to prevent further kidney injury and promote recovery of kidney function are vital, as dialysis dependence at hospital discharge poses significant challenges to patients’ daily lives, and is a barrier to appropriate physical and psychologic rehabilitation therapy and meaningful family and social interactions. Measures to promote recovery of kidney function include: avoidance of nephrotoxins, optimization of hemodynamics, judicious ultrafiltration, and avoidance of hypotension, especially during KRT.
Recovery of Kidney Function
There is no single consensus definition for recovery of kidney function after AKI. Recovery can be broadly defined as partial or complete, depending on the degree of improvement in kidney function (16). It has been suggested that “complete recovery” denotes improvement in eGFR to within 90% of baseline value (17,18). This degree of recovery of kidney function probably occurs in only a minority of patients, usually those with milder forms of AKI (17). Patients who recover kidney function tend to be younger and have higher kidney function and fewer comorbid conditions at baseline (19,20). For example, a 25-year-old man with previously normal kidney function who had rhabdomyolysis and AKI after a motor vehicle accident, but did not require KRT, is more likely to have complete recovery of kidney function than a 75-year-old with underlying CKD in the same scenario (20). Patients who progress from AKI to acute kidney disease after 7 days of nonrecovery may have partial or complete kidney function recovery over the subsequent 90 days (21). After 90 days, patients with persistent kidney function are considered to have CKD, and those who remain dialysis dependent after 90 days are considered to have kidney failure. Achieving dialysis independence after 90 days is exceedingly rare (22).
Timing of recovery, as it relates to the onset of AKI, also has significant prognostic implications (23,24). Early recovery of kidney function can occur within days of onset of AKI, usually in the hospital itself, and delayed recovery of kidney function can occur weeks to even months later. Early recovery of kidney function is associated with excellent long-term prognosis, compared with those who never recover kidney function (23,25). In one study of 16,968 patients with stage 2 or 3 AKI, patients in early recovery had a 90.2% 1-year survival compared with those who never recovered kidney function (39.2% 1-year survival) or who had relapse of AKI (41.9% 1-year survival) (25). In a recent study of >47,000 patients, recovery of kidney function within 4 days of AKI was associated with lower risk for persistent kidney dysfunction when compared with longer recovery periods (23).
Predicting recovery from AKI has important implications regarding decisions such as frequency of dialysis sessions or cessation of dialysis (26). Functional biomarkers (serum creatinine [SCr] or cystatin C) and/or damage biomarkers (kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, interleukin-18, tissue inhibitor of metalloprotease-2/insulin-like growth factor binding protein-7, etc.) have been investigated in predicting kidney function recovery and/or risk of persistent AKI (27). There are complexities and confounding variables associated with these studies (definition of recovery, duration of ICU stay, severity of AKI, and preexisting CKD) that limit conclusive evidence for their utility as predictors of recovery from AKI (28). Loss of muscle mass during critical illness may deem SCr even less sensitive as a marker of kidney function. Creatinine clearances are not routinely performed in critically ill patients even in early stages of kidney recovery. Although some studies have shown a significant association between biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) (29), plasma proenkephalin–A (30), and urinary C-C motif chemokine ligand 14 (31) and recovery of kidney function, these show variable rates of false positivity, and limited value over usual markers, clinical score, or kinetic change in SCr concentrations (28). As recommended by the Acute Disease Quality Initiative (ADQI) 16 and 23 Workgroups, additional studies in well-designed clinical trials assessing the value of clinical risk scores, biomarkers, imaging, and functional studies will be needed to determine the utility of these tools (21,27).
Posthospitalization Care of AKI Patients
In the posthospitalization management of a patient after critical illness and/or AKI, various factors must be considered, including: timing of follow-up; whether follow-up should be with a nephrologist, a primary care physician, or both; coordination of care among various medical services; communication with outpatient physicians; management of dialysis-dependent AKI patients in outpatient facilities; and patient perspective and social implications. The appropriate timing of follow-up after discharge from hospital depends on several aspects such as severity of AKI, presence of comorbidities, dialysis dependence on discharge, and whether patients are discharged to home versus intermediary facilities such as skilled nursing facilities and long-term acute care hospitals. Patients with significant kidney dysfunction may need to be seen as soon as 1–2 weeks post hospital discharge, whereas those who have less severe AKI may not need follow-up for up to 12 weeks after discharge (17). An inpatient nephrology consultation, involvement of the nephrologist in discharge planning, and measurement of SCr and proteinuria at the time of hospital discharge will be helpful in deciding the timing of outpatient follow-up. A dedicated clinic for follow-up of patients with AKI has been established at some centers, but more data are needed before this can be widely recommended (32).
As stated earlier, patients with AKI follow different trajectories, ranging from mild AKI with recovery early in the course of the hospitalization, to dialysis dependence requiring outpatient dialysis at discharge (Figure 1). Interdisciplinary coordination of care and discharge communication are crucial to ensure appropriate postdischarge nephrology follow-up, because patients are frequently discharged to intermediary facilities (skilled nursing facilities, long-term acute care hospitals) and may be lost to follow-up. The presence of a serious complication such as AKI is frequently missing in discharge summaries, and, therefore, the physician managing the patient postdischarge will not know it occurred and cannot ensure appropriate post-AKI follow-up. Greer and colleagues audited the documentation of AKI at hospital discharge in 75 randomly selected patients and noted that only 44% of physician discharge summaries documented a diagnosis of AKI and even fewer listed the cause of AKI (43%) and its course (31%). Even more concerning, only 13% documented AKI-specific patient instructions and only 6% had follow-up treatment plans (33). Quality improvement projects have demonstrated that documentation of AKI-specific information in after-visit summaries can be improved through a systematic, educational initiative. In a single-center quality improvement initiative in the United Kingdom, compliance of documentation with AKI-specific information improved from 22% to 92% at the end of a 12-month period and was sustained for up to 14 months after conclusion of the project (34). This quality improvement project was conducted in only a small representative sample, so it is unclear if the improvement in documentation translated to improvement in overall postdischarge patient care.
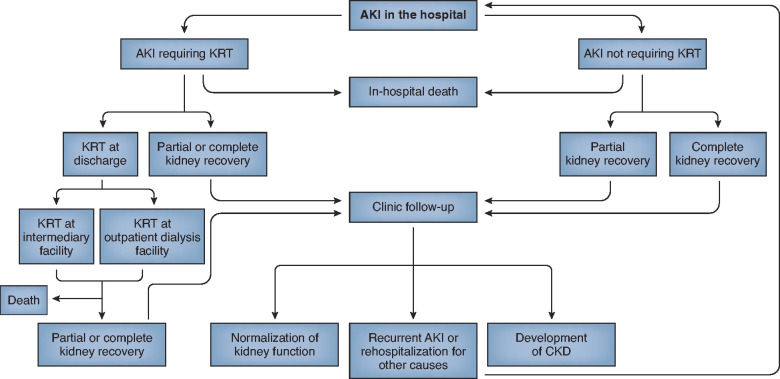
Figure 1.
Clinical course of patients who develop AKI in the hospital. Patients who develop AKI in the hospital may have complete or partial recovery of kidney function in the hospital or after discharge. Those patients who do not recover kidney function in the hospital may need ongoing KRT at an intermediary facility or outpatient dialysis facility. Patients with AKI are at risk for progression to CKD and readmission to the hospital.
Patients with AKI are at high risk for all-cause readmission within 30 days after discharge (35). One large health care database analysis with approximately 150,000 patients from 197 hospitals in Canada revealed that approximately 20% of patients required readmission and about 10% were seen in the emergency room within 30 days after discharge (35). Patients with AKI had a higher likelihood for readmission compared with those without AKI (hazard ratio [HR] 1.53; 95% confidence interval [95% CI], 1.50 to 1.57). This study suggests that early follow-up for patients with AKI may be beneficial in preventing readmissions or emergency room visits. For patients who have achieved dialysis independence at discharge or never required KRT, Vanmassenhove and colleagues designed a postdischarge follow-up algorithm (36). They proposed that patients who had complete recovery of kidney function (return of eGFR to within 90% of baseline) before discharge should be seen in approximately 3 months, whereas those with incomplete recovery of kidney function should be seen within 3 weeks of discharge. Harel and colleagues demonstrated that those who had severe AKI needing temporary, in-hospital KRT and who had nephrology evaluation within 90 days postdischarge had lower 2-year mortality compared with those who did not have nephrology follow-up (15.5% versus 18.9%; HR 0.76; 95% CI, 0.62 to 0.93) (37). Patients with AKI during their hospitalization are at high risk for recurrent AKI necessitating rehospitalization (38). Recurrent AKI usually occurs in patients with other comorbidities—malignancy, liver disease, or heart failure. It is plausible that close follow-up and management of AKI patients after initial hospitalization may decrease their risk for recurrent AKI.
The ADQI consensus statement recommends that health systems develop a structure and process for follow-up of patients with AKI, instituting appropriate monitoring measures to assess for recovery and to prevent further kidney injury (39). At a minimum, we recommend that during each ambulatory visit, a basic metabolic panel and urine albumin-to-creatinine ratio be obtained to assess kidney function and the degree of proteinuria, respectively. The frequency of the ambulatory visits will depend on the severity of AKI, underlying CKD, and other comorbidities, because patients with more severe and complex illnesses will need to be evaluated on a more frequent basis. The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury study, which matched 769 patients who had AKI in the hospital with those who did not have AKI, documented that higher urine albumin-to-creatinine ratio at the 3-month posthospitalization visit was associated with a significant risk for progression of kidney disease (40). On the basis of SCr, an estimation of GFR using the Modification of Diet in Renal Disease or Chronic Kidney Disease Epidemiology Collaboration formula can be performed if SCr is in a steady state. These equations are not reliable in the setting of AKI with frequent fluctuations of SCr. Caution must also be used in using these equations with extremes of age, body mass index, and muscle mass. A review of medications should be performed at each visit to ensure appropriate medication dosing in the setting of kidney dysfunction, and to discontinue those that are potentially nephrotoxic, e.g., nonsteroidal anti-inflammatory medications. Blood pressure and volume status assessment should be performed, with judicious use of renin-angiotensin-aldosterone system (RAAS) blockers and diuretics. Combination of nonsteroidal anti-inflammatory medications, diuretics (including potassium-sparing diuretics), and RAAS blockers is associated with higher risk for AKI, as may be the sacubitril/valsartan combination (41,42). The BP target for patients within the 90-day window after AKI is unclear, but hypotension with resultant renal hypoperfusion should be avoided. If kidney function remains stable, reinitiation or de novo initiation of RAAS blockers should be considered, but the exact timing of starting these drugs remains controversial. A large retrospective cohort study demonstrated that a prescription of either angiotensin-converting enzyme inhibitor or angiotensin receptor blocker within 180 days of discharge after AKI was associated with lower mortality (39.2% versus 50.6%; adjusted HR 0.85; 95% CI, 0.81 to 0.89). However, it was also associated with higher risk for hospitalization for AKI, volume overload, and hyperkalemia (5.8% versus 5.2%; HR 1.31; 95% CI, 1.15 to 1.49) (43). Other large retrospective cohort studies have not demonstrated a higher risk for hospitalizations with reinitiation or continuation of RAAS blockers after AKI (44,45), and one even suggested better survival among patients started on RAAS blockers versus the nonusers (46). A large prospective interventional trial is needed to confirm or refute the potential benefit, and appropriate timing, of initiating angiotensin-converting enzyme inhibitor and angiotensin receptor blocker after hospitalization complicated by AKI. Until then, these medications should be prescribed on the basis of cardiovascular risk factors and other indications.
There is considerable debate as to whether patients with AKI in the hospital require follow-up with nephrologists or general practice physicians. This probably depends on the severity of AKI and other comorbidities. As stated earlier, patients with dialysis-dependent AKI who have only a brief duration of KRT in the hospital have better outcomes than those with a more prolonged course of AKI (23). In a retrospective study, Stoumpos demonstrated that of 396 patients with AKI who recovered kidney function within 1 year, only 8.8% of patients progressed to CKD after a median of 5.3 years. The risk factors for progression to CKD were older age and presence of diabetes mellitus and vascular disease (47). This study implies that patients who achieve close to complete recovery of kidney function within 1 year may not need nephrology follow-up for an extended period.
Patients with AKI who remain dialysis dependent at discharge receive intermittent hemodialysis treatments either at an in-center facility or at an intermediary location like a rehabilitation hospital. The care of the dialysis-dependent AKI patient is fundamentally different from that of a patient with kidney failure, and it is imperative that this distinction be reflected in the management of the patients in the outpatient setting. Abdel-Rahman and colleagues adopted a protocol for care of dialysis-dependent patients with AKI in the outpatient hemodialysis facility. Patients with dialysis-dependent AKI were seen by a nephrologist at each dialysis session, and ultrafiltration rate and blood pressure and volume status were closely monitored. Medications were reviewed for their nephrotoxic potential and adverse effects. Assessments of kidney function using 24-hour combined creatinine and urea clearance were obtained weekly. Dialysis was discontinued upon sustaining average urea and creatinine clearances of >15 ml/min, whereas transitioning to a diagnosis of kidney failure was done if no signs of recovery of kidney function were noted within 90 days. Using this protocol, 42% of patients became dialysis independent within 90 days (48). Longer follow-up revealed that 75% of those who recovered kidney function remained alive or dialysis independent at a median of 2.4 years (49). Hypotensive episodes during intermittent hemodialysis are associated with delay in kidney function recovery (50–52). It is imperative that ultrafiltration rates are adjusted each session for patients with AKI on the basis of hemodynamic and volume status, as opposed to using a designated target weight that is considered routine care for patients with kidney failure.
Patient-Centered Care and Education
Patients recovering from critical illness are coping with multiple comorbidities, and their understanding of AKI and the potential for recovery of kidney function may be insufficient for coping with the disease (53). Many patients who experience AKI during their hospitalization may not be aware of its significance and its prognostic implications, and some may not even realize that they suffered kidney injury during their hospitalization (53). Patients and caregivers may prioritize other aspects of their overall illness that they deem to be more important than AKI. Intense education and raising awareness of AKI and its consequences in the community are vital to prevent recurrent AKI, and to promote kidney function and overall recovery. A single-center study demonstrated that a multipronged approach to patient understanding of AKI during the first follow-up visit can significantly influence the patient’s understanding of the severity of AKI and its consequences (54). Patients, family members, and other caregivers are under considerable stress as patients recover from their critical illness and AKI. It is important to remember that education and awareness need to be multidimensional and must be targeted at all health care workers and the public. Patients who have recovered from critical illness and AKI have recently been heard at national meetings discussing their harrowing experiences with outpatient care (55). Physicians, social workers, nurses, rehabilitation personnel, and others should understand and acknowledge the patients’ hardships as they try to regain normalcy and their prior quality of life (Figure 2). Nephrologists and dialysis staff taking care of dialysis-dependent AKI patients in the outpatient setting may not be cognizant of the complexities of managing an AKI patient with potential for recovery of kidney function, and therefore, education and a culture shift are required in this regard as well. Lack of interoperability among electronic medical records (EMR) systems, especially between outpatient dialysis facilities and hospitals, results in information silos and fragmented care, leading to errors in medication and hemodialysis prescription and lack of communication between dialysis centers and other health care facilities (56–58). Appropriate peer-to-peer and interdisciplinary communication remains vital in the coordination of care of patients with AKI after discharge.
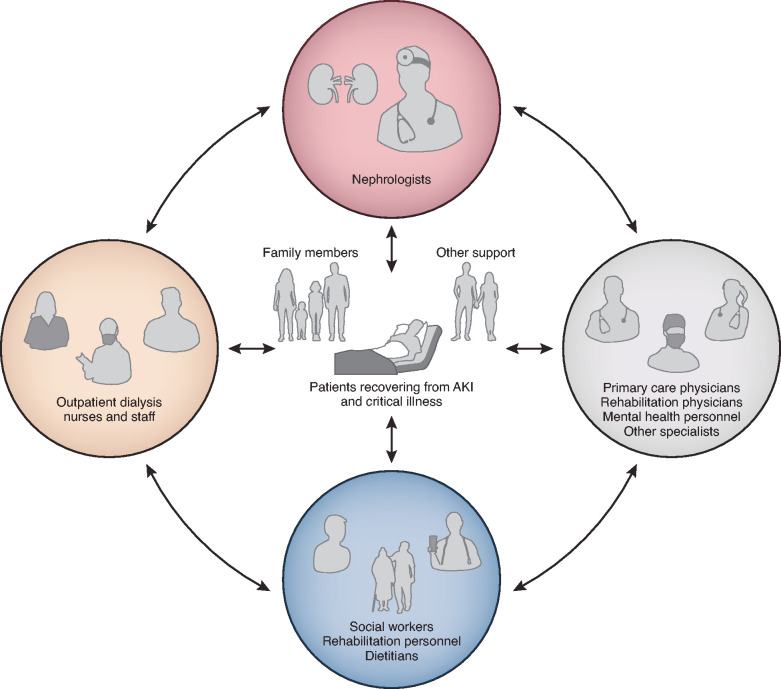
Figure 2.
Coordination of care for patients with critical illness and AKI after discharge. Multidisciplinary care and coordination among multiple specialties, with close involvement of patient and family, are essential for patient recovery after prolonged hospitalization complicated by AKI.
Care of the Pediatric Patient
Hospitalized children are also at risk for development of AKI, both in the ICU and on the wards. As with adults, ICU admission, sepsis, cardiac surgery, and exposure to nephrotoxic medications represent the most common causes of AKI in children. One-quarter of neonates and children admitted to an ICU develop AKI, and Kidney Disease Improving Global Outcomes stage 2 or 3 AKI is independently associated with mortality (59,60). Thirty to 40 percent of children develop AKI after cardiac surgery (61), and once again AKI is independently associated with poor outcomes in these children. As with adults, the need for KRT is associated with higher risk for mortality.
One could argue that follow-up of children who survive an AKI episode entails a greater imperative given their longer projected lifespan compared with adults. As mentioned above, just as in adults, follow-up has not been carried out in a systematic manner for pediatric AKI survivors. Yet, numerous pediatric studies have demonstrated a high prevalence of CKD after an AKI episode, irrespective of the cause of AKI. For example, Garg and colleagues demonstrated in a systematic review that 25% of children who experienced diarrhea-associated hemolytic uremic syndrome had CKD or proteinuria/hypertension 4 years after the episode (62). Menon and colleagues showed that >50% of children who experienced nephrotoxic medication–associated AKI had evidence of CKD or proteinuria 6 months after the AKI episode (63). Importantly, none of these children had any evidence of kidney injury before the AKI episode. Mammen and colleagues found that >50% of children who survived AKI in a pediatric ICU had evidence of CKD or kidney injury 1 year later (64). Finally, children who suffer AKI after cardiac surgery are at higher risk of CKD 5 years later, as evidenced by decreased GFR or persistence of elevated urinary concentrations of novel biomarkers (65,66). These studies are confounded by an ascertainment bias, in that the data were available from patients who had kidney function assessment as part of clinical care, and not in a prospective systematic fashion. As a result, an AKI survivor clinic was developed at two centers to follow AKI survivors prospectively (67). This program also educated patients and their caregivers regarding avoidance of nephrotoxic medications and discussed their history of AKI during evaluation for future surgical procedures.
Recovery from Critical Illness and AKI Secondary to Coronavirus Disease 2019
Coronavirus disease 2019 (COVID-19)–associated AKI came to the forefront of nephrology issues in 2020, and numerous papers have outlined the pathophysiology, course of illness, and recovery after AKI. One study from New York documented a 50% in-hospital mortality among 1825 patients with AKI, and, of the survivors, 35% had persistent acute kidney disease on discharge (68). Similar data showing very low rates of recovery of kidney function after COVID-19–associated AKI were reported from other centers (69,70). The lower rate of recovery of kidney function after COVID-19 probably reflects the overall severity of AKI and underlying comorbidities such as diabetes mellitus, hypertension, and obesity, along with possible direct kidney involvement from the severe acute respiratory syndrome coronavirus 2 virus itself (71). Long-term prognoses such as progression to advanced CKD and kidney failure among patients with COVID-19–associated AKI remain guarded. A cohort study of 1612 patients with AKI noted that GFR declined by 11.3 ml/min per 1.73 m2 faster in patients with COVID-19–associated AKI as compared with AKI from other causes, even after adjustment for other baseline comorbidities and severity of AKI (72). COVID-19 patients also require comprehensive care after discharge as they experience significant physical and mental health disturbances for several weeks after hospitalization (H. Weerahandi et al., unpublished observations).
Summary
In summary, recovery from critical illness and AKI follows a prolonged and winding course and imposes a significant toll on patients and caregivers. An interdisciplinary, well-established, collaborative approach is needed to improve the care and experiences of AKI patients after discharge from the hospital. Our recommendations on appropriate follow-up of patients with AKI after discharge are outlined in Table 1. Patients with AKI undergoing dialysis at outpatient dialysis facilities should have closer and AKI-specific monitoring to assess for and promote kidney function recovery. Prospective data collection on the management of dialysis-dependent patients with AKI in outpatient dialysis facilities will help us to understand and outline the best practices required to care for this population. The National Institutes of Health has placed emphasis on research in this area, specifically to test interventions to reduce posthospitalization complications and morbidity in patients with stage 2 and 3 AKI (55). The request for proposals for the Caring for Outpatients after Acute Kidney Injury was recently announced (https://grants.nih.gov/grants/guide/rfa-files/RFA-DK-20-012.html). Research focusing on better definition of recovery of kidney function, precise predictors of outcomes, patient and caregiver perspectives, timely interventions, and optimal postdischarge care will have a positive effect on the care of patients with AKI.
Table 1.
Recommendations for posthospitalization evaluation of patients with AKI
| Patients Who Recover Kidney Function in the Hospital | Patients Who Are Dialysis Dependent at Discharge | |
| Discharge planning and communication | Coordinate discharge planning with primary team Ensure appropriate follow-up with PCP and other specialists as needed Educate patient and family on AKI and its current and future implications |
Coordinate care with accepting nephrologist and dialysis facility staff Communicate with outpatient nephrologist regarding prognosis for kidney function recovery Educate patient and family on AKI and its current and future implications |
| Outpatient follow-up | Nephrology follow-up within 4 weeks after discharge, depending on severity of AKI and comorbid diseases | Follow up for outpatient dialysis at a dialysis facility per dialysis schedule Preferably allocate in an AKI recovery outpatient unit dedicated to promote recovery |
| Clinical and laboratory parameters at follow-up | Assess blood pressure and volume status, SCr, eGFR, and proteinuria at visit Review medications and initiate or reinitiate diuretics, ACEI, ARB, and SGLT-2 inhibitors on the basis of comorbidities and indications Coordinate with other specialties regarding mediation dosing of other agents, e.g., chemotherapy medications, immunosuppressive agents, and antibiotics |
Assess for kidney function recovery at weekly intervals, e.g., decreasing SCr trends, increase in urine output Avoid aggressive ultrafiltration to prevent hypotensive episodes Avoid establishing a target weight Review medications regularly to prevent further nephrotoxicity, and avoid side effects from renally cleared medications |
| Prognosis for progression to CKD and kidney failure | Determine risk for progression to CKD and kidney failure and arrange subsequent follow-up If complete recovery of kidney function and no proteinuria on initial evaluation, consider discharge to primary care physician for further follow-up |
Determine risk for progression to CKD and kidney failure and plan for long-term dialysis or refer for transplant if poor prognosis for kidney function recovery If patient recovers kidney function sufficient to discontinue dialysis, establish nephrology clinic follow-up |
PCP, primary care physician; SCr, serum creatinine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; SGLT-2, sodium-glucose cotransporter 2.
Disclosures
E.M. Abdel-Rahman reports receiving research funding from Covance and serving as a member of the Kidney Health Initiative. A. Agarwal reports consultancy agreements with Akebia Therapeutics (serving on an expert panel to review new therapeutics on the basis of the Hypoxia Inducible Factor pathway for AKI), Dynamed (reviewing content related to AKI for Dynamed and reviewing updated materials prepared by the Dynamed editorial team for AKI topics), and Reata Pharmaceuticals; ownership interest in Goldilocks Therapeutics, Inc.; receiving research funding through the Genzyme/Sanofi Fabry Fellowship Award; receiving honoraria from Akebia Therapeutics, Emory, University of Southern California, and Vanderbilt; serving on the editorial boards of the American Journal of Physiology–Renal Physiology, Kidney International, and Lab Investigation; serving on the advisory board of Goldilocks Therapeutics, a New York–based company investigating delivery of drugs in the kidney using nanotechnology for acute and chronic kidney disease; serving on the external evaluation panel for the Kidney Precision Medicine Program; and serving on the Advisory Board of Angion and Alpha Young, LLC. Additionally, A. Agarwal’s spouse will be President-Elect for Women in Nephrology (2018–2019). J. Cerda reports employment with Capital District Renal Physicians, Albany Medical College, St Peter’s Health Partners; being a shareholder in Capital District Renal Physicians; receiving research funding from University of Alabama/University of California, San Diego O’Brien Research Center on AKI–National Institutes of Health (NIH); and serving as Co-Chair of the International Society of Nephrology AKI Committee of the 0by25 Initiative, Chair of the American Society of Nephrology AKI!Now Initiative, Co-Chair of the Advisory Committee of the International Society of Nephrology, member of the ASN Online AKI Community, and Associate Director of the International Society of Nephrology 0by25 Initiative. S.L. Goldstein reports consultancy agreements with Baxter Healthcare, Bioporto, Inc., CHF Solutions, Fresenius, Kaneka, Inc., La Jolla Pharmaceuticals, MediBeacon, Medtronic, Otsuka, Reata, and Renibus; ownership interest in MediBeacon; receiving research funding from Baxter Healthcare, Bioporto, and CHF Solutions; receiving honoraria from Baxter Healthcare and Fresenius; patents and inventions with Vigilanz; serving as a scientific advisor or member of MediBeacon; and serving on the speakers bureau with Baxter Healthcare and Fresenius. K.D. Liu reports consultancy agreements with Astute and BOA Medical; holding stock in Amgen; receiving honoraria from the American Society of Nephrology; and serving on the editorial boards of the American Journal of Respiratory and Critical Care Medicine, the American Journal of Kidney Diseases, and CJASN. M.D. Okusa reports receiving a research grant from the NIH and research funding from AM Pharma/Pfizer; receiving honoraria from UpToDate; patents and inventions with the University of Virginia Patent Office; serving as a scientific advisor or member of AM Pharma, Janssen, and Pfizer; and other interests/relationships with the John Bower Foundation. A. Vijayan reports consultancy agreements with Boehringer Ingelheim, NxStage, and Sanofi; receiving research funding from Astellas and Spectral; receiving honoraria from Astute and Sanofi; serving as a scientific advisor or member of NxStage; and serving as a member of the National Kidney Foundation.
Funding
None.
Acknowledgments
The authors would like to acknowledge the dedication and support of the following American Society of Nephrology (ASN) staff members who were crucial to the mission and success of the AKI!NOW Initiative: Ms. Susan Stark, Acting Vice President, Excellence in Patient Care; Ms. Bonnie Freshly, Project Specialist; Ms. Kerry Leigh, Project Specialist; and Ms. Darlene Rodgers, Nurse Clinical Consultant. ASN has received a Baxter Healthcare Corporation unrestricted educational grant to support the AKI!Now ASN initiative.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Srisawat N, Sileanu FE, Murugan R, Bellomod R, Calzavacca P, Cartin-Ceba R, Cruz D, Finn J, Hoste EE, Kashani K, Ronco C, Webb S, Kellum JA; Acute Kidney Injury-6 Study Group : Variation in risk and mortality of acute kidney injury in critically ill patients: A multicenter study. Am J Nephrol 41: 81–88, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML: Incidence and outcomes of acute kidney injury in intensive care units: A Veterans Administration study. Crit Care Med 37: 2552–2558, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, Powe NR, Pavkov ME, Williams DE, Saran R, Shahinian VB; Centers for Disease Control and Prevention CKD Surveillance Team : Acute kidney injury recovery pattern and subsequent risk of CKD: An analysis of Veterans Health Administration data. Am J Kidney Dis 67: 742–752, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT: Five-year risk of end-stage renal disease among intensive care patients surviving dialysis-requiring acute kidney injury: A nationwide cohort study. Crit Care 17: R145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, Klarenbach SW, Manns BJ, Hemmelgarn BR: Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 78: 803–809, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Cerdá J, Liu KD, Cruz DN, Jaber BL, Koyner JL, Heung M, Okusa MD, Faubel S; AKI Advisory Group of the American Society of Nephrology : Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol 10: 1859–1867, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heung M, Faubel S, Watnick S, Cruz DN, Koyner JL, Mour G, Liu KD, Cerda J, Okusa MD, Lukaszewski M, Vijayan A; American Society of Nephrology Acute Kidney Injury Advisory Group : Outpatient dialysis for patients with AKI: A policy approach to improving care. Clin J Am Soc Nephrol 10: 1868–1874, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawal G, Yadav S, Kumar R: Post-intensive care syndrome: An overview. J Transl Int Med 5: 90–92, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azoulay E, Vincent JL, Angus DC, Arabi YM, Brochard L, Brett SJ, Citerio G, Cook DJ, Curtis JR, Dos Santos CC, Ely EW, Hall J, Halpern SD, Hart N, Hopkins RO, Iwashyna TJ, Jaber S, Latronico N, Mehta S, Needham DM, Nelson J, Puntillo K, Quintel M, Rowan K, Rubenfeld G, Van den Berghe G, Van der Hoeven J, Wunsch H, Herridge M: Recovery after critical illness: Putting the puzzle together—A consensus of 29. Crit Care 21: 296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group : One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, Penley L, Howard A, Dixon L, Leach S, Small R, Hite RD, Haponik E: Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 36: 2238–2243, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kress JP, Hall JB: ICU-acquired weakness and recovery from critical illness. N Engl J Med 370: 1626–1635, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Major ME, van Nes F, Ramaekers S, Engelbert RHH, van der Schaaf M: Survivors of critical illness and their relatives. A qualitative study on hospital discharge experience. Ann Am Thorac Soc 16: 1405–1413, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Duff S, Murray PT: Defining early recovery of acute kidney injury. Clin J Am Soc Nephrol 15: 1358–1360, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, Schetz M, Joannidis M: Renal recovery after acute kidney injury. Intensive Care Med 43: 855–866, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Lee BJ, Hsu CY, Parikh R, McCulloch CE, Tan TC, Liu KD, Hsu RK, Pravoverov L, Zheng S, Go AS: Predicting renal recovery after dialysis-requiring acute kidney injury. Kidney Int Rep 4: 571–581, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR: Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis 52: 262–271, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16 : Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Bhandari S, Turney JH: Survivors of acute renal failure who do not recover renal function. QJM 89: 415–421, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Siew ED, Abdel-Kader K, Perkins AM, Greevy RA Jr, Parr SK, Horner J, Vincz AJ, Denton J, Wilson OD, Hung AM, Robinson-Cohen C, Matheny ME: Timing of recovery from moderate to severe AKI and the risk for future loss of kidney function. Am J Kidney Dis 75: 204–213, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Bhatraju PK, Zelnick LR, Chinchilli VM, Moledina DG, Coca SG, Parikh CR, Garg AX, Hsu CY, Go AS, Liu KD, Ikizler TA, Siew ED, Kaufman JS, Kimmel PL, Himmelfarb J, Wurfel MM: Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw Open 3: e202682, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS: Recovery after acute kidney injury. Am J Respir Crit Care Med 195: 784–791, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endre ZH: Recovery from acute kidney injury: The role of biomarkers. Nephron Clin Pract 127: 101–105, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, Bell M, Forni L, Guzzi L, Joannidis M, Kane-Gill SL, Legrand M, Mehta R, Murray PT, Pickkers P, Plebani M, Prowle J, Ricci Z, Rimmelé T, Rosner M, Shaw AD, Kellum JA, Ronco C: Recommendations on acute kidney injury biomarkers from the Acute Disease Quality Initiative consensus conference: A consensus statement. JAMA Netw Open 3: e2019209, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Darmon M, Truche AS, Abdel-Nabey M, Schnell D, Souweine B: Early recognition of persistent acute kidney injury. Semin Nephrol 39: 431–441, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA; Genetic and Inflammatory Markers of Sepsis (GenIMS) Study Investigators : Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int 80: 545–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caironi P, Latini R, Struck J, Hartmann O, Bergmann A, Bellato V, Ferraris S, Tognoni G, Pesenti A, Gattinoni L, Masson S; ALBIOS Study Investigators : Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem 64: 1361–1369, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Hoste E, Bihorac A, Al-Khafaji A, Ortega LM, Ostermann M, Haase M, Zacharowski K, Wunderink R, Heung M, Lissauer M, Self WH, Koyner JL, Honore PM, Prowle JR, Joannidis M, Forni LG, Kampf JP, McPherson P, Kellum JA, Chawla LS; RUBY Investigators : Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensive Care Med 46: 943–953, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hines A, Li X, Ortiz-Soriano V, Saleh S, Litteral J, Ruiz-Conejo M, Wald R, Silver SA, Neyra JA: Use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and acute kidney disease after an episode of AKI: A multicenter prospective cohort study. Am J Nephrol 51: 266–275, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE: Hospital discharge communications during care transitions for patients with acute kidney injury: A cross-sectional study. BMC Health Serv Res 16: 449, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reschen ME, Vaux E: Improving the completeness of acute kidney injury follow-up information in hospital electronic discharge letters. BMJ Open Qual 6: e000022, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver SA, Harel Z, McArthur E, Nash DM, Acedillo R, Kitchlu A, Garg AX, Chertow GM, Bell CM, Wald R: 30-Day readmissions after an acute kidney injury hospitalization. Am J Med 130: 163–172.e4, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Vanmassenhove J, Vanholder R, Lameire N: Points of concern in post acute kidney injury management. Nephron 138: 92–103, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, Hung AM, Fly J, Speroff T, Ikizler TA, Matheny ME: Predictors of recurrent AKI. J Am Soc Nephrol 27: 1190–1200, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashani K, Rosner MH, Haase M, Lewington AJP, O’Donoghue DJ, Wilson FP, Nadim MK, Silver SA, Zarbock A, Ostermann M, Mehta RL, Kane-Gill SL, Ding X, Pickkers P, Bihorac A, Siew ED, Barreto EF, Macedo E, Kellum JA, Palevsky PM, Tolwani AJ, Ronco C, Juncos LA, Rewa OG, Bagshaw SM, Mottes TA, Koyner JL, Liu KD, Forni LG, Heung M, Wu VC: Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 14: 941–953, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu CY, Chinchilli VM, Coca S, Devarajan P, Ghahramani N, Go AS, Hsu RK, Ikizler TA, Kaufman J, Liu KD, Parikh CR, Reeves WB, Wurfel M, Zappitelli M, Kimmel PL, Siew ED; ASSESS-AKI Investigators : Post-acute kidney injury proteinuria and subsequent kidney disease progression: The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) study. JAMA Intern Med 180: 402–410, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S: Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. BMJ 346: e8525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dreischulte T, Morales DR, Bell S, Guthrie B: Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 88: 396–403, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N; Interdisciplinary Chronic Disease Collaboration : Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 178: 1681–1690, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu CY, Liu KD, Yang J, Glidden DV, Tan TC, Pravoverov L, Zheng S, Go AS: Renin-angiotensin system blockade after acute kidney injury (AKI) and risk of recurrent AKI. Clin J Am Soc Nephrol 15: 26–34, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bidulka P, Fu EL, Leyrat C, Kalogirou F, McAllister KSL, Kingdon EJ, Mansfield KE, Iwagami M, Smeeth L, Clase CM, Bhaskaran K, van Diepen M, Carrero JJ, Nitsch D, Tomlinson LA: Stopping renin-angiotensin system blockers after acute kidney injury and risk of adverse outcomes: Parallel population-based cohort studies in English and Swedish routine care. BMC Med 18: 195, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang CY, Liu JS, Tseng WC, Tsai MT, Lin MH, Kao ZK, Lin YP, Hsu CC, Tarng DC: Effect of renin-angiotensin-aldosterone system blockade on long-term outcomes in postacute kidney injury patients with hypertension. Crit Care Med 48: e1185–e1193, 2020 [DOI] [PubMed] [Google Scholar]
- 47.Stoumpos S, Mark PB, McQuarrie EP, Traynor JP, Geddes CC: Continued monitoring of acute kidney injury survivors might not be necessary in those regaining an estimated glomerular filtration rate >60 mL/min at 1 year. Nephrol Dial Transplant 32: 81–88, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Gautam SC, Brooks CH, Balogun RA, Xin W, Ma JZ, Abdel-Rahman EM: Predictors and outcomes of post-hospitalization dialysis dependent acute kidney injury. Nephron 131: 185–190, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Rathore AS, Chopra T, Ma JZ, Xin W, Abdel-Rahman EM: Long-term outcomes and associated risk factors of post-hospitalization dialysis-dependent acute kidney injury patients. Nephron 137: 105–112, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Pajewski R, Gipson P, Heung M: Predictors of post-hospitalization recovery of renal function among patients with acute kidney injury requiring dialysis. Hemodial Int 22: 66–73, 2018 [DOI] [PubMed] [Google Scholar]
- 51.Vijayan A, Delos Santos RB, Li T, Goss CW, Palevsky PM: Effect of frequent dialysis on renal recovery: Results from the acute renal failure trial network study. Kidney Int Rep 3: 456–463, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAdams M, Ortiz-Soriano V, Jordan M, Armentrout B, Vasquez-Rios G, Lima F, Sawaya BP, Neyra JA: Kidney recovery in patients discharged to an acute rehabilitation facility with acute kidney injury requiring hemodialysis. Clin Nephrol 92: 15–24, 2019 [DOI] [PubMed] [Google Scholar]
- 53.Silver SA, Saragosa M, Adhikari NK, Bell CM, Harel Z, Harvey A, Kitchlu A, Neyra JA, Wald R, Jeffs L: What insights do patients and caregivers have on acute kidney injury and posthospitalisation care? A single-centre qualitative study from Toronto, Canada. BMJ Open 8: e021418, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ortiz-Soriano V, Alcorn JL 3rd, Li X, Elias M, Ayach T, Sawaya BP, Malluche HH, Wald R, Silver SA, Neyra JA: A survey study of self-rated patients’ knowledge about AKI in a post-discharge AKI clinic. Can J Kidney Health Dis 6: 2054358119830700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siew ED, Liu KD, Bonn J, Chinchilli V, Dember LM, Girard TD, Greene T, Hernandez AF, Ikizler TA, James MT, Kampschroer K, Kopp JB, Levy M, Palevsky PM, Pannu N, Parikh CR, Rocco MV, Silver SA, Thiessen-Philbrook H, Wald R, Xie Y, Kimmel PL, Star RA: Improving care for patients after hospitalization with AKI. J Am Soc Nephrol 31: 2237–2241, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams KT, Howe JL, Fong A, Puthumana JS, Kellogg KM, Gaunt M, Ratwani RM: An analysis of patient safety incident reports associated with electronic health record interoperability. Appl Clin Inform 8: 593–602, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutton PR, Payne TH: Interoperability of electronic health information and care of dialysis patients in the United States. Clin J Am Soc Nephrol 14: 1536–1538, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly YP, Kuperman GJ, Steele DJR, Mendu ML: Interoperability and patient electronic health record accessibility: Opportunities to improve care delivery for dialysis patients. Am J Kidney Dis 76: 427–430, 2020 [DOI] [PubMed] [Google Scholar]
- 59.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ; Neonatal Kidney Collaborative (NKC) : Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1: 184–194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, Jefferies JL: Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg 143: 368–374, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF: Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: A systematic review, meta-analysis, and meta-regression. JAMA 290: 1360–1370, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Menon S, Kirkendall ES, Nguyen H, Goldstein SL: Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 165: 522–7.e2, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M: Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 92: 751–756, 2017 [DOI] [PubMed] [Google Scholar]
- 66.Cooper DS, Claes D, Goldstein SL, Bennett MR, Ma Q, Devarajan P, Krawczeski CD: Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI). Clin J Am Soc Nephrol 11: 21–29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silver SA, Goldstein SL, Harel Z, Harvey A, Rompies EJ, Adhikari NK, Acedillo R, Jain AK, Richardson R, Chan CT, Chertow GM, Bell CM, Wald R: Ambulatory care after acute kidney injury: An opportunity to improve patient outcomes. Can J Kidney Health Dis 2: 36, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Bottinger EP, Glicksberg BS, Coca SG, Nadkarni GN, Mount Sinai CIC, Li L: AKI in hospitalized patients with COVID-19. J Am Soc Nephrol: 32, 151–160, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, Ross DW, Sharma P, Sakhiya V, Fishbane S, Jhaveri KD, Northwell Nephrology C-RCM: Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 77: 204–215, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, Kluge S, Gross O, Pueschel K, Schröder AS, Edler C, Aepfelbacher M, Puelles VG, Huber TB: SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A, Ghazi L, Greenberg J, Mansour S, Moledina D, Wilson FP: Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open 4: e211095, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]