Abstract
With increasing presentations of headaches following COVID-19 vaccination, we present one of the UK’s earliest proven cases of vaccine-induced thrombotic thrombocytopaenia (VITT), with the aim of giving colleagues a case to compare other patients against. Our patient was a 48-year-old man who presented with frank haematuria, a widespread petechial rash, and headaches, 2 weeks after receiving the first dose of the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine. He had a platelet count of 14×109/L and an extensive cerebral venous sinus thrombosis (CVST) with subarachnoid haemorrhage on imaging. He developed localising neurological signs and experienced a cardiopulmonary arrest. He was successfully resuscitated and transferred to a tertiary care centre for urgent thrombectomy. This case illustrates how the diagnosis of VITT should be based on the platelet count and imaging—and how patients with VITT should be cared for in centres with urgent neurosurgical and interventional radiology services.
Keywords: COVID-19, emergency medicine, general practice / family medicine, haematology (incl blood transfusion), public health
Background
With increasing presentations of patients with headaches following COVID-19 vaccination, we present one of the UK’s earliest confirmed cases of vaccine-induced thrombotic thrombocytopaenia (VITT) that was seen at our hospital during the UK’s spring 2021 vaccination programme.
This case illustrates how VITT presents and how the diagnosis can be made. We hope that it will serve as a guide to differentiate true cases of VITT from patients who are well but otherwise worried.
Case presentation
We present the case of a 48-year-old man who, 13 days after receiving the first dose of his Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine, attended with a sudden onset of frank haematuria and a widespread fine petechial rash. He also complained of an evolving generalised headache.
He had a background of antibiotic-treated prostatitis, eczema, asthma and essential hypertension only. The patient had no previous infection with COVID-19, no exposure to a COVID-19 vaccine prior to this first dose of the AstraZeneca vaccine, and no background or family history of hypercoagulable states or thrombocytopaenia.
A routine blood panel revealed a platelet count of 14×109/L and a D-dimer level of 62 342 ng/mL. Clotting function and haemoglobin were in the normal range. A blood film demonstrated left shift myeloid cells with metamyelocytes and myelocytes, with an occasional giant platelet.
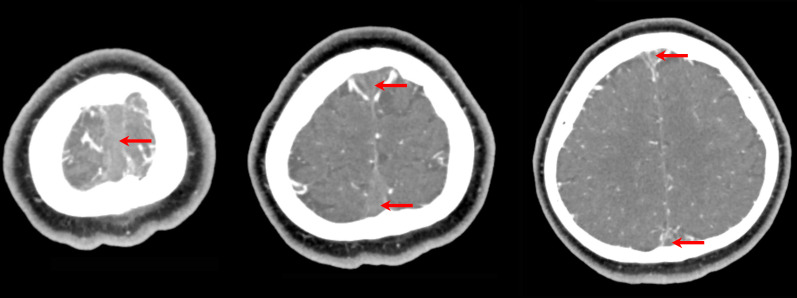
A CT scan of the brain with contrast venography demonstrated an extensive thrombosis of the superior sagittal sinus and the superficial cortical veins, with no intracranial haemorrhage (figure 1). The patient had no focal neurology at this time, and was transferred to the haematology services for treatment of a suspected idiopathic thrombocytopaenic purpura.
Figure 1.
CT of the brain with contrast venography, demonstrating an extensive thrombosis of the superior sagittal sinus and the superficial cortical veins - seen as filling defects of the contrast (red arrows). There is no intracranial haemorrhage.
The initial treatment plan included steroid therapy and intravenous immunoglobulins (IVIG). A transfusion of platelets was briefly considered to allow a subsequent anticoagulation of the thrombus, but this plan was abandoned after a multidisciplinary team discussion. The patient was also commenced on eltrombopag and prophylactic enoxaparin, but these too were cancelled after a single dose of each. A bone marrow biopsy done at this point demonstrated a reactive marrow with plentiful eosinophils, macrophage activity and some reactive plasma cells.
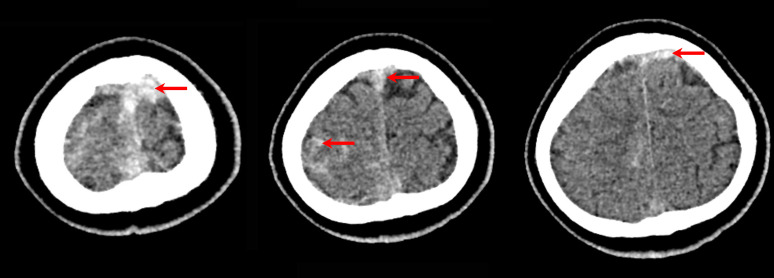
On the following day, the patient developed focal neurological signs, including left-sided weakness and dysdiadochokinesia. A repeat CT of the brain demonstrated a new mild acute subarachnoid haemorrhage in the right frontal and parietal sulci, adjacent to the thrombosis seen on the previous CT scan (figure 2).
Figure 2.
CT of the brain without contrast, demonstrating a new mild acute subarachnoid haemorrhage in the right frontal and parietal sulci (red arrows), adjacent to the superior sagittal venous sinus thrombosis.
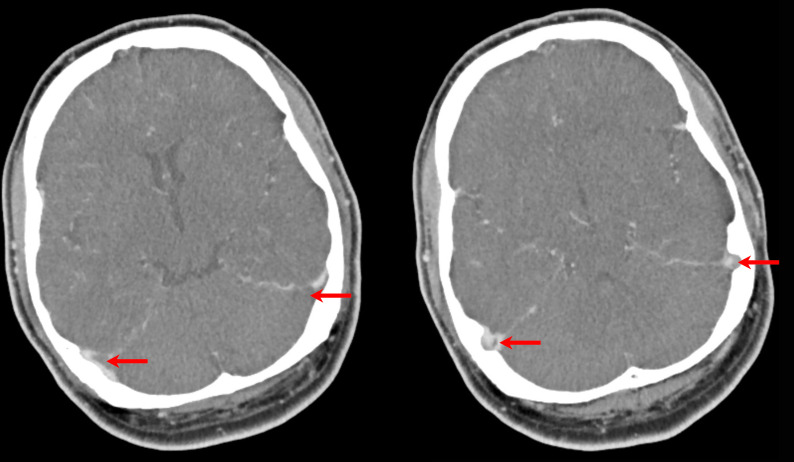
However, during the course of the day, his weakness and discoordination progressed. A further repeat CT of the brain with contrast venography that evening demonstrated a progression of the left transverse sinus thrombosis and a new partial thrombus within the right transverse sinus, with extension into the sigmoid sinuses bilaterally (figure 3).
Figure 3.
CT of the brain with contrast venography demonstrating a progression of the left transverse sinus thrombosis and a new partial thrombus within the right transverse sinus, with extension into the sigmoid sinuses bilaterally. These are seen as filling defects of the contrast (red arrows).
Later that evening, the patient experienced a seizure on the ward, which deteriorated into a cardiorespiratory arrest, where the patient experienced pulseless electrical activity. CPR was undertaken for 5 minutes before a return of spontaneous circulation was achieved. Following this, he had uncontrolled motor activity, increased agitation and poor response to voice or pain. He was intubated and transferred to the intensive care unit (ICU) of a tertiary centre under the care of the neurosurgery services.
Here, he was treated with a heparin infusion, steroids and a further course of IVIG. A wean of sedation resulted in focal seizures, and so he was resedated and commenced on intravenous levetiracetam. At this point, an ELISA for autoimmune heparin-induced thrombocytopaenia (HIT) returned positive for the patient, confirming the diagnosis of VITT.
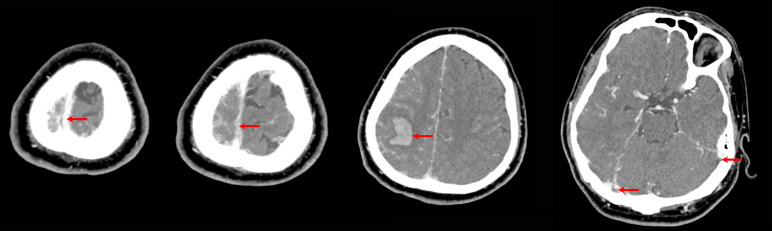
An urgent mechanical thrombectomy was undertaken by interventional radiology. This was technically successful, but fresh clot was noted to be actively forming at the same time as the established clot was being removed. Nevertheless, all clot was eventually removed and a subsequent CT of the brain with contrast venography demonstrated patent sinuses with no extension of the intracranial haemorrhage. This scan also revealed that a right venous cortical infarct had formed following earlier events (figure 4).
Figure 4.
CT of the brain with contrast venography demonstrating patent sinuses (leftmost two red arrows) and a right venous cortical infarct (middle red arrow), with no extension of the intracranial haemorrhage; some persisting filling defects in the transverse sinuses (rightmost two red arrows).
The patient was continued on the heparin infusion, but the partial thromboplastin time became labile. He was subsequently switched to argobatran and then fondaparinux.
On day 7 in ICU, a tracheostomy was inserted due to a difficult respiratory wean. Progress was then further complicated by a ventilator-associated pneumonia, which was successfully treated with intravenous piperacillin/tazobactam. He made good recovery from then on.
On waking, the patient experienced a short-lived delirium and low mood. He was successfully weaned from the ventilator and had his tracheostomy decannulated on day 16 of ICU stay. The platelets improved to 599×109/L, and the patient was discharged from hospital on oral dabigatran after 21 days in ICU.
Investigations
| Haemoglobin | 148 g/L (135–180) |
| White cell count | 7.0×109/ L (4–11) |
| Platelets | 14×109/ L (150–400) |
| Internationalised normalised ratio | 1.2 |
| D-dimer | 62 342 ng/mL |
| Creatinine | 125 μmol/L (62–106) |
| Estimated glomerular filtration rate (e-GFR) | 58 mL/min |
| Adjusted calcium | 2.34 mmol/L (2.2–2.6) |
| Phosphate | 1.09 mmol/L (0.8–1.5) |
| Potassium | 4.2 mmol/L (3.5–5.3) |
| Fibrinogen assay | 2.12 g/L (2.0–6.0) |
| C reactive protein | 30 mg/L |
| Lactate dehydrogenase (LDH) | 291 IU/L (135–225) |
| C3 | 1.77 g/L (0.9–1.8) |
| C4 | 0.50 g/L (0.1–0.4) |
| COVID-19 PCR | Negative |
| Cyclic citrullinated peptide (CCP) antibodies | Negative |
| Antinuclear antibodies | Negative |
| Double-stranded deoxyribonucleic acid (dsDNA) antibodies | Negative |
| Anti-neutrophil cytoplasm (ANCA) antibodies | Negative |
| Immunoglobulins (IgG, IgA and IgM) | Normal range |
| B12, folate and ferritin | Normal range |
| Homocysteine | Normal range |
| Paroxysmal nocturnal haemoglobinuria (PNH) | No evidence of PNH clone |
| Serum protein electrophoresis | Trace monoclonal band in gamma region |
| ELISA for heparin-induced thrombocytopaenia (HIT) | Positive inhibitory IgG: optical density 2.453 (>0.400 indicates positive result) |
| Thrombophilia screen and antiphospholipid screen were not completed, but | |
| Anticardiolipin antibodies | Negative – 6 u/mL (0–10) |
| Beta-2-glycoprotein-1 IgG antibodies | Negative – 1.4 U/mL (0–7) |
| Dil Russell viper venom time | 53.0 s (29-51) |
| Lupus-sensitive APTT | 28 s (25-41) |
| Blood film | Left shifted myeloid cells with metamyelocytes and myelocytes seen. True thrombocytopaenia with very occasional giant platelet. |
| Bone marrow histology | Particulate and hypercellular trails. Trilineage haemopoiesis seen with maturation and no dysplastic features. A reactive marrow with eosinophils, macrophages and plasma cells seen. No abnormal infiltrate seen. Plentiful megakaryocytes with normal morphology. |
| Bone marrow immunophenotyping | There is no excess of blasts at 3.2% of total cells by flow cytometry; myeloid series is left-shifted, no apparent infiltrates; 88% of total cells are CD13+CD33+myeloid: 65% CD11b+, 4% CD14+. There is no excess of myeloid blasts, 3.2% CD34+CD117+CD45int+. There are 1.2% CD19+ B cells and 7.6% CD3+ T cells; these appear polyclonal. |
| Cytogenetic analysis of blood | Negative for the missense variant c.1849G>T p.(Val617Phe) (aka JAK2 V617F) in the JAK2 gene. No evidence of a clinically significant variant in targeted regions of JAK2, CALR and MPL. |
| Urine cytology | Reactive/degenerate urothelial cells and contaminating squames, no malignant cells |
| Ultrasound of the urinary tract | No significant findings, no obstruction |
| Chest X-ray | Clear |
Outcome and follow-up
The patient was discharged back home and remains well. He was commenced on oral dabigatran, to take for 6 months in total, and on oral levetiracetam, to be reviewed at the neurology clinic. He was also commenced on amlodipine for blood pressure control.
The plan from haematology clinic was for weekly full blood count (FBC) to monitor platelets. If platelets were to drop, the patient would receive a further course of IVIG. However, the platelets remained stable, and so the FBC checks were made fortnightly and now monthly.
One month after discharge, the patient re-presented to the emergency department with an episode of haemoptysis. Fine nasal endoscopy identified a right vocal cord granuloma, likely secondary to intubation. The patient was commenced on a proton pump inhibitor and discharged by otorhinolaryngology.
The patient has recently reported difficulty in making a fist with his left hand, particularly with the fourth and fifth digits. This is suspected to be spasticity from his venous cortical infarct and he has been referred for neurophysiotherapy.
The patient remains under haematology and neurology follow-up. This case was reported to the Medicines and Healthcare Products Regulatory Agency and Public Health England as one of the first cases of VITT in the UK.
Discussion
VITT is a newly recognised phenomenon,1 with only a handful of cases being described in the literature.2 Patients described in these cases are often younger adults who have the diagnostic hallmark of a low platelet count and thrombosis following inoculation with the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine.
Patients with VITT commonly present with headaches, and such patients often have a CVST that may also deteriorate into an intracranial haemorrhage. However, other rarer thromboses can also occur with VITT, such as deep vein thromboses in the abdomen, pulmonary emboli, or arterial thrombi that can cause ischaemia.3
Our case was unique from other reported cases as our patient presented with frank haematuria and a widespread petechial rash, which has not been commonly described in the literature. It was also unique in revealing how rapidly clot can form —this was highlighted when clot was seen rapidly forming in the cerebral venous sinuses at the same time as established clot was being mechanically removed during thrombectomy.
As of June 2021, 389 cases of VITT were reported in the UK following vaccination with the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine, occurring almost equally in men and women. 31 of these reported cases were after the second dose of the vaccine. CVSTs have been reported in 140 of all VITT cases—at an average age of 46 years—while the remaining 249 cases have had other thromboses.3
The estimated incidence of VITT after the first dose currently stands at 14.6 per million doses, with a higher reported incidence rate in younger adults. A lower incidence of VITT is reported following the second dose, of 1.6 per million doses, with all cases here being in patients aged 50 years or older. There have been 68 reported deaths from VITT in the UK thus far (case fatality rate of 17%), with 4 of these occurring after receipt of the second dose of the vaccine.3
The aetiology of VITT is thought to be a prothrombotic state similar to HIT, with thrombocytopaenia and thrombosis occurring in the presence of platelet-activating antibodies against multimolecular platelet factor 4 (PF4) complexes. Accordingly, cases of VITT are found to have high levels of anti-PF4 IgG antibodies and show strong reactivity in both PF4 and PF4-heparin ELISA, as is also seen in HIT. The HIT ELISA assay is therefore considered to be a diagnostic test for VITT.4
The fact that patients have anti-PF4 IgG antibodies and a positive HIT ELISA assay after receiving a dose of the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine, and without any exposure to heparin, points to an autoimmune process linked to the vaccine.
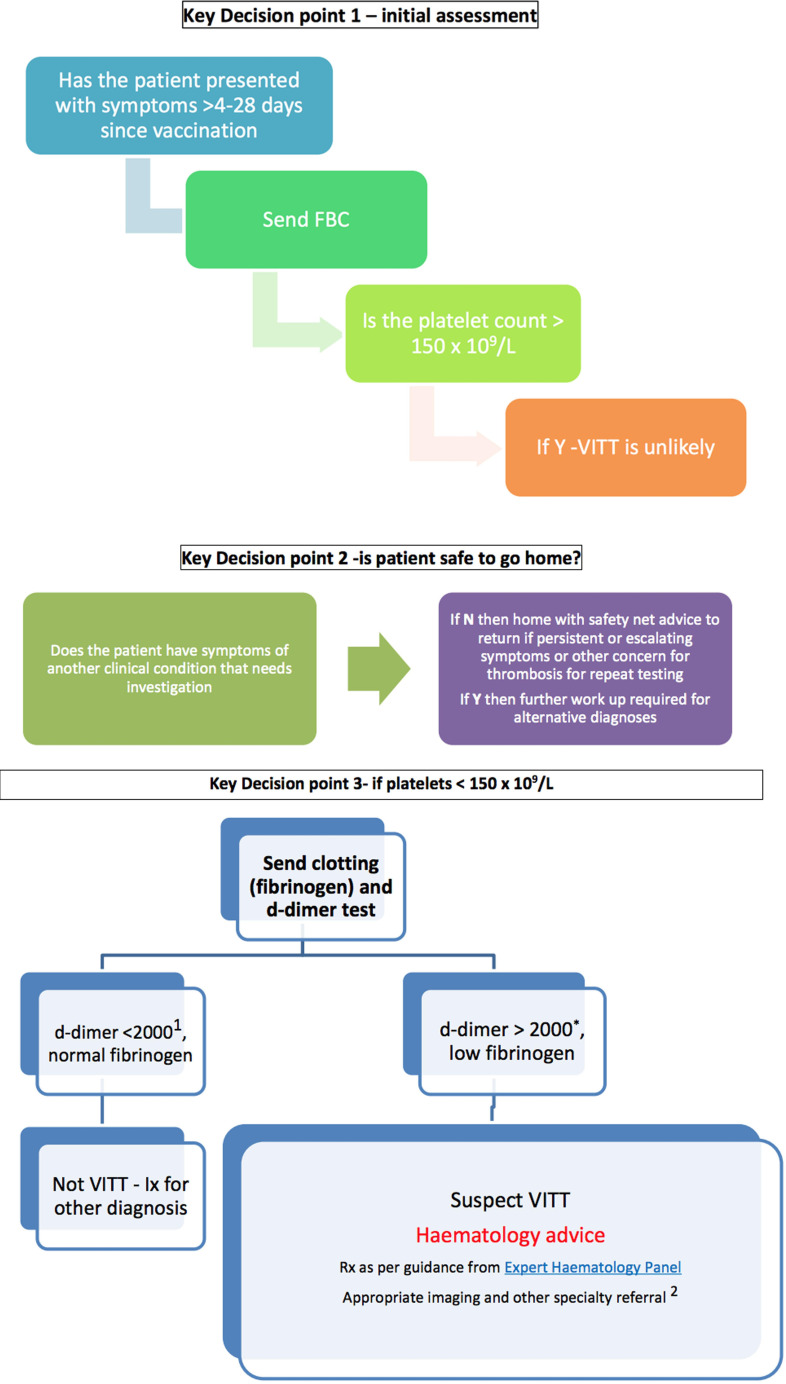
To assess patients presenting to the emergency department or acute medical units with symptoms of VITT, recently released guidance by the Royal Colleges of Physicians and Emergency Medicine prioritises an FBC to screen for VITT, using the platelet count to triage patients.5 According to this guidance, if platelet levels are found to be below 150×109/L and the time frame of the thrombocytopaenia is between 4 and 28 days after inoculation with the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine, further haematology workup is advised to assess for VITT (figure 5).
Figure 5.
Management flowchart for patients presenting to the emergency department or acute medicine with symptoms of VITT.5 1D Dimer as mcg/L, (includes FEU or DDU) = 2mg/L *D Dimers 2000-4000 mcg/L need to be discussed as probable cases. FBC, full blood count; VITT, vaccine-induced thrombotic thrombocytopaenia; Ix, Investigation. Image by: Royal College of Emergency Medicine, Society for Acute Medicine, Royal College of Physicians.
This workup includes testing D-dimer and fibrinogen levels. If D-dimer is below 2000 mcg/L and fibrinogen levels are within the normal reference range, VITT becomes unlikely. However, if D-dimer is elevated above 2000 mcg/L and fibrinogen levels are reduced, VITT should be suspected and appropriate further investigation and treatment should be commenced in discussion with the haematology services.
To confirm a diagnosis, guidance from the UK’s Expert Haematology Panel recommends that serum should be tested using the HIT ELISA assay. It also recommends appropriate imaging, depending on the suspected site of the thrombosis.6
For patients with headaches, a dedicated CT of the brain with contrast venography is recommended as a rapid, accurate and sensitive test to detect CVST; however, an MRI venogram of the brain is considered equally accurate and sensitive if that is more appropriate for the patient.5
Treatment options include urgent IVIGs and full non-heparin-based anticoagulation, with agents such as direct oral anticoagulants or fondaparinux. The risk of using heparin is unknown, but it is considered best not used as VITT mimics HIT closely in aetiology. Nonetheless, replacing fibrinogen through fibrinogen concentrate or cryoprecipitate is recommended. The guidance also recommends early transfer to a neurosurgery specialist centre for more timely investigation and intervention if needed.6
The risk from platelet transfusions, or the usefulness of them, is unclear, and thus there is no clear consensus on their use, even in cases of secondary cerebral bleeds. However, plasma exchange may be undertaken daily (for up to 5 days) in resistant cases, as well as the use of rituximab. Both IVIGs and plasma exchange can be further repeated as necessary.6
In marginal cases of VITT, where there is thrombocytopaenia in the absence of thrombosis, the Panel recommends thromboprophylaxis (as opposed to full anticoagulation) with non-heparin-based anticoagulants.6
In our case, we mainly adhered to the aforementioned guidance, even though they were not published at the time. Due to the initial uncertainty surrounding the diagnosis and a lack of knowledge around VITT at the time, treatments outside the aforementioned guidelines were briefly trialled, such as eltrombopag, prophylactic enoxaparin and a heparin infusion in ICU. Additionally, we had briefly considered using treatment dose enoxaparin if the platelet count recovered, and considered prioritising platelet transfusions and eltrombopag over steroids and IVIG. However, through multidisciplinary team discussions involving colleagues at other centres, these treatments were abandoned.
We had also transferred our patient to a tertiary hospital for neurosurgery services after a cardiorespiratory arrest had occurred and while they were intubated. An earlier transfer would have been better, if possible, to lower the risk of the transfer. Furthermore, given the potential need for interventional radiology to care for patients with VITT, we would recommend involving the radiology department at the chosen tertiary centre early too.
Although most discussion around VITT—including this case—involves the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine, other adenovirus vector vaccines have now also been shown to also have a small risk of inducing VITT.7 We would recommend that the Panel add these vaccines to the guidance as the COVID-19 vaccine programme continues and more data become available.
Learning points.
Vaccine-induced thrombotic thrombocytopaenia (VITT) commonly manifests as cerebral venous sinus thrombosis and presents with headaches, but be alert to alternative presentations that may point to VITT in other sites. If suspecting VITT, refer to the clear guidelines on managing VITT to avoid confusion and minimise risks to the patient.
Triage patients using platelet count and the time frame of presentation following inoculation with the Oxford AstraZeneca ChAdOx1 nCoV-19 vaccine or other adenovirus vector vaccines. After triaging, diagnoses can be worked up using D-dimer and fibrinogen levels, and confirmed through appropriate imaging and the heparin-induced thrombocytopaenia ELISA assay. This assay is widely available and there should be a low threshold to request it.
Immediate treatment comprises urgent IVIGs and full non-heparin-based anticoagulation. Also replace fibrinogen and involve local haematology for further treatment.
Heparin should be avoided in patients with VITT, and there is no consensus on the risks or benefits of platelet transfusions.
Mechanical thrombectomy may be needed. Patients with VITT should thus be cared for in centres that offer urgent neurosurgical and interventional radiology services, with early transfers to these centres recommended if appropriate.
Acknowledgments
We acknowledge Dr Saleem Shafeek (Consultant Haematologist) and the entire haematology department at Worcester Royal Hospital for their care and management of the patient. We acknowledge the critical care and interventional radiology services for for their care and management of the patient both in out hospital and the tertiary centre we dealt with.
Footnotes
Twitter: @ammarwaraich
Contributors: GW wrote the initial case report draft and acquired patient results and consent. AW rewrote the draft into the final version, and added the remaining sections to the report, including summary, investigations, discussion and lessons. He also acquired additional results, haematological results, and images.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021;384:2254–6. 10.1056/NEJMe2106315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz N, Sørvoll I, Michelsen A. Thrombosis and thrombocytopaenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicines and Healthcare products Regulatory Agency . Coronavirus vaccine - weekly summary of Yellow Card reporting, 2021. Available: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting [Accessed 25 Jun 2021].
- 4.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of emergency medicine, Society for acute medicine, Royal College of physicians. management of patients presenting to the emergency Department/ acute medicine with symptoms. Royal College of emergency medicine, Society for acute medicine, Royal College of physicians, 2021. Available: https://www.rcem.ac.uk/docs/Policy/ED-AM%20%20Vaccine%20pathway%20concerns%20-%20RCP%20-%20SAM%20-%20RCEM.pdf
- 6.Pavord S, Lester W, Makris M. Guidance from the expert haematology panel on Covid-19 vaccine-induced immune Thrombocytopaenia and thrombosis. United Kingdom expert haematology panel, 2021. Available: https://b-s-h.org.uk/media/19590/guidance-version-17-on-mngmt-of-vitt-20210420.pdf
- 7.Public Health England . Blood clotting following COVID-19 vaccination: information for health professionals. public health England, 2021. Available: assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/992072/PHE_COVID-19_AZ_vaccine_and_blood_clots_factsheet_8June21.pdf