Visual Abstract
Keywords: kidney transplantation, gene expression, rejection, diagnostic tests, routine, graft rejection, cellfree nucleic acid
Abstract
Background and objectives
Subclinical acute rejection is associated with poor outcomes in kidney transplant recipients. As an alternative to surveillance biopsies, noninvasive screening has been established with a blood gene expression profile. Donor-derived cellfree DNA (cfDNA) has been used to detect rejection in patients with allograft dysfunction but not tested extensively in stable patients. We hypothesized that we could complement noninvasive diagnostic performance for subclinical rejection by combining a donor-derived cfDNA and a gene expression profile assay.
Design, setting, participants, & measurements
We performed a post hoc analysis of simultaneous blood gene expression profile and donor-derived cfDNA assays in 428 samples paired with surveillance biopsies from 208 subjects enrolled in an observational clinical trial (Clinical Trials in Organ Transplantation-08). Assay results were analyzed as binary variables, and then, their continuous scores were combined using logistic regression. The performance of each assay alone and in combination was compared.
Results
For diagnosing subclinical rejection, the gene expression profile demonstrated a negative predictive value of 82%, a positive predictive value of 47%, a balanced accuracy of 64%, and an area under the receiver operating curve of 0.75. The donor-derived cfDNA assay showed similar negative predictive value (84%), positive predictive value (56%), balanced accuracy (68%), and area under the receiver operating curve (0.72). When both assays were negative, negative predictive value increased to 88%. When both assays were positive, positive predictive value increased to 81%. Combining assays using multivariable logistic regression, area under the receiver operating curve was 0.81, significantly higher than the gene expression profile (P<0.001) or donor-derived cfDNA alone (P=0.006). Notably, when cases were separated on the basis of rejection type, the gene expression profile was significantly better at detecting cellular rejection (area under the receiver operating curve, 0.80 versus 0.62; P=0.001), whereas the donor-derived cfDNA was significantly better at detecting antibody-mediated rejection (area under the receiver operating curve, 0.84 versus 0.71; P=0.003).
Conclusions
A combination of blood-based biomarkers can improve detection and provide less invasive monitoring for subclinical rejection. In this study, the gene expression profile detected more cellular rejection, whereas donor-derived cfDNA detected more antibody-mediated rejection.
Introduction
Subclinical acute rejection in a kidney transplant is associated with worse clinical outcomes, including higher risk of subsequent clinical acute rejection, de novo donor-specific antibody (DSA) formation, and graft fibrosis (1–6). Although large trials are still lacking, there are several trials suggesting that treating subclinical rejection improves outcomes (6–9). Monitoring patients for subclinical rejection typically involves serial surveillance biopsies to detect the disease. However, despite clinical evidence, only about half of high-volume transplant programs in the United States perform surveillance biopsies (10). Recently, a noninvasive blood gene expression profile biomarker (TruGraf; Transplant Genomics, Inc., Mansfield, MA) has been developed to detect or exclude subclinical rejection (1,11–13).
Donor-derived cellfree DNA (cfDNA) has been developed as a biomarker for rejection in kidney recipients (14). At currently defined thresholds, donor-derived cfDNA is more sensitive to vascular injury typified by antibody-mediated rejection (15) or more severe forms of cellular rejection (Banff 1B and greater) (14). Sigdel et al. (16) reported diagnostic performance of donor-derived cfDNA on a subset of protocol biopsy samples. Their reported sensitivity, specificity, prevalence-adjusted positive predictive value (PPV), and negative predictive value (NPV) were 0.92, 0.75, 0.55, and 0.97, respectively. They, however, did not include borderline histology as rejection, despite data suggesting the adverse effects of this type of subclinical rejection (1,6,17). A recent editorial suggested similar caution in relying on donor-derived cfDNA to exclude clinical or subclinical acute cellular rejection in the absence of antibody-mediated rejection (18). Previous estimates of antibody-mediated rejection in stable patients were low, and assays developed to detect antibody-mediated rejection in stable patients were on the basis of this assumption. Given the known effect of subclinical antibody-mediated rejection on graft outcomes (19,20) and the recognition that the prevalence is higher when including the “suspicious antibody-mediated rejection” category, we sought to assess the role of a cfDNA assay using these more inclusive clinical phenotype definitions. Thus, there are no publications combining a gene expression profile assay with plasma donor-derived cfDNA or supporting the routine use of donor-derived cfDNA tests to monitor stable patients for subclinical rejection, including borderline rejection.
We, therefore, undertook an analysis to describe the performance of the TruGraf gene expression profile individually and combined with measurements of plasma donor-derived cfDNA to complement the diagnostic accuracy of either test alone to monitor stable kidney transplant recipients for subclinical rejection.
Materials and Methods
Study Population
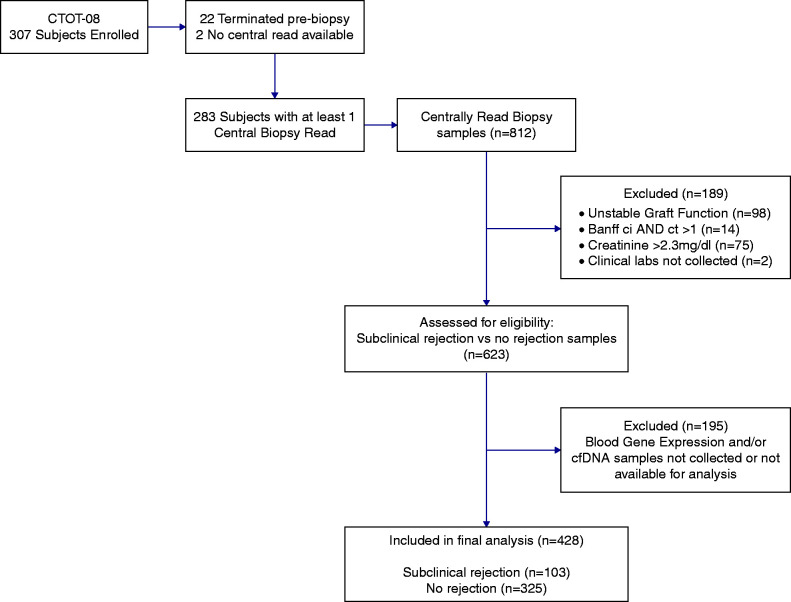
This post hoc analysis was performed on 428 (325 no rejection and 103 subclinical rejection) samples previously collected from 208 patients paired with surveillance kidney biopsies in the setting of stable kidney function who enrolled between 2011 and 2014 in the Clinical Trials in Organ Transplantation-08 (CTOT-08; NCT01289717) study, which was previously described (1). In brief, CTOT-08 was a prospective, multicenter, 2-year observational study of 307 subjects. Surveillance biopsies were performed at 2–6, 12, and 24 months after transplant. We used an independent Northwestern biorepository cohort (n=105 samples, 76 no rejection and 29 subclinical rejection) of subjects (n=85) who underwent surveillance biopsy in the first 2 years post-transplant (NCT01531257) for external validation (Table 1). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism, were subject to institutional review board approval, and adhered to the Declaration of Helsinki, and informed consent was obtained from all subjects.
Table 1.
Characteristics of study participants and kidney donors
| Demographics | Clinical Trials in Organ Transplantation-08 Cohort | Northwestern University Validation Cohort | ||||
| Only Subclinical Rejection, n=22 | No Subclinical Rejection, n=123 | Mixed Subclinical Rejection and No Rejection, n=63 | Only Subclinical Rejection, n=21 | No Subclinical Rejection, n=59 | Mixed Subclinical Rejection and No Rejection, n=5 | |
| Kidney donors | ||||||
| Deceased donor, n (%) | 16 (73) | 46 (37) | 22 (35) | 11 (52) | 20 (34) | 2 (40) |
| Donor age, yr, mean (± SD) | 35 (16) | 41 (13) | 41 (13) | 39 (14) | 36 (13) | 46 (17) |
| Donor sex, women, n (%) | 10 (46) | 62 (50) | 29 (46) | 9 (43) | 30 (51) | 3 (60) |
| Donor race, n (%) | ||||||
| White | 17 (77) | 82 (67) | 50 (80) | 12 (57) | 32 (54) | 3 (60) |
| Black | 2 (9) | 21 (17) | 2 (3) | 2 (10) | 11 (19) | 0 (0) |
| American Indian or Alaska Native | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Native Hawaiian/other Pacific Islander | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 1 (5) | 4 (3) | 2 (3) | 1 (5) | 3 (5) | 0 (0) |
| Unknown | 2 (9) | 14 (11) | 8 (13) | 3 (14) | 0 (0) | 0 (0) |
| Donor ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 16 (73) | 91 (74) | 47 (75) | 8 (38) | 22 (37) | 2 (40) |
| Hispanic or Latino | 3 (14) | 17 (14) | 8 (13) | 4 (19) | 1 (2) | 0 (0.0) |
| Unknown | 3 (14) | 15 (12) | 8 (13) | 9 (43) | 36 (61) | 3 (60) |
| Kidney recipients | ||||||
| Recipient age, yr, mean (± SD) | 48 (16) | 51 (15) | 53 (14) | 49 (12) | 50 (13) | 58 (15) |
| Recipient sex, women, n (%) | 6 (27) | 44 (36) | 21 (33) | 10 (48) | 25 (42) | 3 (60) |
| Recipient race, n (%) | ||||||
| White patients | 14 (64) | 73 (59) | 43 (68) | 12 (57) | 31 (53) | 2 (40) |
| Black patients | 6 (27) | 29 (24) | 7 (11) | 5 (24) | 10 (17) | 0 (0) |
| American Indian or Alaska Native patients | 2 (9) | 0 (0) | 2 (3) | 0 (0) | 1 (2) | 0 (0) |
| Native Hawaiian/other Pacific Islander patients | 0 (0) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Asian patients | 0 (0) | 10 (8) | 1 (2) | 1 (5) | 5 (9) | 0 (0) |
| Unknown | 0 (0) | 9 (7) | 8 (13) | 2 (10) | 4 (7) | 0 (0) |
| Recipient ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 19 (86) | 101 (82) | 47 (75) | 17 (81) | 47 (80) | 2 (40) |
| Hispanic or Latino | 2 (9) | 18 (15) | 11 (18) | 2 (10) | 9 (15) | 3 (60) |
| Unknown | 1 (5) | 4 (3) | 5 (8) | 2 (10) | 3 (5) | 0 (0) |
| Recipient primary reason for kidney failure, n (%) | ||||||
| Cystic (includes PKD) | 0 (0) | 12 (10) | 10 (16) | 2 (10) | 7 (12) | 1 (20) |
| Diabetes mellitus | 5 (23) | 26 (21) | 11 (18) | 2 (10) | 10 (17) | 2 (40) |
| GN | 6 (27) | 36 (29) | 14 (22) | 8 (38) | 22 (37) | 1 (20) |
| Hypertension | 2 (9) | 25 (20) | 11 (18) | 6 (29) | 15 (25) | 1 (20) |
| Other | 9 (41) | 24 (20) | 17 (27) | 3 (14) | 5 (9) | 0 (0) |
| Recipient PRA at transplant | ||||||
| PRA class I, % median [IQR] | 0 [0–9] | 0 [0–0] | 0 [0–0] | 11 [0–51] | 4 [0–15] | 4 [0–20] |
| PRA class II, % median [IQR] | 0 [0–6] | 0 [0–0] | 0 [0–0] | 2 [0–75] | 0 [0–18] | 0 [0–4] |
| cPRA, % median [IQR] | 18 [0–97] | 9 [0–54] | 0 [0–64] | 12 [0–54] | 0 [0–0] | 0 [0–0] |
| Induction therapy, n (%) | ||||||
| Basiliximab | 4 (18) | 21 (17) | 16 (25) | 4 (19) | 8 (14) | 1 (20) |
| Alemtuzumab | 9 (41) | 59 (48) | 33 (52) | 17 (81) | 51 (86) | 5 (100) |
| Antithymocyte globulin | 10 (46) | 37 (30) | 13 (21) | 0 (0) | 0 (0) | 0 (0) |
| Steroid | 18 (82) | 97 (79) | 52 (83) | 21 (100) | 59 (100) | 5 (100) |
| IVIG | 1 (5) | 3 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rituximab | 0 (0) | 0 (0) | 0 (0) | 5 (24) | 6 (10) | 0 (0) |
| Received desensitization therapy, n (%) | 0 (0) | 5 (4) | 8 (13) | 5 (24) | 7 (12) | 0 (0) |
| Maintenance therapy, n (%) | ||||||
| Steroid | 18 (82) | 56 (46) | 42 (67) | 7 (33) | 9 (15) | 1 (20) |
| Tacrolimus | 22 (100) | 122 (99) | 63 (100) | 16 (76) | 51 (86) | 5 (100) |
| Cyclosporine | 2 (9) | 5 (4) | 4 (6) | 1 (5) | 0 (0) | 0 (0) |
| Azathioprine | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Mycophenolate | 22 (100) | 121 (98) | 62 (98) | 16 (76) | 47 (80) | 5 (100) |
| Sirolimus | 1 (5) | 8 (7) | 7 (11) | 2 (10) | 1 (2) | 0 (0) |
| Leflunomide | 0 (0) | 1 (1) | 2 (3) | 0 (0) | 0 (0) | 0 (0) |
| Belatacept | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 8 (14) | 0 (0) |
In total, 208 subjects with stable kidney function underwent surveillance biopsies; 22 (11%), 123 (59%), and 63 (30%) had only subclinical rejection, no rejection, and mixed either no rejection or subclinical rejection (e.g., one or more episodes of subclinical rejection during the study period), respectively. The median PRA and cPRA were reported because they were not normally distributed. In the Northwestern validation cohort, 85 subjects with stable kidney function underwent surveillance biopsies; 21 (25%), 59 (69%), and five (6%) had only subclinical rejection, no rejection, and mixed either no rejection or subclinical rejection (e.g., one or more episode of subclinical rejection during the study period), respectively. PKD, polycystic kidney disease; PRA, panel reactive antibody; IQR, interquartile range; cPRA, calculated panel reactive antibody; IVIG, intravenous Ig.
Histologic Phenotypes and Subject Selection Criteria
The study samples were chosen to represent a screening cohort of stable patients with good kidney function. Histologic phenotypes for this analysis included subclinical rejection and no rejection (1). All biopsies were analyzed and scored by a blinded, central pathologist. Biopsies done for cause, biopsies done in patients with serum creatinine ≥2.3 mg/dl, or biopsies read as having chronic fibrosis (Banff interstitial fibrosis score more than one and tubular atrophy score more than one) with or without inflammation were excluded from the analysis (Figure 1). The subclinical rejection clinical phenotype was defined by histology with acute rejection (greater than or equal to borderline cellular rejection by Banff 2007 criteria and/or antibody-mediated rejection) and stable kidney function. Stable kidney function was specified as serum creatinine <2.3 mg/dl and <20% increase in creatinine compared with a minimum of two or three prior values. The no rejection clinical phenotype included stable kidney function and normal histology on surveillance biopsy and was updated according to the recent changes to the Banff 2019 criteria (21) (no evidence of rejection: Banff i=0 with t=0 or 1, g=0, ptc=0, interstitial fibrosis =0 or 1, tubular atrophy =0 or 1). We believe that tubulitis (t2/t3) with i0 represents significant inflammation and shares traits with newly defined borderline changes. Therefore, we classified tubulitis (t2/t3) with i0 as borderline changes in this study. Because we did not have DSA information available on the majority of samples, we relied on histologic criteria for diagnosing antibody-mediated rejection in samples missing paired DSA information. The biopsy was classified as antibody-mediated rejection if two histologic criteria of acute antibody-mediated rejection according to the 2019 Banff classification were present. If a specimen met one of two histologic criteria of acute antibody-mediated rejection, we classified it as “suspicious antibody-mediated rejection.” We analyzed the gene expression profile and donor-derived cfDNA performance by including the “suspicious antibody-mediated rejection” in the antibody-mediated rejection group to capture biopsies with even low levels of microvascular inflammation. For example, a sample with ptc=1 was considered “suspicious antibody-mediated rejection.”
Figure 1.
CONSORT diagram illustrating the number of patients and the samples available for analysis on the basis of inclusion and exclusion criteria in addition to sample availability. CONSORT, Consolidated Standards of Reporting Trials; cfDNA, cellfree DNA; ci, interstitial fibrosis; ct, tubular atrophy; CTOT-08, Clinical Trials in Organ Transplantation-08.
Gene Expression Profile
Blood samples for the gene expression profile assay were drawn directly into PAXgene (BD BioSciences, San Jose, CA) tubes at the time of surveillance biopsy. The samples were processed as previously described (22) using Affymetrix HT HG‐U133+PM Array Plates on the Gene Titan MC instrument (Thermo Fisher Scientific, Waltham, MA; GEO accession no. GSE107509) (1). The gene expression profiles were analyzed with the TruGraf algorithm—a DNA microarray–based gene expression algorithm analyzing differential expression of 120 genes (12,23)—and assigned a result of either TX or not TX. Gene expression profile results were provided as a probability score normalized on a 0–100 scale. The TruGraf assay (Eurofins–Transplant Genomics, Mansfield, MA) has a previously defined probability threshold of 50 to differentiate the TX (normal, no rejection) from the not TX phenotype (including subclinical rejection).
Donor-Derived Cellfree DNA
Blood for donor-derived cfDNA analysis was also drawn at the time of surveillance biopsy in plasma separation tubes (BD Vacutainer PPT Plasma Preparation Tube; BD BioSciences, San Jose, CA). Next generation sequencing data were mapped to a reference genome and, along with recipient genotype data (see below), analyzed for percentage donor-derived cfDNA by a bioinformatics pipeline licensed from Stanford University (24).
Recipient genotyping was performed on PBMC samples. The donor-derived cfDNA results were provided as a percentage of the donor-derived fraction as compared with total cfDNA by using panels of single-nucleotide polymorphisms (approximately 70,000 single-nucleotide polymorphisms) to differentiate between donor and recipient cfDNA without requiring knowledge of donor genotypes. The TRAC assay (Eurofins–Viracor, Lee’s Summit, MO) reports the fraction of donor-derived cfDNA as a percentage with >0.7% being considered positive. We also report results of the assay with additional thresholds to allow comparison with other commercial donor-derived cfDNA assays.
Statistical Analyses
Demographic characteristics were compared among the three groups with different histologic phenotypes using one-way ANOVA for continuous variables and chi-squared tests for nominal variables. Biopsies, gene expression profile, and donor-derived cfDNA results were treated as binary outcomes for performance analyses. Sensitivity, specificity, PPV, NPV, area under the receiver operating curve (AUROC), accuracy, and balanced accuracy were calculated. Bootstrapping with 10,000 iterations was used to calculate a 95% confidence interval (95% CI) for each performance metric. To assess the performance of combined gene expression profile and donor-derived cfDNA continuous scores, multivariable logistic regression was performed using the continuous scores of both assays. External validation was performed using an independent Northwestern biorepository cohort. We considered a P value of 0.05 as statistically significant in a two-tailed test. All statistical analyses were performed using R version 4.0.0 via RStudio.
Results
In total, 428 blood samples were analyzed from 208 unique subjects who had surveillance biopsies paired with gene expression profiles and donor-derived cfDNA. Of 208 subjects, 11% (n=22) had only subclinical rejection (i.e., no normal biopsies), 59% (n=123) had no rejection only (i.e., no biopsies with subclinical rejection), and 30% (n=63) had either subclinical rejection or no rejection (e.g., one or more episodes of subclinical rejection during the study period) on the basis of histologic phenotypes (Table 1). There was no significant difference in patient-level demographics between the three groups except for race, desensitization therapy, and steroid use as a maintenance therapy. Of 428 samples, 76% (n=325) and 24% (n=103) were classified no rejection and subclinical rejection, respectively, by histologic phenotypes. The 103 subclinical rejection biopsies consisted of borderline at 32% (n=33), Banff≥1A at 5% (n=5), borderline plus suspicious antibody-mediated rejection at 13% (n=13), Banff≥1A plus suspicious antibody-mediated rejection at 4% (n=4), suspicious antibody-mediated rejection at 21% (n=22), antibody-mediated rejection only at 20% (n=20), and antibody-mediated rejection with greater than or equal to borderline at 6% (n=6). Of 65 antibody-mediated rejection biopsy samples, 23 (35%) had cellular rejection components and 42 cases (65%) had antibody-mediated rejection only. Subclinical rejection occurred almost evenly at the different biopsy time points in the study period. Of 103 subclinical rejection cases, 21%, 32%, 31%, and 16% were identified at 3–6 months, 12 months, 24 months, and the intensive monitoring period or other time, respectively (Table 2).
Table 2.
Subclinical acute rejection types, timing of rejections, and number positive by each assay
| No. and Timing of Rejection/Type of Rejection, n=103 | 3–6 mo, 21%; n=22 | 12 mo, 32%; n=33 | 24 mo, 31%; n=32 | Intense Monitoring Visit or Suspected Rejection Visit, 16%; n=16 |
| Acute cellular rejection Banff borderline or ≥1A, 37%; n=38 | 10 | 10 | 12 | 6 |
| (6 gene expression profile, 0 cfDNA) | (5 gene expression profile, 0 cfDNA) | (7 gene expression profile, 3 cfDNA) | (1 gene expression profile, 1 cfDNA) | |
| Mixed acute cellular rejection + antibody-mediated rejection, 22%; n=23 | 5 | 10 | 3 | 5 |
| (1 gene expression profile, 3 cfDNA) | (3 gene expression profile, 6 cfDNA) | (2 gene expression profile, 1 cfDNA) | (4 gene expression profile, 3 cfDNA) | |
| Suspicious antibody-mediated rejection + antibody-mediated rejection, 41%; n=42 | 7 | 13 | 17 | 5 |
| (2 gene expression profile, 3 cfDNA) | (5 gene expression profile, 10 cfDNA) | (4 gene expression profile, 13 cfDNA) | (4 gene expression profile, 5 cfDNA) |
A total of 103 subclinical rejection cases were identified by histologic evaluation. The acute cellular rejection cases consisted of borderline (n=33) and Banff≥1A (n=5). The mixed group included borderline plus suspicious antibody-mediated rejection (n=13), Banff≥1A plus suspicious antibody-mediated rejection (n=4), and antibody-mediated rejection with greater than or equal to borderline (n=6). There were 22 cases of suspicious antibody-mediated rejection and 20 of antibody-mediated rejection in the suspicious antibody-mediated rejection plus antibody-mediated rejection group. Columns depict the timing post-transplant when rejection episodes occurred (with percentage of total rejections and numbers in parentheses). Each square demonstrates the total number of rejection cases on the basis of the clinical/histologic phenotype followed by the number of true-positive tests detected by each assay in parentheses. Subclinical rejection indicates subclinical acute rejection. Suspected rejection visit indicates suspected rejection visit (but found to have stable function meeting the definition of subclinical acute rejection). cfDNA, donor-derived cellfree DNA assay.
Diagnostic Performance of Gene Expression, Donor-Derived Cellfree DNA, and the Combination of the Two Tests
Gene Expression Profile Performance.
In 103 subclinical rejection cases, the gene expression profile was positive (not TX) in 43% (n=44) and negative (TX) in 57% (n=59). Of the 325 normal biopsy cases, the gene expression profile was negative in 85% (n=275) and positive in 15% (n=50). Full performance metrics are listed in Table 3. Of true-positive gene expression profile samples (n=44), 66% (n=29) of subclinical rejection cases detected were either acute cellular rejection or acute cellular rejection with antibody-mediated rejection. The remaining 34% (n=15) were antibody-mediated rejection alone. The timing post-transplant and type of rejection episodes detected by the gene expression profile are presented in Table 2.
Table 3.
Summary of diagnostic metrics to detect subclinical acute rejection
| Diagnostic Performance | Gene Expression Profile Alone | Donor-Derived Cellfree DNA Alone | Positive = Gene Expression Profile+ or Donor-Derived Cellfree DNA+ | Positive = Gene Expression Profile+ and Donor-Derived Cellfree DNA+ | Logistic Regression at 0.35 Cutoff |
| Sensitivity | 0.43 | 0.47 | 0.69 | 0.20 | 0.51 |
| 95% Confidence interval | (0.32 to 0.53) | (0.34 to 0.59) | (0.58 to 0.79) | (0.12 to 0.30) | (0.40 to 0.62) |
| Specificity | 0.85 | 0.88 | 0.74 | 0.98 | 0.87 |
| 95% Confidence interval | (0.80 to 0.89) | (0.84 to 0.92) | (0.69 to 0.80) | (0.97 to 1) | (0.83 to 0.91) |
| Positive predictive value | 0.47 | 0.56 | 0.46 | 0.81 | 0.56 |
| 95% Confidence interval | (0.35 to 0.59) | (0.44 to 0.67) | (0.37 to 0.55) | (0.63 to 0.95) | (0.45 to 0.67) |
| Negative predictive value | 0.82 | 0.84 | 0.88 | 0.80 | 0.85 |
| 95% Confidence interval | (0.78 to 0.86) | (0.80 to 0.88) | (0.84 to 0.92) | (0.75 to 0.84) | (0.81 to 0.89) |
| Accuracy | 0.75 | 0.78 | 0.73 | 0.80 | 0.79 |
| Balanced accuracy | 0.64 | 0.68 | 0.72 | 0.59 | 0.69 |
Performance metrics (with 95% confidence interval) of the individual gene expression profile and donor-derived cellfree DNA assays (columns 1 and 2, respectively). Column 3 presents the combination with either or both tests being positive to diagnose subclinical reject (both tests have to be negative to be called negative). Column 4 presents the combination with both tests required to be positive to diagnose subclinical rejection, and in the last column, the performance of the multivariable logistic regression model combining the two assays using their continuous output scores. Subclinical rejection indicates subclinical acute rejection.
Donor-Derived Cellfree DNA Performance.
Of the 103 subclinical rejection cases, the donor-derived cfDNA assay was positive in 47% (n=48) and negative in 53% (n=55). Of the 325 normal biopsy cases, the donor-derived cfDNA assay was negative in 88% (n=287) and positive in 12% (n=38). Full performance metrics are listed in Table 3. Of true-positive donor-derived cfDNA samples (n=48), 92% (n=44) of subclinical rejection cases detected were either antibody-mediated rejection or acute cellular rejection mixed with antibody-mediated rejection (Table 2). In terms of timing, only 13% (n=6) of the true-positive donor-derived cfDNA results occurred before the 1-year post-transplant biopsy, and all were antibody-mediated rejection or acute cellular rejection mixed with antibody-mediated rejection (Table 2).
When a donor-derived cfDNA threshold of >1% was used as a positive cutoff, sensitivity (41%) was lower than using the 0.7% threshold (47%). However, specificity, PPV, NPV, accuracy, and balanced accuracy were similar or higher at 91%, 60%, 83%, 79%, and 66%, respectively (Supplemental Table 2).
A scatterplot of all samples on the basis of the gene expression profile and donor-derived cfDNA scores to present the distribution of scores for each assay is provided in Supplemental Figure 1.
Performance of Gene Expression and Donor-Derived Cellfree DNA Depending on Rejection Type.
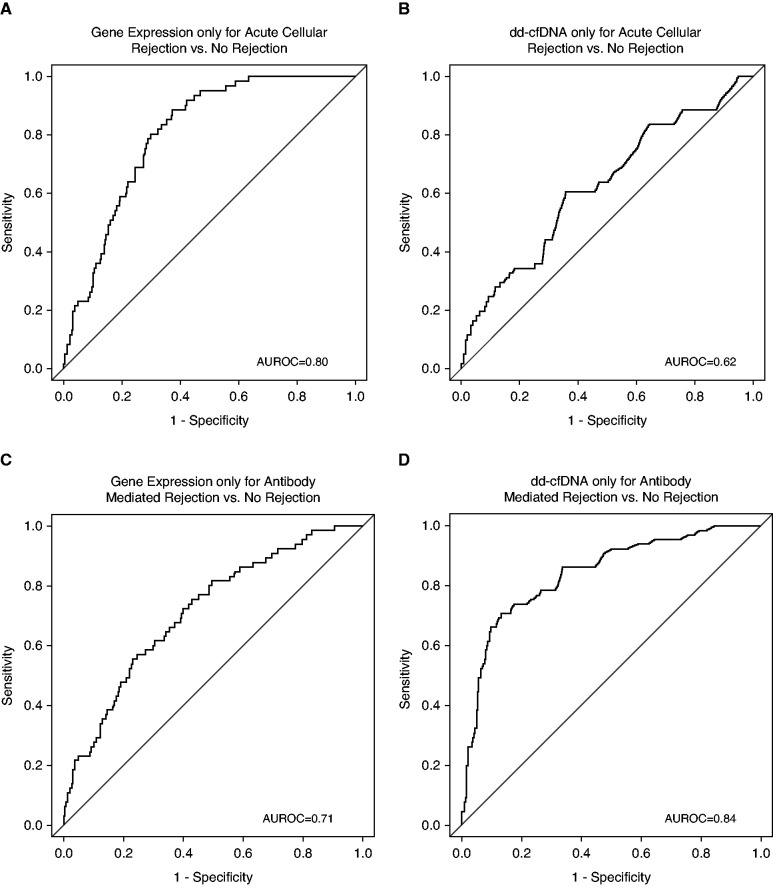
Significant differences were seen in diagnostic performance on the basis of rejection type. The gene expression profile outperformed donor-derived cfDNA on acute cellular rejection cases (that included acute cellular rejection alone and acute cellular rejection plus antibody-mediated rejection cases) on the basis of AUROC (0.80 versus 0.62; P<0.001) and balanced accuracy (0.67 versus 0.58; P=0.10) (Figure 2, A and B). Conversely, donor-derived cfDNA showed higher performance when compared with the gene expression profile in the antibody-mediated rejection cases (that included antibody-mediated rejection alone and antibody-mediated rejection plus acute cellular rejection cases) on the basis of AUROC (donor-derived cfDNA, 0.84 versus gene expression profile, 0.71; P=0.003) and balanced accuracy (0.78 versus 0.62; P<0.001) (Figure 2, C and D).
Figure 2.
Differential performance of the gene expression profile and donor-derived cfDNA based on rejection type (acute cellular versus acute antibody-mediated rejection). (A) AUROC of the gene expression profile only for distinguishing acute cellular rejection versus no rejection. (B) AUROC of donor-derived cfDNA only for distinguishing acute cellular rejection versus no rejection. (C) AUROC of the gene expression profile only for antibody-mediated rejection versus no rejection. (D) AUROC of donor-derived cfDNA only for antibody-mediated rejection versus no rejection. AUROC, area under the receiver operating curve; dd-cfDNA, donor-derived cellfree DNA.
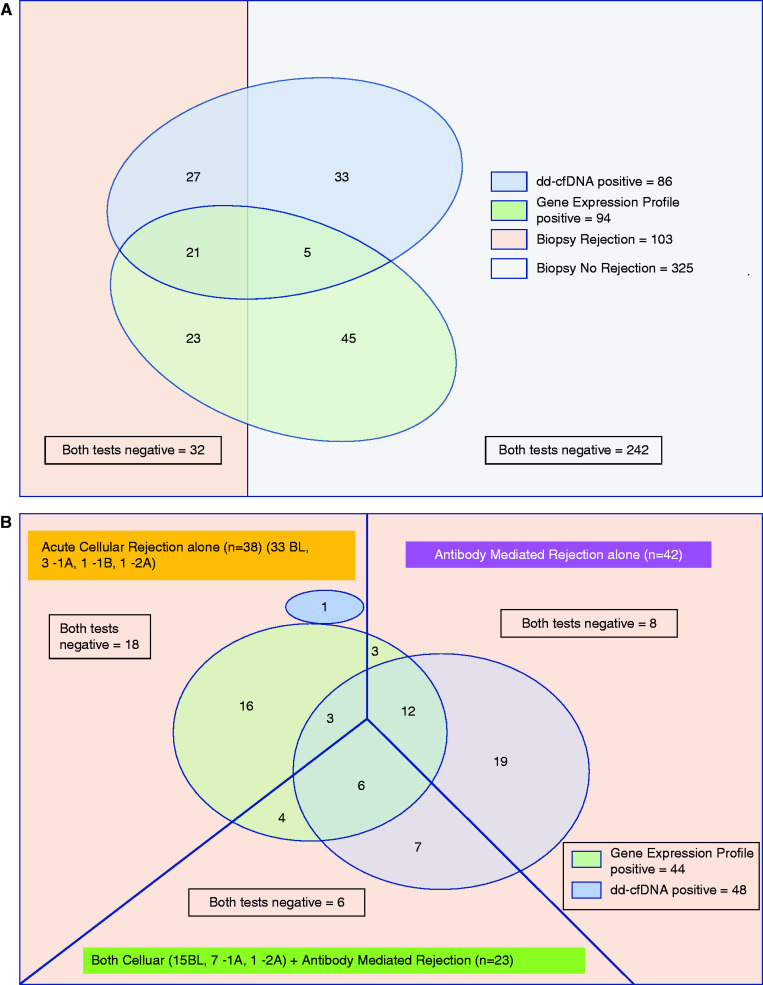
The overall summary of gene expression profile and donor-derived cfDNA performance on the basis of biopsy phenotype is summarized in Figure 3A. Importantly, the figure highlights the overlap (or lack thereof) in which cases of subclinical rejection are accurately identified by the different tests, with the gene expression profile picking up more cases of earlier acute cellular rejection and donor-derived cfDNA picking up more cases of antibody-mediated rejection (Figure 3B, Table 2). In addition, there is nonoverlap of the biopsy-paired samples that were called falsely positive by each test. The summary of diagnostic metrics for each rejection type is shown in Supplemental Tables 3–6.
Figure 3.
Gene expression profile and donor-derived cellfree DNA preferentially detect different types of rejection. (A) All cases of subclinical rejection versus no rejection by biopsy and assay result. (B) All cases of subclinical rejection by rejection type and assay result. (A) Summary of the gene expression profile and donor-derived cfDNA performance at sample levels. Of 428 samples, the subclinical rejection group (n=103) consists of gene expression profile alone positive (n=23), donor-derived cfDNA alone positive (n=27), both gene expression profile and donor-derived cfDNA positive (n=21), and both gene expression profile and donor-derived cfDNA negative (n=32). Of the normal biopsies (n=325), both tests were negative for n=242, both were positive for n=5, gene expression profile alone was positive for n=45, and donor-derived cfDNA alone was positive for n=33. (B) Of 103 subclinical rejection cases, they are divided by histology phenotypes into antibody-mediated rejection alone (n=42), acute cellular rejection alone (n=38), and combined antibody-mediated rejection plus acute cellular rejection (n=23) cases, with breakdown of acute cellular rejection Banff grade as shown. The numbers demonstrate the true positives and false negatives with each assay. Although some overlap exists, the two assays tend to detect different types of rejection. 1A, Banff 1A acute cellular rejection; 1B, Banff 1B acute cellular rejection; 2A, Banff 2A acute cellular rejection; BL, borderline cellular rejection.
Combined Gene Expression Profile and Donor-Derived Cellfree DNA Performance.
We investigated the diagnostic performance related to biopsy phenotypes with different combinations of groups of gene expression profile and donor-derived cfDNA tests. Of time points with subclinical rejection on biopsy and both gene expression profile and donor-derived cfDNA positive (n=21), 12 were antibody-mediated rejection alone, three were acute cellular rejection alone, and six were combined antibody-mediated rejection and acute cellular rejection by histology (Figure 3). Thirty-two subclinical rejection cases found on biopsy were both gene expression profile and donor-derived cfDNA negative (Figure 3A). The histology of those associated samples was borderline (n=15), Banff 1A (n=3), Banff 1A with antibody-mediated rejection including suspicious antibody-mediated rejection (n=2), borderline with suspicious antibody-mediated rejection (n=4), suspicious antibody-mediated rejection (n=7), and antibody-mediated rejection (n=1).
First, to evaluate combining the results of both tests to detect the presence or absence of rejection, the group with either gene expression profile or donor-derived cfDNA positive (including both tests positive) was considered as a positive test and compared with those where both gene expression profile and donor-derived cfDNA were negative to be called negative (Table 3). When both tests had to be negative to call a time point a negative test, the NPV increased to 88% (95% CI, 0.84 to 0.92).
When requiring both gene expression profile and donor-derived cfDNA to be positive (positive = gene expression profile and donor-derived cfDNA positive) to call a time point a positive test, the sensitivity dropped to 20% (95% CI, 0.12 to 0.30), but the specificity increased to 98% (95% CI, 0.97 to 1), with a PPV of 0.81 (95% CI, 0.63 to 0.95). A summary of the performance characteristics is presented in Table 3.
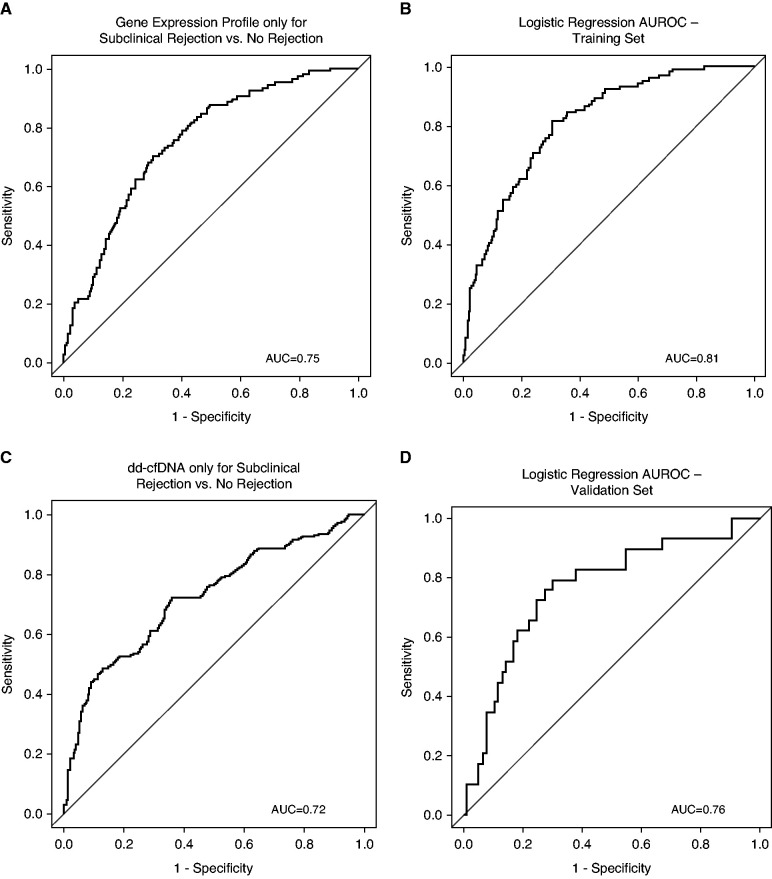
When the gene expression profile and donor-derived cfDNA were combined using a multivariable logistic regression using their continuous scores rather than binary output on the basis of thresholds, the AUROC improved to 0.81, which was significantly higher than gene expression profile alone (0.81 versus 0.75; P<0.001) or donor-derived cfDNA alone (0.81 versus 0.72; P=0.006) (Figure 4, A–C).
Figure 4.
Performance metrics of individual gene expression profile and donor-derived cfDNA assays compared with the logistic regression model with continuous variables for combined gene expression profile and donor-derived cfDNA to distinguish subclinical rejection versus no rejection. (A) AUROC of the gene expression profile only for subclinical rejection versus no rejection. (B) AUROC of the combined gene expression profile and donor-derived cfDNA performance on the CTOT-08 cohort (training set) by the multivariable logistic regression model using the continuous score output of both tests. (C) AUROC of donor-derived cfDNA only for subclinical rejection versus no rejection. (D) AUROC of an external validation with an independent cohort (n=105 samples) by the multivariable logistic regression model using the continuous score output of both tests. AUC, area under the curve.
Examining the continuous scores, a 1%-higher donor-derived cfDNA is associated with an odds ratio of 1.76 (95% CI, 1.38 to 2.23; P<0.001) for subclinical rejection, and each ten-point higher gene expression profile probability score is associated with an odds ratio of 1.66 (95% CI, 1.43 to 1.92; P<0.001) for subclinical rejection. This suggests that the donor-derived cfDNA and gene expression profile assays are independently associated with subclinical rejection.
Logistic Regression Model External Validation.
The external validation AUROC (0.76) for the combined test logistic regression model maintained good performance compared with the CTOT-08 dataset (0.81) (Figure 4D). The prediction by rejection type using gene expression profile, donor-derived cfDNA, and logistic regression on CTOT-08 and the external validation set is available as Supplemental Tables 3 and 4.
Discussion
We investigated the diagnostic performance of a blood gene expression profile biomarker and plasma donor-derived cfDNA assay in stable patients with kidney transplants and either normal surveillance biopsies or subclinical rejection. Importantly, this is the first study to define the performance characteristics of donor-derived cfDNA in a large, prevalent cohort of stable patients who all had surveillance kidney biopsies. With recent updates to the Banff classification (21), the bar was raised for the diagnosis of cellular rejection (with the elimination of the i0, t1 lesions in the borderline category). Conversely, we chose to be more inclusive for diagnosing antibody-mediated rejection (by including cases in the suspicious antibody-mediated rejection category, which we and others [19] believe represents consequential microvascular inflammation). The net effect was to increase the number of cases of subclinical antibody-mediated rejection and reduce the number of subclinical acute cellular rejection cases seen in our observational cohort. Our results need to be considered with this in mind, understanding that by providing the objective criteria used to assign clinical phenotypes, the performance of these biomarkers can be reinterpreted with potential changes to diagnostic categories in the future.
Whereas other groups have published the performance of donor-derived cfDNA in patients with graft dysfunction and for-cause biopsies (14) or in a high immunologic risk cohort (15), this series establishes the performance of donor-derived cfDNA as a screening test in a prevalent cohort of stable kidney recipients. In addition, this is the first publication to characterize the combined performance of donor-derived cfDNA and a gene expression profile test at the same time points, with all clinical phenotypes confirmed by surveillance biopsy. Although the two tests have similar overall sensitivity and specificity, the gene expression profile preferentially detects subclinical acute cellular rejection, whereas donor-derived cfDNA preferentially detects subclinical antibody-mediated rejection (Supplemental Tables 3–6). In the observational trial, subclinical acute cellular rejection tended to occur earlier in the 2-year follow-up than antibody-mediated rejection (except for patients who underwent positive crossmatch desensitization). This has important implications for their use in clinical practice in that detecting early cellular rejection using the gene expression profile may prevent antibody formation later post-transplant.
Our data also suggest that the reported performance of donor-derived cfDNA assays developed in cohorts of patients with both for-cause and protocol biopsies (16) should be interpreted with caution when applied to a population of stable patients.
Although both assays have similar rates of false positives (15% for gene expression profile and 12% for donor-derived cfDNA, respectively), they call a different set of samples falsely positive (Figure 3A). Similarly, there is a fair amount of nonoverlap in true positives called by each test (Figure 3). These data suggest that the tests can be used in a complementary fashion, detecting different cases of rejection. The performance metrics improved when both gene expression profile and donor-derived cfDNA results were considered together, primarily on the basis of the detection of specific histologic subtypes. Although the gene expression profile assay is currently given as a binary output, there is a graded risk of rejection as the value of the continuous output of both assays increases.
Our study has multiple strengths including that (1) the gene expression profile and donor-derived cfDNA were evaluated simultaneously with each biopsy, (2) the clinical trial used strictly defined and objective clinical criteria for subclinical rejection, (3) the study population was a representative US kidney transplant population, and (4) biopsies were read by a central pathologist to reduce interobserver variability.
Our study has several limitations. We focused on subclinical rejection and did not include for-cause biopsies. Additional analysis is ongoing to determine the performance of gene expression profile and donor-derived cfDNA in patients biopsied for graft dysfunction. Next, these samples represent a single point in time, not longitudinal changes of gene expression profile or donor-derived cfDNA. Third, active cases of infection (pyelonephritis and BK nephropathy) were excluded from the analysis. Therefore, the effects of active infection on gene expression profile and donor-derived cfDNA are not available. Lastly, de novo DSA information was not available at all biopsy time points in the trial, limiting the comparisons between de novo DSA as a biomarker for subclinical rejection compared with the gene expression profile and donor-derived cfDNA assays.
Blood-based biomarkers may allow less invasive, more frequent monitoring of kidney transplant recipients for subclinical rejection. Donor-derived cfDNA was significantly better at detecting subclinical antibody-mediated rejection when compared with the gene expression profile, and conversely, the gene expression profile was significantly better at detecting subclinical acute cellular rejection. When both gene expression profile and donor-derived cfDNA are negative or positive, their NPV or PPV is higher than either test alone. Combining the continuous output scores of both tests using a novel multivariable logistic regression model significantly improved the AUROC when compared with either test alone. Although neither positive test can yet replace a biopsy, their routine use can better inform the need for biopsy, reducing risk to patients and allowing more personalized management. Additional studies are required to determine the optimal timing and frequency of testing as well as the predictive ability of these biomarkers.
Disclosures
M.A. Abecassis reports consultancy agreements with, receiving research funding from, and serving as a scientific advisor or member of Eurorofins Transplant Diagnostics US–Transplant Genomics Inc. J.J. Friedewald reports consultancy agreements with Eurofins–Transplant Genomics Inc., Novartis, and Sanofi; receiving research funding from Abbvie, CSL Behring, Eurofins Viracor, Inc., Hansa, the National Institutes of Health, Veloxis, and Viela Bio; receiving honoraria from Novartis and Sanofi; patents and inventions with Northwestern University/Scripps Research Institute; serving as a scientific advisor or member of Eurofins–Transplant Genomics Inc.; and speakers bureau for Novartis and Sanofi. J. Holman reports employment with Transplant Genomics, Inc. and ownership interest in Eurofins. S. Kleiboeker reports employment with Eurofins Viracor Clinical Diagnostics and ownership interest in Eurofins. S.M. Kurian reports employment with Scripps Health, consultancy agreements with Transplant Genomics Inc., patents and inventions with Transplant Genomics Inc., and serving as a scientific advisor or member of MindX Sciences and Transplant Genomics Inc. E.D. Poggio reports consultancy agreements with Renalytix; receiving honoraria from CareDx, Novartis, and Reata; and serving on the advisory board for CareDx. R. Sinha reports employment with Eurofins Viracor; ownership interest in Amazon, Apple, Microsoft, Quantumscape, and TESLA; and serving on the reviewer board of Microorganisms. D.J. Taber reports receiving research funding from Astellas, CareDx, Novartis, and Veloxis; serving as a scientific advisor or member of the American Society of Transplantation Community of Practice; and serving on the advisory board for Sanofi-Aventis. J. Weems reports employment with Transplant Genomics Inc. All remaining authors have nothing to disclose.
Funding
S. Park acknowledges National Institute of Diabetes and Digestive and Kidney Diseases grant T32DK108738. The authors also acknowledge financial support in the form of National Institute of Allergy and Infectious Diseases grants U01 AI084146, 3U01 AI063594‐07S1, 1U01AI088635, 2U19 AI063603, and R34 AI118493 and from Eurofins–Viracor.
Supplementary Material
Acknowledgments
Portions of these data were presented in abstract form at the American Transplant Congress, June 2021.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Two Can Be Better Than One: Improving Noninvasive Diagnostics in Kidney Transplantation,” on pages 1462–1463.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05530421/-/DCSupplemental.
Supplemental Table 1. Summary of biopsy phenotypes on the basis of the prior Banff grading and the updated Banff 2019 classification.
Supplemental Table 2. Diagnostic performance of the donor-derived cfDNA assay depending on variable donor-derived cfDNA cutoff levels.
Supplemental Table 3. Summary of test results on all CTOT 08 samples by rejection type using the gene expression profile, donor-derived cfDNA, and the logistic regression model.
Supplemental Table 4. Prediction by rejection type using the gene expression profile, donor-derived cfDNA, and logistic regression on a 105-sample external validation set.
Supplemental Table 5. Gene expression profile and donor-derived cfDNA performance on the antibody-mediated rejection (n=65) and no rejection (n=325) groups.
Supplemental Table 6. Gene expression profile and donor-derived cfDNA performance on the acute cellular rejection (n=61) and no rejection (n=325) groups.
Supplemental Figure 1. Distribution of samples by clinical phenotype, gene expression profile probability score, and percentage donor-derived cfDNA.
References
- 1.Friedewald JJ, Kurian SM, Heilman RL, Whisenant TC, Poggio ED, Marsh C, Baliga P, Odim J, Brown MM, Ikle DN, Armstrong BD, Charette JI, Brietigam SS, Sustento-Reodica N, Zhao L, Kandpal M, Salomon DR, Abecassis MM; Clinical Trials in Organ Transplantation 08 (CTOT-08) : Development and clinical validity of a novel blood-based molecular biomarker for subclinical acute rejection following kidney transplant. Am J Transplant 19: 98–109, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Yi Z, Keung KL, Shang H, Wei C, Cravedi P, Sun Z, Xi C, Woytovich C, Farouk S, Huang W, Banu K, Gallon L, Magee CN, Najafian N, Samaniego M, Djamali A, Alexander SI, Rosales IA, Smith RN, Xiang J, Lerut E, Kuypers D, Naesens M, O’Connell PJ, Colvin R, Menon MC, Murphy B: A peripheral blood gene expression signature to diagnose subclinical acute rejection. J Am Soc Nephrol 30: 1481–1494, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seifert ME, Yanik MV, Feig DI, Hauptfeld-Dolejsek V, Mroczek-Musulman EC, Kelly DR, Rosenblum F, Mannon RB: Subclinical inflammation phenotypes and long-term outcomes after pediatric kidney transplantation. Am J Transplant 18: 2189–2199, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta R, Bhusal S, Randhawa P, Sood P, Cherukuri A, Wu C, Puttarajappa C, Hoffman W, Shah N, Mangiola M, Zeevi A, Tevar AD, Hariharan S: Short-term adverse effects of early subclinical allograft inflammation in kidney transplant recipients with a rapid steroid withdrawal protocol. Am J Transplant 18: 1710–1717, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Mehta RB, Tandukar S, Jorgensen D, Randhawa P, Sood P, Puttarajappa C, Zeevi A, Tevar AD, Hariharan S: Early subclinical tubulitis and interstitial inflammation in kidney transplantation have adverse clinical implications. Kidney Int 98: 436–447, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Seifert ME, Agarwal G, Bernard M, Kasik E, Raza SS, Fatima H, Gaston RS, Hauptfeld-Dolejsek V, Julian BA, Kew CE, Kumar V, Mehta S, Ong S, Rosenblum F, Towns G, Mannon RB: Impact of subclinical borderline inflammation on kidney transplant outcomes. Transplant Direct 7: e663, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szederkényi E, Iványi B, Morvay Z, Szenohradszki P, Borda B, Marofka F, Kemény E, Lázár G: Treatment of subclinical injuries detected by protocol biopsy improves the long-term kidney allograft function: A single center prospective randomized clinical trial. Transplant Proc 43: 1239–1243, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Kurtkoti J, Sakhuja V, Sud K, Minz M, Nada R, Kohli HS, Gupta KL, Joshi K, Jha V: The utility of 1- and 3-month protocol biopsies on renal allograft function: A randomized controlled study. Am J Transplant 8: 317–323, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Parajuli S, Joachim E, Alagusundaramoorthy S, Blazel J, Aziz F, Garg N, Muth B, Mohamed M, Mandelbrot D, Zhong W, Djamali A: Subclinical antibody-mediated rejection after kidney transplantation: Treatment outcomes. Transplantation 103: 1722–1729, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Lee DM, Abecassis MM, Friedewald JJ, Rose S, First MR: Kidney graft surveillance biopsy utilization and trends: Results from a survey of high-volume transplant centers. Transplant Proc 52: 3085–3089, 2020 [DOI] [PubMed] [Google Scholar]
- 11.First MR, Rose S, Schieve C, Lee D, Lewis P, Pierry D, David J, McNulty M, Clark D, Weiss G, Kurian S, Whisenant T, Friedewald JJ, Abecassis MM: Value of the TruGraf blood test as a biomarker for monitoring renal transplant recipients. Insights Biomed 3: 8, 2018 [Google Scholar]
- 12.Marsh CL, Kurian SM, Rice JC, Whisenant TC, David J, Rose S, Schieve C, Lee D, Case J, Barrick B, Peddi VR, Mannon RB, Knight R, Maluf D, Mandelbrot D, Patel A, Friedewald JJ, Abecassis MM, First MR: Application of TruGraf v1: A novel molecular biomarker for managing kidney transplant recipients with stable renal function. Transplant Proc 51: 722–728, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Peddi VR, Patel PS, Schieve C, Rose S, First MR: Serial peripheral blood gene expression profiling to assess immune quiescence in kidney transplant recipients with stable renal function. Ann Transplant 25: e920839, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloom RD, Bromberg JS, Poggio ED, Bunnapradist S, Langone AJ, Sood P, Matas AJ, Mehta S, Mannon RB, Sharfuddin A, Fischbach B, Narayanan M, Jordan SC, Cohen D, Weir MR, Hiller D, Prasad P, Woodward RN, Grskovic M, Sninsky JJ, Yee JP, Brennan DC; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators : Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 28: 2221–2232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang E, Sethi S, Peng A, Najjar R, Mirocha J, Haas M, Vo A, Jordan SC: Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am J Transplant 19: 1663–1670, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Sigdel TK, Archila FA, Constantin T, Prins SA, Liberto J, Damm I, Towfighi P, Navarro S, Kirkizlar E, Demko ZP, Ryan A, Sigurjonsson S, Sarwal RD, Hseish SC, Chan-On C, Zimmermann B, Billings PR, Moshkevich S, Sarwal MM: Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J Clin Med 8: E19, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiebe C, Rush DN, Gibson IW, Pochinco D, Birk PE, Goldberg A, Blydt-Hansen T, Karpinski M, Shaw J, Ho J, Nickerson PW: Evidence for the alloimmune basis and prognostic significance of Borderline T cell-mediated rejection. Am J Transplant 20: 2499–2508, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crew RJ, Khairallah P, Husain SA: Cell-free DNA: Proceed, but with caution. J Am Soc Nephrol 31: 2491–2492, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callemeyn J, Ameye H, Lerut E, Senev A, Coemans M, Van Loon E, Sprangers B, Van Sandt V, Rabeyrin M, Dubois V, Thaunat O, Kuypers D, Emonds MP, Naesens M: Revisiting the changes in the Banff Classification for antibody-mediated rejection after kidney transplantation. Am J Transplant 21: 2413–2423, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Arias M, Serón D, Herrero I, Rush DN, Wiebe C, Nickerson PW, Ussetti P, Rodrigo E, de Cos MA: Subclinical antibody-mediated rejection. Transplantation 101: S1–S18, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M: The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant 20: 2318–2331, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurian SM, Williams AN, Gelbart T, Campbell D, Mondala TS, Head SR, Horvath S, Gaber L, Thompson R, Whisenant T, Lin W, Langfelder P, Robison EH, Schaffer RL, Fisher JS, Friedewald J, Flechner SM, Chan LK, Wiseman AC, Shidban H, Mendez R, Heilman R, Abecassis MM, Marsh CL, Salomon DR: Molecular classifiers for acute kidney transplant rejection in peripheral blood by whole genome gene expression profiling. Am J Transplant 14: 1164–1172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First M, Kleiboeker S, Rose S, Friedewald JJ, Abecassis MM: The real-life experience of developing and commercializing TruGraf, a validated non-invasive transplant biomarker. J Biochem Anal. Stud 4: 1–8, 2020 [Google Scholar]
- 24.Sharon E, Shi H, Kharbanda S, Koh W, Martin LR, Khush KK, Valantine H, Pritchard JK, De Vlaminck I: Quantification of transplant-derived circulating cell-free DNA in absence of a donor genotype. PLOS Comput Biol 13: e1005629, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.