Abstract
We have used fluorescent amplified-fragment length polymorphism (FAFLP) analysis to subtype clinical isolates of Streptococcus pyogenes serotype M1. Established typing methods define most M1 isolates as members of a clone that has a worldwide distribution and that is strongly associated with invasive diseases. FAFLP analysis simultaneously sampled 90 to 120 loci throughout the M1 genome. Its discriminatory power, precision, and reproducibility were compared with those of other molecular typing methods. Irrespective of disease symptomatology or geographic origin, the majority of the clinical M1 isolates shared a single ribotype, pulsed-field gel electrophoresis macrorestriction profile, and emm1 gene sequence. Nonetheless, among these isolates, FAFLP analysis could differentiate 17 distinct profiles, including seven multi-isolate groups. The FAFLP profiles of M1 isolates reproducibly exhibited between 1 and more than 20 amplified fragment differences. The high discriminatory power of genotyping by FAFLP analysis revealed genetic microheterogeneity and differentiated otherwise “identical” M1 isolates as members of a clone complex.
Streptococcus pyogenes, the group A streptococcus (GAS), is the etiological agent of diverse invasive and noninvasive diseases. A primary virulence factor, the M protein, is encoded by the emm gene and provides the basis for seroepidemiology (11, 12).
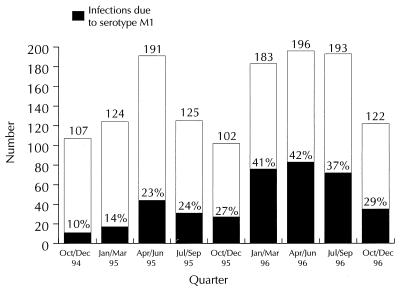
The last 10 years have seen a worldwide resurgence of severe invasive GAS disease caused predominantly by serotypes M1 and M3. M1 has been strongly associated with rapidly fulminating invasive infections (21). The Public Health Laboratory Service Enhanced Surveillance Study of invasive GAS infections (1994 to 1997) for England and Wales (Fig. 1) indicated an increase, with the overall predominant serotype (30%) being M1 (6). A similar proportion of M1 infections has been reported in North America (3).
FIG. 1.
Proportion of invasive GAS disease due to serotype M1 over a 2-year period (PHLS Enhanced Surveillance Study data [6]). Open columns and numbers represent the total of invasive GAS infections, while the shaded areas indicate the percentage of infections caused by M1 isolates.
Cleary et al. (2) reported on the worldwide emergence of a highly virulent M1 “clone” expressing the streptococcal pyogenic exotoxin A (SPEA). Musser et al. (15) further characterized the genotype of this M1 “subclone” as multilocus electropherotype ET1 and pulsed-field gel electrophoresis (PFGE) type 1a and found that all members of the subclone possessed identical sequences for the emm1, speA, and ska genes. It was identified from many patients with invasive disease in Finland and Norway (13).
Established molecular methods previously applied to GAS include multilocus enzyme electrophoresis (14), restriction endonuclease analysis (2), ribotyping (19), PCR-restriction fragment length polymorphism (PCR-RFLP) analysis or sequencing of the emm gene (1, 19), and PFGE (18, 20).
Amplified-fragment length polymorphism analysis, a PCR-based technique (25), has been used with radioactive labelling to demonstrate strain heterogeneity in several bacterial genera (9, 10, 24). Those studies made no attempt to quantify its discriminatory power. We have previously shown that fluorescent amplified-fragment length polymorphism (FAFLP) analysis, in which one PCR primer is labelled with a fluorescent dye and the products are separated on an automated DNA sequencer, can successfully resolve a cluster of isolates recovered from a temporally and geographically related outbreak of GAS (5). The objective of the present study, on the other hand, was to establish whether FAFLP analysis could accurately and reproducibly demonstrate microheterogeneity within a “strain” which by all other molecular techniques was regarded as a clone. We chose to analyze the established M1 subclone of S. pyogenes and compare FAFLP analysis with existing molecular typing methods.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The type strain (NCTC 8198), 2 reference strains (NCTC 2218 and NCTC 8370), and 37 clinical isolates (recovered from 1994 to 1995) of S. pyogenes serotype M1 were analyzed (Table 1). Clinical isolates were from the Streptococcus and Diphtheria Reference Unit, while type and reference strains were from the National Collection of Type Cultures (NCTC; Central Public Health Laboratory, London, United Kingdom). These 3 strains and 35 of the 37 clinical isolates contained the pyrogenic exotoxin gene speA. All 40 lacked the insertion element IS1239 (19). Streptococci were cultured aerobically at 37°C for 18 to 24 h on horse blood agar plates, and stock cultures were preserved in blood glycerol (16%; vol/vol) broth (Oxoid, Basingstoke, United Kingdom) at −70°C. Isolates were serotyped before and after genotyping by conventional methods (11, 12).
TABLE 1.
S. pyogenes isolates and their genotypes
| Isolate no. | Source of isolation/ disease | Combined 16S ribo-typea | Combined mrp typeb | FAFLP profilec |
|---|---|---|---|---|
| R2590 | Hampshire/cellulitis | R-2 | mrp1.6 | A1 |
| R2335 | Wigan/cellulitis | R-2 | mrp1.6 | A1 |
| R2242 | Oxford/septicemia | R-2 | mrp1.6 | A1 |
| R0027 | Nottingham/pneumonia | R-2 | mrp1.6 | A1 |
| R0214 | Norwich/septicemia | R-2 | mrp1.6 | A1 |
| R2588 | Doncaster/pneumonia | R-2 | mrp1.6 | A1 |
| R2657 | London/bacteremia | R-2 | mrp1.6 | A2 |
| R2419 | Salisbury/septicemia | R-2 | mrp1.6 | A2 |
| R1919 | Bishop Auckland/skin infection | R-2 | mrp1.6 | A3 |
| R1567 | Kent/umbilical swab | R-2 | mrp1.6 | A3 |
| R1990 | Basildon/pharyngitis | R-2 | mrp1.6 | A3 |
| R1763 | Blackpool/sore throat | R-2 | mrp1.6 | A3 |
| R0112 | Blackpool/unknown | R-2 | mrp1.6 | A4 |
| R0109 | Nottingham/cellulitis | R-2 | mrp1.6 | A4 |
| R2420 | Salisbury/cellulitis | R-2 | mrp1.6 | A5 |
| R1870 | Manchester/renal disease | R-2 | mrp1.6 | A5 |
| R1848 | Barnsley/vaginitis | R-2 | mrp1.6 | A5 |
| R2193 | Nottingham/sore throat | R-2 | mrp1.6 | A5 |
| R1934 | Exeter/tonsillitis | R-2 | mrp1.6 | A6 |
| R2179 | Nottingham/pneumonia | R-2 | mrp1.6 | A6 |
| R2424 | Macclesfield/necrotizing fasciitis | R-3 | mrp1.6 | A6 |
| R2656 | London/bacteremia | R-2 | mrp1.6 | A6 |
| R2132 | Surrey/septicemia | R-2 | mrp1.6 | A7 |
| R0129 | West Suffolk/pharyngitis | R-2 | mrp1.6 | A7 |
| R2646 | Manchester/septic arthritis | R-2 | mrp1.6 | A7 |
| R0181 | West Suffolk/impetigo | R-2 | mrp1.6 | A8 |
| R2211 | Carmarthen/vaginitis | R-2 | mrp1.6 | A9 |
| R1630 | Leeds/rheumatic fever | R-2 | mrp1.6 | A10 |
| R2468 | Manchester/toxic shock syndrome | R-2 | mrp1.6 | A11 |
| R0019 | London/infected burns | R-2 | mrp1.6 | A12 |
| R2326 | Salisbury/cellulitis | R-2 | mrp1.6 | A13 |
| R2609 | Plymouth/necrotizing fasciitis | R-2 | mrp1.6 | A14 |
| R2509 | Rhyl/bacteremia | R-2 | mrp1.6 | A15 |
| R1968 | Durham/eye infection | R-2 | mrp1.6 | A16 |
| R2437 | Lancaster/pyrexia | R-2 | mrp1.6a | A17 |
| NCTC 8370d | London/scarlet fever | R-2 | mrp1.5 | A18 |
| R583d | Unknown/unknown | R-5 | mrp1.4 | A19 |
| NCTC 2218d | Aberdeen/scarlet fever | R-6 | mrp1.3 | A20 |
| R2518d | Unknown/unknown | R-4 | mrp1.2 | A21 |
| NCTC 8198d | Manchester/scarlet fever | R-1 | mrp1.1 | A22 |
Numbers following the combined ribotype (R; obtained with EcoRI and SacI) indicate different patterns of hybridization with the probe.
Numbers following the combined mrp indicate a unique RFLP obtained by combining the results of digestion with three endonucleases (SmaI, SfiI, and NgoAIV).
FAFLP profiles obtained with the EcoRI+0 and MseI+T primer pair.
Isolates with early isolation dates.
emm gene polymorphism (PCR-RFLP analysis and sequencing).
The “all-M” PCR primers and conditions of Podbielski et al. (17) were used to amplify the emm gene. RFLP analysis of emm1 amplicons was carried out as described previously (19). All amplicons were purified with GeneClean (Bio 101) and were subjected to cycle sequencing with the all-M forward primer of Podbielski et al. (17) with the PRISM Dye Terminator Cycle Sequencing kit. Analysis was performed on an ABI 373A DNA sequencer.
PFGE and tree construction.
PFGE was carried out following macrorestriction with three enzymes (SmaI, SfiI, and NgoAIV) as described previously (4). Genetic relationships between the isolates were then estimated by applying the equation of Nei and Li (16) to calculate distance (D) values (additive for all enzymes), and a distance matrix was constructed. The resulting estimates of overall restriction site similarity were used to construct an unrooted tree by applying the FITCH option of the PHYLIP computer package (8).
Minipreparation of genomic DNA and 16S ribotyping.
Genomic DNA was extracted from streptococcal plate cultures as described previously (20), digested with an endonuclease (XhoI, EcoRI, or SacI), electrophoresed, blotted, and hybridized with a 1,500-bp S. pyogenes 16S rRNA gene probe as described previously (20). Membrane filters were developed colorimetrically and were scanned directly (ScanMaker IIG; Microtek Lab, Redondo Beach, Calif.) into a Power Macintosh 6100/60 (Apple Computer, Cupertino, Calif.).
FAFLP analysis.
FAFLP analysis was performed with DNA extracted from M1 isolates as described previously (5). FAFLP products were separated on an ABI Prism 377 automated DNA sequencer as described previously (5), with modifications as follows. The Premix Long Ranger polyacrylamide gel solution (FMC BioProducts, Vallensbaek Strand, Denmark) was used for the gel. The reaction mixtures used for FAFLP analysis were diluted 1:3, and 1.5 μl was added to 3.5 μl of loading dye (2.5 μl of formamide, 0.5 μl of dextran blue, and 0.5 μl of ROX-2500 internal lane standard). The electrophoresis conditions were as described previously (5).
RESULTS
Polymorphism of the emm1 gene.
PCR amplicons were generated from the emm1 genes of all isolates and analyzed for HaeIII polymorphisms as described previously (19). Two RFLPs (subtypes 1.H1 and 1.H2) were found among the 40 isolates. All but one isolate (NCTC 2218) shared subtype 1.H1. The sizes of the two amplicons calculated from the sum of the sizes of the HaeIII fragments were approximately 1,460 bp (emm1.H1) and 1,200 bp (emm1.H2).
The 5′ regions (280 bp) of the amplicons from all isolates were sequenced and aligned with the MegAlign module of the Lasergene software (DNASTAR). All amplicons contained the nucleotide sequence 5′-TCGCTTAGAAAATTAA-3′, which distinguishes sequences in the conserved regions of the emm genes from the corresponding regions of enn or mrp genes (26, 27). Thirty-five of the 39 isolates which exhibited RFLP subtype 1.H1 had nucleotide sequences identical to that previously published for the M1 type strain (27). Of the remaining four amplicons with RFLP subtype 1.H1, two had a single-base substitution (G→T; 1 at position 144 [isolate R2609] and the other at position 256 [isolate R1968]), one (isolate R2193) had a 3-base deletion (AAA) at position 34 and a single base substitution at position 80 (A→G), while the fourth one (isolate R2437) had two single-base substitutions at position 139 (T→C) and position 144 (G→T). RFLP subtype 1.H2 exhibited 34% sequence divergence from the predominant (and type strain) emm1.H1 sequence (data not shown).
16S ribotypes.
Two XhoI RFLPs were detected; 95% of isolates shared one of these ribotypes, ribotype X1. Of two EcoRI ribotypes detected, 98% of isolates shared ribotype E1. Among six SacI ribotypes detected, 88% of isolates shared ribotype S2. By sequential addition of data, six combined ribotypes were derived; thus, the ribotype for isolates with ribotypes E1, X1, and S1 was termed combined ribotype R-1, while the ribotype for those with ribotypes E11, X2, and S24 was termed combined ribotype R-6. By this analysis, 35 M1 isolates were found to share combined ribotype R-2. The remaining five isolates, four of which had early isolation dates, had unique combined ribotypes.
PFGE and macrorestriction profiles.
Six macrorestriction profiles (mrps) were found among the 40 isolates with SmaI, six were found with SfiI, and six were found with NgoAIV. Eighty-five percent of isolates shared a principal SmaI mrp and one principal SfiI mrp. Eighty-eight percent shared one NgoAIV mrp.
The individual mrps obtained with three endonucleases were combined to give an mrp type (Table 1). Each designated mrp type was differentiated by at least three band differences with at least one endonuclease. If there were fewer than three band differences with any endonuclease, then the type was designated clonally related by a postscript letter. For example, mrp1.6a was related to mrp1.6 by fewer than three band differences in its SmaI and SfiI profiles. Again, isolates with SmaI mrp1, SfiI mrp1, and NgoAIV mrp1 were designated mrp1.1, while mrp1.2 represented the combination SmaI mrp5, SfiI mrp4, and NgoAIV mrp4.
Analysis of genetic relationships by established techniques.
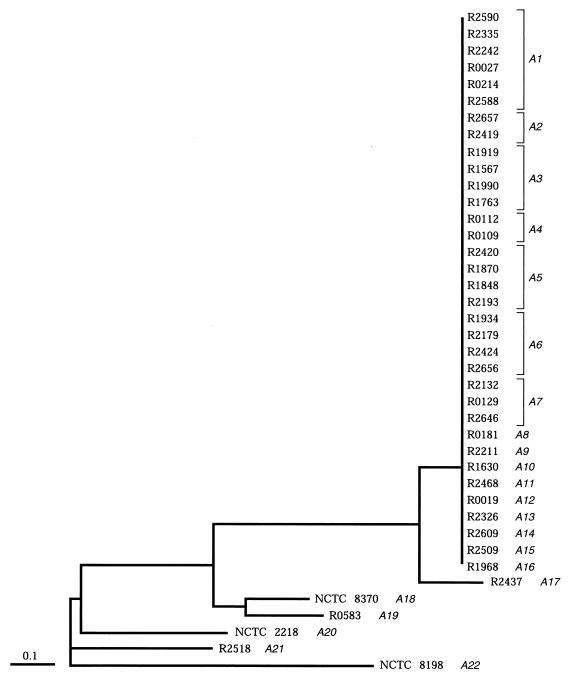
Distance matrices for the three macrorestriction profiles were added for all isolates and were used to construct a dendrogram (Fig. 2). Its striking feature was that a large group (34 contemporary isolates) had identical macrorestriction profiles for all three enzymes. These isolates also shared a ribotype (R-2) and an emm gene subtype (1.H1). One contemporary isolate with a minor mrp difference (fewer than three bands) also carried this emm1 subtype and was therefore considered to be closely related to the main mrp grouping. The five remaining strains had distinct mrps and ribotypes. Four strains (isolated in 1950, 1952, 1972, and 1973, respectively) shared emm subtype 1.H1, but one strain (isolated in 1926) had a unique emm subtype, subtype 1.H2.
FIG. 2.
Dendrogram showing the relationships between serotype M1 isolates, inferred from overall restriction site similarities (on PFGE) estimated with the equation of Nei and Li (16). These were used to construct an unrooted tree with the FITCH option of the PHYLIP package. The corresponding FAFLP profiles are shown in italics, next to the strain numbers. All isolates except strain NCTC 2218 exhibited emm1 RFLP type 1.H1.
FAFLP analysis.
FAFLP analysis with a nonselective primer (EcoRI+0) and a selective primer (MseI+T) (5) generated 90 to 120 amplified fragments ranging in size from 60 to 600 bp. Experiments with a subset of 15 isolates established that reactions performed from the same DNA extract(s) or from serial DNA extracts made from the same isolate(s) at different times yielded reproducible amplified fragment profiles. When these reactions were run on the same and on different gels, the sizes of the fragments did not differ by more than 1.0 bp. In-house studies have established the reproducibility of FAFLP analysis in experiments in which five different scientists have performed the technique from DNA extraction to final profiling for the same isolate (3a). Primary fluorescent band profiles were transformed to electropherograms, and these were overlaid for visual scrutiny. Peak height is a measure of the fluorescence detected at a given datum point (±1.0 bp for the ABI 377 automated sequencer). The heights of all peaks constituting a profile are governed by PCR efficiency and are normalized by the GeneScan analysis software.
Twenty-two distinct profiles were detected among the 40 isolates. Fifteen isolates had unique FAFLP profiles; they included five of the isolates that were also differentiated by PFGE. The other 25 isolates were assigned to seven FAFLP profiles: 6 were assigned to profile A1, 4 each were assigned to profiles A3, A5, and A6, 3 were assigned to profile A7, and 2 each were assigned to profiles A2 and A4 (Table 1; Fig. 2). The majority of isolates, those having mrp1.6 and emm subtype 1.H1 (i.e., members of the M1 subclone), exhibited various combinations of 11 polymorphic amplified fragments (summarized in Table 2). Five isolates with unique mrp profiles and early isolation dates (Table 1) exhibited further FAFLP polymorphisms (data not shown).
TABLE 2.
Polymorphisms of FAFLP profiles exhibited by M1 subclone isolatesa
| FAFLP profile | Presence of polymorphic fragments of the following size (bp)b:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 73 | 99 | 129 | 258 | 316 | 328 | 365 | 367 | 382 | 510 | 511 | |
| A1 | + | − | + | − | + | − | + | + | − | − | + |
| A2 | + | − | + | − | + | − | + | − | − | − | + |
| A3 | + | − | + | + | + | − | + | + | − | + | + |
| A4 | + | − | + | − | − | − | + | + | − | + | + |
| A5 | + | − | + | + | + | − | + | + | + | + | + |
| A6 | + | − | + | + | + | − | + | − | − | + | + |
| A7 | + | − | + | + | + | − | + | − | + | + | + |
| A8 | + | − | + | − | + | − | − | + | − | − | + |
| A9 | + | − | + | − | + | − | + | + | − | + | + |
| A10 | + | − | + | + | − | − | + | + | + | + | + |
| A11 | + | − | + | + | + | − | + | − | − | + | − |
| A12 | + | − | + | + | − | − | + | − | − | + | + |
| A13 | + | − | + | − | − | − | + | − | − | + | + |
| A14 | + | + | + | + | + | − | + | + | + | + | + |
| A15 | + | − | − | + | + | + | + | + | + | + | + |
| A16c | − | + | + | − | + | + | + | + | − | + | + |
| A17c | − | + | + | − | + | − | + | + | − | − | − |
FAFLP profiles were obtained with the EcoRI+0 and MseI+T primer pair. All the isolates are identical by PFGE, ribotyping, and emm1 subtype (Table 1).
The presence or absence of differential fragments is shown. +, a fragment characteristically present in that FAFLP profile; −, characteristic absence of fragment from that FAFLP profile.
Isolates with these two FAFLP profiles exhibited further polymorphisms (data not shown).
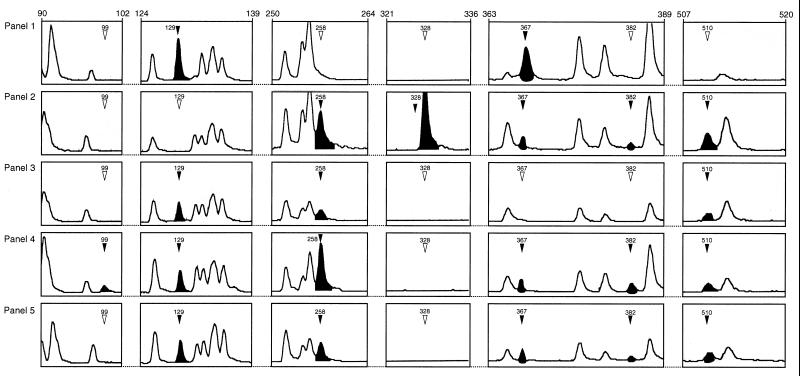
Examples of areas of FAFLP polymorphism within the M1 subclone are shown in Fig. 3. These five electropherograms represent some of the 17 FAFLP profiles found within the subclone defined by PFGE (mrp1.6 or mrp1.6a). For example, FAFLP profile A1 (Fig. 3, panel 1) contained six characteristic fragments of 73, 129, 316, 365, 367, and 511 bp and lacked five amplified fragments found in other FAFLP profiles for the subclone. FAFLP profile A15 contained nine characteristic fragments of 73, 258, 316, 328, 365, 367, 382, 510, and 511 bp, while it lacked two fragments found in the FAFLP profiles of other subclones. A fragment of 352 bp was present in M1 subclone isolates but not in all type and reference strains. Conversely, fragments of 244, 358, and 364 bp were absent from M1 subclone isolates but were present in some type and reference strains.
FIG. 3.
GeneScan 2.1 software-derived electropherograms showing examples of areas of polymorphism within FAFLP profiles for EcoRI+0 plus MseI+T amplifications of five GAS genomes. Panels 1 to 5 correspond to FAFLP profiles A1, A15, A6, A14, and A5, respectively. The solid arrowheads and peaks indicate a fragment characteristic of that profile (sizes are indicated in base pairs). Open arrowheads indicate the absence of a polymorphic fragment from that profile.
DISCUSSION
We found that 35 contemporary United Kingdom M1 isolates had identical ribotypes, mrps, and (with two minor variations) emm1 gene sequences. They were etiological agents of diseases ranging from pharyngitis to pneumonia and renal disease to necrotizing fasciitis (Table 1) but could not be differentiated by published methods. By contrast, FAFLP analysis readily subtyped them, grouping 25 of the 35 isolates into seven (multi-isolate) profiles and assigning further individual profiles to the remainder of the isolates. The number of band differences defining FAFLP profiles (within the M1 subclone) varied from 1 to more than 20 (examples are shown in Fig. 3). These were reproducible and made up of precisely sized (±1.0-bp) amplified fragments.
The present study is too small to allow us to reach general conclusions about disease and strain genotype. Nonetheless, we observed three kinds of associations with multi-isolate FAFLP profiles. These were the association of profile A1 and all invasive disease isolates, profile A3 and all superficial disease isolates, and profile A6 and a mixture of invasive and superficial disease isolates. PFGE, by contrast, typed all M1 subclone isolates into the third (mixed) class. The discriminatory power and reproducibility of FAFLP analysis therefore offer a valuable tool for epidemiological analysis of M1. For example, isolates with identical PFGE profiles from the same geographical area (e.g., Nottingham, Blackpool, or Manchester, United Kingdom; Table 1) had distinct FAFLP profiles and could not have constituted sources of an outbreak.
As further studies are completed, interpretative criteria should be defined for FAFLP profiles by appropriate interlaboratory and international collaboration. Criteria for assessment of the genetic relatedness of PFGE profiles of isolates were proposed (23). These criteria relate to macrorestriction band shifts on PFGE gels. In FAFLP analysis, by contrast, even a single fragment which distinguishes a profile is a PCR amplicon which was precisely sized, reproducibly found, and amplified under stringent conditions. For either technique, in clinical and public health practice, the determinants of a profile cannot be separated from the epidemiological data. The present study shows that FAFLP analysis found considerable microheterogeneity among M1 isolates which shared a single PFGE profile and implies that they constitute a clone complex. Hypervariability of the sic gene (which encodes the streptococcal inhibitor of complement), recently found for strains of serotype M1 (22), is concordant with the FAFLP analysis data. The latter are based on simultaneous PCR sampling of multiple anonymous loci throughout the genome rather than one gene.
As the power to characterize strains improves, the number of differences that can be detected increases and the degree of apparent clonality recedes (7). The results presented in this study suggest that FAFLP analysis could subtype other pathogenic bacterial clones such as epidemic phage types of methicillin-resistant Staphylococcus aureus or of Salmonella enteritidis.
ACKNOWLEDGMENTS
We thank Jon Clewley for assistance with the dendrogram and Philip Mortimer for critically reading the manuscript.
REFERENCES
- 1.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet. 1992;339:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 3.Davies H D, McGeer A, Schwartz B, Green K, Cann D, Simor A, Low A E, Low D E Ontario Group A Streptococcal Study Group. Invasive group A streptococcal infections in Ontario, Canada. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 3a.Desai, M., and J. Stanley. Unpublished data.
- 4.Desai M, Tanna A, Efstratiou A, George R, Clewley J, Stanley J. Extensive genetic diversity among clinical isolates of Streptococcus pyogenes serotype M5. Microbiology. 1998;144:629–637. doi: 10.1099/00221287-144-3-629. [DOI] [PubMed] [Google Scholar]
- 5.Desai M, Tanna A, Wall R, Efstratiou A, George R, Stanley J. Fluorescent amplified-fragment length polymorphism analysis of an outbreak of group A streptococcal invasive disease. J Clin Microbiol. 1998;36:3133–3137. doi: 10.1128/jcm.36.11.3133-3137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efstratiou A, George R C, Gaworzewska E T, Hallas G, Tanna A, Blake W A, Monnickendam M A, McEvoy M B. Group A streptococcal invasive disease in England and Wales. Adv Exp Med Biol. 1997;418:207–210. doi: 10.1007/978-1-4899-1825-3_49. [DOI] [PubMed] [Google Scholar]
- 7.Eisenstein B I. New molecular techniques for microbial epidemiology and the diagnosis of infectious diseases. J Infect Dis. 1990;161:595–602. doi: 10.1093/infdis/161.4.595. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein J. PHYLIP: phylogenetic inference package, version 3.0. Seattle: University of Washington; 1988. [Google Scholar]
- 9.Huys G, Kersters I, Coopman R, Janssen P, Kersters K. Genotypic diversity among Aeromonas isolates recovered from drinking water production plants as revealed by AFLPTM analysis. Syst Appl Microbiol. 1996;19:428–435. [Google Scholar]
- 10.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D R, Kaplan E L, Sramek J, Bicova R, Havlicek J, Havlickova H, Motlova J, Kriz P. Laboratory diagnosis of group A streptococcal infections. Geneva, Switzerland: World Health Organization Oriental Press; 1996. [Google Scholar]
- 12.Lancefield R C. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 1933;57:571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muotiala A, Seppala H, Huovinen P, Vuopio-Varkila J. Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis. 1997;175:392–399. doi: 10.1093/infdis/175.2.392. [DOI] [PubMed] [Google Scholar]
- 14.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musser J M, Kapur V, Szeto J, Pan X, Swanson D S, Martin D R. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect Immun. 1995;63:994–1003. doi: 10.1128/iai.63.3.994-1003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nei M, Li W. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podbielski A, Melzer B, Lutticken R. Application of the polymerase chain reaction to study the M protein (-like) gene family in beta-hemolytic streptococci. Med Microbiol Immunol. 1991;180:213–227. doi: 10.1007/BF00215250. [DOI] [PubMed] [Google Scholar]
- 18.Single L A, Martin D R. Clonal differences within M-types of the group A Streptococcus revealed by pulsed field gel electrophoresis. FEMS Microbiol Lett. 1992;70:85–89. doi: 10.1016/0378-1097(92)90567-8. [DOI] [PubMed] [Google Scholar]
- 19.Stanley J, Desai M, Xerry J, Tanna A, Efstratiou A, George R. High-resolution genotyping elucidates the epidemiology of group A streptococcal outbreaks. J Infect Dis. 1996;174:500–506. doi: 10.1093/infdis/174.3.500. [DOI] [PubMed] [Google Scholar]
- 20.Stanley J, Linton D, Desai M, Efstratiou A, George R. Molecular subtyping of prevalent M serotypes of Streptococcus pyogenes causing invasive disease. J Clin Microbiol. 1995;33:2850–2855. doi: 10.1128/jcm.33.11.2850-2855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 22.Stockbauer K E, Grigsby D, Pan X, Fu Y X, Mejia L M, Cravioto A, Musser J M. Hypervariability generated by natural selection in an extracellular complement-inhibiting protein of serotype M1 strains of group A Streptococcus. Proc Natl Acad Sci USA. 1998;95:3128–3133. doi: 10.1073/pnas.95.6.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valsangiacomo C, Baggi F, Gaia V, Balmelli T, Peduzzi R, Piffaretti J. Use of amplified fragment length polymorphism in molecular typing of Legionella pneumophila and application to epidemiological studies. J Clin Microbiol. 1995;33:1716–1719. doi: 10.1128/jcm.33.7.1716-1719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kulper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whatmore A M, Kapur V, Sullivan D J, Musser J M, Kehoe M A. Non-congruent relationships between variation in emm gene sequences and the population genetic structure of group A streptococci. Mol Microbiol. 1994;14:619–631. doi: 10.1111/j.1365-2958.1994.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 27.Whatmore A M, Kehoe M A. Horizontal gene transfer in the evolution of group A streptococcal emm-like genes: gene mosaics and variation in Vir regulons. Mol Microbiol. 1994;11:363–374. doi: 10.1111/j.1365-2958.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]