Abstract
Visceral leishmaniosis (VL) due to Leishmania infantum (L. chagasi) is a lethal disease if untreated, but asymptomatic L. infantum infections have been reported previously. A better understanding of parasite transmission, dissemination, and survival in the human host is needed. The purpose of this study was to assess whether L. infantum circulated in peripheral blood of subjects with no history of VL. Sera from 565 blood donors were screened by Western blotting to detect Leishmania-specific antibodies and identify individuals with probable past exposure to Leishmania. Seropositivity was found in 76 donors whose buffy coats were examined by PCR and direct culture. The parasite minicircle kinetoplast DNA was amplified from blood samples of nine donors. Promastigotes were detected by culture in blood samples from nine donors. Only two donors were PCR and culture positive. These results indicate that L. infantum circulates intermittently and at low density in the blood of healthy seropositive individuals, who thus appear to be asymptomatic carriers. Implications for the safety of blood transfusion are discussed.
The visceral form of leishmaniosis (VL) affects approximately half a million new patients each year. Due to systemic parasite dissemination, the disease is fatal if untreated (27). The main areas of concern are Sudan, Eastern India, Bangladesh, and Nepal (Leishmania donovani) and Brazil and the area around the Mediterranean (Leishmania infantum) (34). Those infected with the viscerotropic Leishmania species may, however, remain asymptomatic (3, 27). The documentation of individuals who have no history of VL but whose leishmanin skin tests (LST) are positive—specific evidence of delayed antileishmanial hypersensitivity—is not new (3, 21, 26). The mechanisms implicated in susceptibility in humans are not fully elucidated, although much has been learned about leishmaniosis in a murine model (29). Immunosuppression, such as in AIDS patients, is one of the factors responsible for increased vulnerability to a primary Leishmania infection or to reactivation of a latent infection (14; for a review, see reference 1). Coinfection with human immunodeficiency virus (HIV) and L. infantum is becoming increasingly frequent; to date, 1,400 VL-AIDS cases have been reported in southern Europe (34).
The documentation of occult Leishmania in healthy subjects is important so that researchers can obtain more knowledge about parasite reservoir and transmission and a better understanding of the pathways of parasite dissemination and its capacity for surviving in the host. Parasite circulation in peripheral blood has been reported in asymptomatic Leishmania donovani and Leishmania tropica infections (6, 9, 31) and in cured and inapparent Leishmania braziliensis infection (10, 11, 19), but except for our preliminary report (15), no documentation of parasitemia in healthy L. infantum-seropositive individuals exists.
We showed previously (21) that the positivity of the LST correlated with the presence of specific antileishmanial antibodies evidenced by Western blotting as typical 14- and/or 18-kDa bands. More recently, the detection of these antibodies before VL diagnosis proved effective in distinguishing cases of VL following primary Leishmania infection from cases originating from the reactivation of a latent infection in HIV-positive patients (14). In this study, the 14- and/or 18-kDa bands revealed by Western blotting with sera of blood donors were used to identify individuals who were probably exposed to Leishmania. Our aim was to assess whether L. infantum parasitemia occurred in asymptomatic Leishmania-seropositive subjects with no history of VL.
From the standpoint of public health, demonstration of the parasite circulation in the blood must be followed by documentation of its transmission by blood transfusion, which has not yet been provided. Decisions regarding the implementation of the Leishmania screening test and the safety of blood transfusions are discussed in this context.
MATERIALS AND METHODS
Subjects and experimental protocol.
Blood obtained from the Monaco Blood Bank was from donors living in neighboring areas where L. infantum is endemic (20) and having no history of VL. Blood from 565 donations was screened over a period of 12 months (April 1996 to March 1997). On the day of donation, sera were analyzed by Western blotting, and the buffy coats corresponding to the sera which revealed the typical 14- and/or 18-kDa leishmanial bands (21, 33) were further examined. Three aliquots (3 ml each) of the 1-day-old (stored at 4°C) buffy coats were stored at −20°C in EDTA containing vacutainer tubes (Becton Dickinson, Meylan, France) until DNA extraction and amplification by PCR. The buffy coats or peripheral blood mononuclear cells (PBMC) were seeded for culture.
Control sera.
Presumably negative control sera were obtained from 141 individuals living in areas free of L. infantum; these individuals were blood donors (n = 50, Bourg-en-Bresse, France) or women who were seronegative for toxoplasmosis during their pregnancies (Parasitology Laboratories, University Hospitals of Reims [n = 50] and Angers [n = 41], France). The sera of patients cured of VL were used as positive controls.
Guidelines for human research.
Informed, written consent from all participants and the approval of the local ethics committees at our institutions were obtained for this study.
Western blot analysis.
The antigen preparations were electrophoresed on sodium dodecyl sulfate–14% polyacrylamide gel and transferred and immunodetected with human sera as described previously (33).
Isolation of parasites by culture.
Three to six milliliters of buffy coats from Leishmania-seropositive subjects was cultured in 25 ml of RPMI 1640 complete medium (RPMI 1640 medium supplemented with 2 mM l-glutamine, penicillin [100 U/ml], streptomycin [100 μg/ml], and 10% heat-inactivated fetal calf serum). Alternatively, PBMC were isolated by centrifugation of buffy coats (diluted 1:1 in 0.9% NaCl) over lymphocyte separation medium (Eurobio, Les Ulis, France). The interface cells were washed, and 5 × 106 to 15 × 106 PBMC were seeded at 1 × 106 to 3 × 106 cells per ml in RPMI 1640 complete medium or in Schneider’s medium as described in reference 17. All cultures were maintained at 25°C for 6 months (or until positive) and were inspected by inverted contrast phase microscopy twice per month. The medium was changed twice per month for 2 months and then once per month. We verified that after approximately 1 month most human cells were taking up trypan blue.
DNA preparation.
Buffy coats obtained from Leishmania-seropositive donors were stored at −20°C until the end of the serological screening. Total DNA was then extracted from cells contained in 3-ml aliquots, corresponding roughly to 30 ml of whole blood. DNA extracts were prepared from the buffy coats by following the procedure described in reference 4, except that the volume of lysis and its duration were increased. Briefly, after five washes in saline (10 min, 2,500 × g), the pellet corresponding to 3 ml of a buffy coat was incubated with 3.6 ml of lysis buffer (8 M guanidine thiocyanate in 0.1 M Tris-HCl [pH 6.4], 36 mM EDTA, 2% Triton X-100) for 48 h at room temperature, with constant mixing. The homogenized lysate was centrifuged (10 min, 2,500 × g), and 2.7 ml of the supernatant was incubated with 120 μl of Kieselguhr DG (Riedel-deHaën, Seelze, Germany, sold through Sigma-Aldrich) suspension (20% [wt/vol] in water, 3.6% HCl) for 10 min at room temperature, with intermittent vortexing. The mixture was centrifuged (3 min, 2,500 × g), the supernatant was discarded, and the pellet was washed twice with 3 ml of washing buffer (8 M guanidine thiocyanate in 0.1 M Tris-HCl [pH 6.4]) and three times with 3 ml of 70% ethanol and finally suspended in 1 ml of acetone. After centrifugation (10 min, 10,000 × g), the supernatant was discarded, and the pellet was dried (15 min at 56°C) and then incubated for 10 min at 56°C in 180 μl of water. After additional centrifugation (5 min, 10,000 × g) 100 μl of DNA solution was obtained and used immediately or stored at −20°C.
DNA amplification.
PCR was carried out by using 10 μl of DNA solution in a final volume of 50 μl of reaction mix (Taq polymerase buffer [Appligene], 0.2 mM [each] deoxynucleotide [Promega], 1 μM [each] primer [Eurogentec], 0.014 U of Taq DNA polymerase [Appligene]/ml) containing 6 μl of internal control (see below). Two distinct parasite target sequences, LT1 (145 bp) and LT2 (120 bp), were chosen for amplification in the conserved region of kinetoplast DNA (kDNA) minicircles. The LT1 fragment of kDNA was amplified with the primers adapted from reference 28 as follows: RV1 (sense), 5′-CTTTTCTGGTCCCGCGGGTAGG-3′, and RV2 (antisense), 5′-CCACCTGGCCTATTTTACACCA-3′. After an initial denaturation (2 min at 94°C), 45 cycles (denaturation, 1 min at 94°C; annealing, 1.5 min at 62°C; polymerization, 30 s at 70°C) were carried out, and PCR was terminated by a final extension at 70°C for 10 min. To double-check the results, a second pair of primers (13A [sense] 5′-GTGGGGGAGGGGCGTTCT-3′ and 13B [antisense] 5′-ATTTTACACCAACCCCCAGTT-3′) leading to the target sequence LT2 (30) was used on all DNA samples under the same amplification conditions except for the annealing, which was carried out at 50°C. The positive internal controls (PIC1 and PIC2 for the target sequences LT1 and LT2, respectively) were constructed with M13mp18 phage DNA as described in reference 5. Briefly, for each target sequence, an M13mp18 fragment with Leishmania sequences at the ends was generated in a first PCR with composite Leishmania/phage primers (RV1ph1, 5′-CTTTTCTGGTCCCGCGGGTAGGCGGTTTGCGTATTGGG-3′, and RV2ph2, 5′-CCACCTGGCCTATTTTACACCACAGGATTTTCGCCTG-3′, or 13Aph1, 5′-GTGGGGGAGGGGCGTTCTCGGTTTGCGTATTGGG-3′, and 13Bph2, 5′-ATTTTACACCAACCCCCAGTTCAGGATTTTCGCCTG-3′) and then amplified with the corresponding pair of Leishmania primers (RV1 and RV2 or 13A and 13B, respectively) leading to the PIC1 of 183 bp and the PIC2 of 178 bp. This internal control DNA was added to each amplification reaction mixture, preventing false-negative results which could be induced by excessive loads of cellular material (28). The amount of the PIC DNA was chosen in order to minimize competition with the parasite DNA. Two negative controls (DNA extracted from a buffy coat obtained from a Leishmania-seronegative individual and an aliquot of distilled water) were included in each PCR run to detect contamination which could lead to false-positive results. The amplification products were visualized after electrophoresis on a 3% agarose gel containing ethidium bromide.
RESULTS
Serological screening.
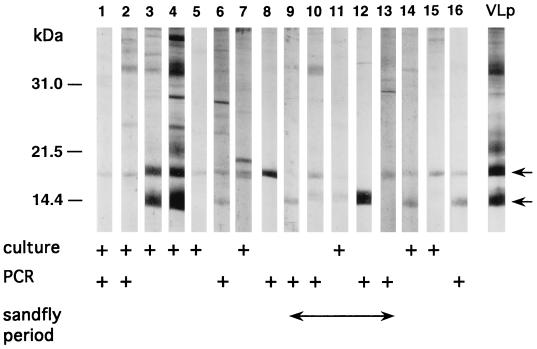
The bands at 14 and/or 18 kDa, indicative of a previous asymptomatic infection with L. infantum (14, 21, 33), were revealed by the sera from 76 donors (13.4%) to Monaco Blood Bank. Antibodies detecting both bands were found in 25 serum samples, and antibodies detecting one band at 14 or 18 kDa were found in 20 or 31 serum samples, respectively. Figure 1 shows the characteristic Western blot profiles obtained with the sera of 16 donors found to be Leishmania positive. Interestingly, among 141 control serum samples obtained from regions where VL is not endemic and thus, presumably, Leishmania seronegative, 6 exhibited a weak 14-kDa (3 samples) or 18-kDa (3 samples) reactivity (data not shown). However, in five cases, previous travel to regions of endemicity (southern France, Portugal, Algeria, or Morocco) was evident on the basis of a retrospective interview. These findings further validate previous observations (21, 22) about the usefulness of detecting antibodies directed against the 14- and/or 18-kDa leishmanial fractions to assess past contact with the parasite and confirm the important (13%) prevalence of asymptomatic L. infantum infection of humans in southern France (14, 20).
FIG. 1.
L. infantum seropositivity and presence in blood samples of 16 asymptomatic blood donors with no history of VL. Parasite-positive donors were identified among 76 L. infantum-seropositive individuals whose Western blots revealed characteristic 14- and/or 18-kDa bands (arrows). The profile of a VL patient (lane VLp) is shown. The presence of Leishmania was indicated either by parasite kDNA amplification (Fig. 2) or by direct culture, as described in Materials and Methods. Blood samples drawn during the Leishmania-transmitting sandflies’ active period (May to October) are indicated.
Amplification of parasite kDNA in buffy coats of Leishmania-seropositive blood donors.
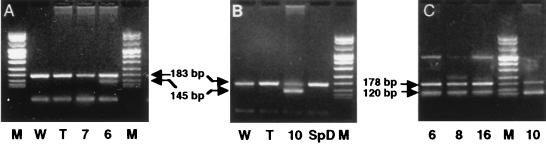
Buffy coats from 73 Leishmania-seropositive donors were analyzed, since buffy coats from 3 Leishmania-seropositive donors found to be hepatitis C virus seropositive were discarded from the study. Under the experimental conditions described in Materials and Methods, the Leishmania kDNA was detected in the buffy coats of nine Leishmania-seropositive blood donors with primers RV1 and RV2. Primers 13A and 13B were found to be less efficient and amplified parasite kDNA in seven of these nine positive samples. Figure 2 shows examples of amplifications with primers RV1 and RV2 (Fig. 2A and B) and primers 13A and 13B (Fig. 2C) for four PCR-positive and three PCR-negative donors. The blood of five PCR-positive donors was drawn during the period when Leishmania-transmitting sandflies (May to October) are active, while the remaining PCR-positive samples were obtained from blood donated outside this period (Fig. 1).
FIG. 2.
PCR amplifications with primers RV1 and RV2 (A and B) and 13A and 13B (C). Lanes 6, 7, 8, and 10 correspond to donors mentioned in the legend for Fig. 1, and lane SpD corresponds to a Leishmania-seropositive donor who was PCR and culture negative. Lanes W and T, two negative controls of PCR runs (distilled water and DNA from a Leishmania-seronegative donor); lanes M, DNA molecular size markers (pUCBM21 cleaved by HpaII and DraI plus HindIII). Upper arrows, PICs constructed with primers RV1 and RV2 (A and B) and 13A and 13B (C) as described in Materials and Methods; lower arrows, Leishmania-specific amplicons.
Detection of parasites by culture.
L. infantum promastigotes were detected in cultures of blood cells from 9 donors. These cultures were not carried out under standardized conditions (see Materials and Methods). Six positive samples were obtained from cultures of 6 ml of buffy coats (corresponding to approximately 60 ml of blood), and the remaining three positive samples were PBMC cultures (corresponding to approximately 10 ml of blood). In all cases a long incubation period (1 to 6 months) was necessary to detect parasites. All isolated strains were typed as Zymodeme MON-1. For one donor the culture was positive in two independent blood donations (a culture of the buffy coat in April and a culture of PBMC in October). For this donor the Leishmania kDNA was also detected by PCR. Evidence of the parasitemia in two PCR-positive donors was also provided by culture. The remaining PCR-positive samples were culture negative (Fig. 1).
Retroactive study of files and sera of VL patients.
We examined the files of 50 VL patients, infected with HIV (32 patients) or not infected with HIV (18 patients), and found that 6 of the former and 2 of the latter had had a blood transfusion. Sera from 10 persons who donated blood to two patients that received transfusions (six donors for one patient and four donors for the second patient) were recovered and analyzed by Western blotting; two persons who donated blood to the same (HIV positive) VL patient were found to be Leishmania seropositive. Analyses of sera drawn previously from the recipient showed that this patient was Leishmania seropositive before the transfusion.
DISCUSSION
In this study we screened blood from the Monaco Blood Bank obtained from 565 donors living in an area where L. infantum is endemic. The Leishmania-specific antibodies were revealed by Western blotting in the sera of 76 donors which showed the characteristic 14- and/or 18-kDa bands. The presence of the parasite was evident in the blood of 16 Leishmania-seropositive donors either by parasite kDNA amplification or by direct culture.
The knowledge that some individuals with no history of VL due to L. infantum present a positive LST (showing past exposure to the parasite) is not new (3, 21, 26). We showed previously (21) that the positivity of LST was in biological concordance (80%) with the presence of antibodies directed against 14- and/or 18-kDa fractions of L. infantum. In this study, six control serum samples of persons living in regions free of leishmaniosis (of 141 samples tested) also detected the typical 14- and/or 18-kDa bands, but five of these six samples should be disregarded, since previous travel to regions where Leishmania is endemic was mentioned by donors in interviews. Although false-positive and false-negative results cannot be excluded when one serological test is used, and since the aim of the study was qualitative, rather than quantitative, we selected the buffy coats of Leishmania-seropositive donors to search for the parasite.
Parasite kDNA was amplified in blood samples from nine donors. The live L. infantum promastigotes were detected by culture in blood samples from nine donors. Only two donors were PCR positive and culture positive. A second blood donation from the culture-positive donors was obtained several months after the first; only one of these samples was culture positive, and all nine samples were PCR negative. These results indicate that the density of Leishmania in the peripheral blood is low and that parasitemia is probably episodic. Why PCR, which is a powerful technique, gives a negative result for a blood sample from a culture-positive donor is puzzling. However, amplification was performed on DNA extracted from approximately 30 ml of blood, so even if minicircle kDNA amplification may, theoretically, detect 1 parasite in 1 million cells (28), our findings indicate that not every 30 ml of blood carried an amastigote kDNA target sequence. The direct culture was performed on the equivalent of 10 to 60 ml of blood, a poor way to amplify Leishmania, and we were unable to determine the optimal culture conditions. In all cases the detection of parasites was made possible by the maintenance of the cultures for unusually long periods. Several nonexclusive hypotheses may be proposed to explain this observation. For instance (i) the intracellular amastigotes might remain trapped until the death of their host cell (9), (ii) the liberated amastigotes might be rephagocytosed before transformation due to a high density of potential host cells, and/or (iii) the promastigotes might proliferate very slowly when the initial parasite load in the culture is low, due to a low concentration of the autocrine growth-regulating factor (16). We did observe in some mixed cultures (PBMC plus promastigotes) the persistence for several weeks of a few detectable parasites, without apparent multiplication (data not shown). Taken together, our results imply that episodes of parasitemia of variable intensities might be the rule rather than the exception in healthy L. infantum carriers.
Evidence of L. infantum parasitemia found in subjects with no history of VL, in several cases before the annual emergence of a new generation of Leishmania-transmitting sandflies, raises questions concerning physiopathology, epidemiology, and public health. First, as in acute disease, the potential target organs of the persisting Leishmania might be the bone marrow, liver and spleen, but it is not known whether all three organs are colonized in an occult infection and might be a source of hematogenous dissemination and whether circulating amastigotes are always intracellular or might be free. Second, unlike L. donovani, which is anthroponotic, L. infantum is anthropozoonotic and has been thought to be transmitted to man via sandfly from an animal reservoir, mostly dogs, but our findings point to the possibility that the healthy human carriers are a reservoir. The recently demonstrated (24) contamination of sandflies fed the blood of Leishmania-HIV-coinfected patients indicates that a new natural anthroponotic cycle should be considered in the epidemiology of L. infantum-HIV coinfection (25). Third, the parasitemia in asymptomatic subjects indicates that the parasite might be transmitted in blood by transfusions. The possibility that blood transfusion might pose a risk of Leishmania transmission was also suggested by the significant increase in the prevalence of anti-Leishmania antibodies in hemodialysis patients in Brazil who received multiple blood transfusions (18). However, to date, the reports of transfusion-transmitted leishmaniosis have been mainly anecdotal, and in no case was the donor identified (2, 7, 8, 12, 23, 32). In an attempt to document a case of transfusion-transmitted VL, we examined the files of 50 VL patients, but even if some donors’ sera were recovered and analyzed, no conclusion could be drawn, since the recipient of blood from the two Leishmania-seropositive donors was Leishmania seropositive before the transfusion.
We are now studying individuals who received blood from Leishmania-seropositive donors, in an attempt to document parasite transmission. However, such a transmission of L. infantum, assuming we are able to document it, does not necessarily result in the transmission of the disease. In our region of high prevalence of Leishmania seropositivity and, thus, of a high density of potential asymptomatic donors, the seemingly transfusion-transmitted leishmaniosis (occurring within a few months after a transfusion) has not been documented. Systematic screening for and discarding of the blood of Leishmania-seropositive donors would decrease the blood supply in our region by 10% without, at present, proof of the efficacy of this practice. To date, no large-scale test for screening blood donors is available, and Western blotting is costly and time-consuming. We are presently trying to identify and clone Leishmania antigens which could be used for such screening by enzyme-linked immunosorbent assay. Informative comments regarding the implementation of donor screening tests have been provided by Kleinman et al. (12).
We estimate that prior to any kind of preparation of packed erythrocytes, a blood unit contains 2 × 109 to 3 × 109 leukocytes. After the first step of preparation, i.e., the removal of the buffy coat after centrifugation, the blood unit contains roughly 5 × 108 leukocytes. After the second step, i.e., deleukocytation by filtration, the unit contains fewer than 106 leukocytes. Therefore, assuming that parasite transmission is proportional to leukocyte concentration, a risk score of 1 assigned to an initial, undeleukocyted unit would decrease to 10−4 −10−3 after deleukocytation. At present, all blood products in Monaco (as of 1 April 1997) and France (as of 1 April 1998) are deleukocyted. Blood is not conserved prior to deleukocytation, preventing the possible release of intracellular agents by cell lysis during storage. Finally, it should be emphasized that screening blood for one pathogenic agent protects against this agent alone, while the deleukocytation of blood products eliminates several pathogens (e.g., cytomegalovirus, but also as-yet-unidentified agents), prevents alloimmunization, and improves the well-being of the blood recipient.
In conclusion, we believe that considering present constraints of time, money, and knowledge, researchers should invest first in the preparation of blood products and then in donor testing to protect recipients of blood transfusions against L. infantum.
ACKNOWLEDGMENTS
We thank the blood donors for their willingness to participate; M. Angué from the Bourg-en-Bresse Blood Bank, C. Chemla from the University Hospital of Reims, and B. Cimon from the University Hospital of Angers for providing us with control sera from subjects living outside an area of endemicity and for conducting additional interviews with Leishmania-seropositive individuals; and F. Pratlong and J. P. Dedet for biochemical characterization of Leishmania strains. We also thank G. Pagliardini and S. Fornasero for excellent technical help, C. Minghelli and A. Grima for illustrations, and B. Ferrua for critical reading of the manuscript.
This work was supported by a PHRC grant from the French Ministry of Health and by gifts from le Groupe d’Action Contre la Leishmaniose (GACL).
REFERENCES
- 1.Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.André R, Brumpt L, Dreyfus B, Passelecq A, Jacob S. Cutaneous leishmaniasis, cutaneous-glandular leishmaniasis and transfusional kala-azar. Trop Dis Bull. 1958;55:379–381. [PubMed] [Google Scholar]
- 3.Badaro R, Jones T C, Carvalho J M, Sampaio D, Reed S G, Barral A, Teixeira R, Johnson W D. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretagne S, Costa J M, Vidaud M, Van Nhieu J T, Fleury-Feith J. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J Infect Dis. 1993;168:1585–1588. doi: 10.1093/infdis/168.6.1585. [DOI] [PubMed] [Google Scholar]
- 6.Chandra J, Rai R N, Mittal S K, Sharma D. Kala-azar without hepatosplenomegaly. Indian Pediatr. 1991;28:1185–1186. [PubMed] [Google Scholar]
- 7.Chung H L, Chow K K, Lu J P. The first two cases of transfusion kala-azar. Chin Med J. 1948;66:325–326. [Google Scholar]
- 8.Cohen C, Corazza F, De Moll P, Brasseur D. Leishmaniasis acquired in Belgium. Lancet. 1991;338:128. doi: 10.1016/0140-6736(91)90129-d. [DOI] [PubMed] [Google Scholar]
- 9.Grogl M, Daugirda J L, Hoover D L, Magill A J, Berman J D. Survivability and infectivity of viscerotropic Leishmania tropica from operation Desert Storm participants in human blood products maintained under blood bank conditions. Am J Trop Med Hyg. 1993;49:308–315. doi: 10.4269/ajtmh.1993.49.308. [DOI] [PubMed] [Google Scholar]
- 10.Guevara P, Ramirez J L, Rojas L, Scorza J V, Gonzales N, Anez N. Leishmania braziliensis in blood 30 years after cure. Lancet. 1993;341:1341. doi: 10.1016/0140-6736(93)90845-8. [DOI] [PubMed] [Google Scholar]
- 11.Guevara P, Rojas L, Gonzales N, Scorza J V, Anez N, Valera M, Ramirez J L. Presence of Leishmania braziliensis in blood samples from cured patients or at different stages of immunotherapy. Clin Diagn Lab Immun. 1994;1:385–389. doi: 10.1128/cdli.1.4.385-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinman S H, Bush M P, Schreiber G B, AuBuchon J P. Declining value of alanine aminotransferase in screening blood donors: missed opportunities. Reply Transfus. 1996;36:847–348. doi: 10.1046/j.1537-2995.1996.36996420768.x. [DOI] [PubMed] [Google Scholar]
- 13.Kostman R, Barr M, Bengtson E, Garnham P C C, Hult G. Proceedings of the Seventh International Congress of Tropical Medicine and Malaria. Geneva, Switzerland: World Health Organization; 1963. Kala-azar transferred by exchange blood transfusion in two Swedish infants; p. 384. [Google Scholar]
- 14.Kubar J, Marty P, Lelièvre A, Quaranta J F, Staccini P, Caroli-Bosc C, Le Fichoux Y. Visceral leishmaniosis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T lymphocytes counts. AIDS. 1998;12:2147–2153. doi: 10.1097/00002030-199816000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Kubar J, Quaranta J F, Aufeuvre J P, Marty P, Lelièvre A, Le Fichoux Y. Transmission of Leishmania infantum by blood donors. Nat Med. 1997;3:368. doi: 10.1038/nm0497-368. [DOI] [PubMed] [Google Scholar]
- 16.Lemesre J L, Rizvi F, Santoro F, Loyens M, Sadigursky M, Capron A. Autorégulation de la croissance in vitro des Trypanosomatidae. C R Acad Sci (Paris) 1988;307:283–288. [Google Scholar]
- 17.Lima H C, Bleyenberg J A, Titus R G. A simple method for quantifying Leishmania in tissues of infected animals. Parasitol Today. 1997;13:80–82. doi: 10.1016/s0169-4758(96)40010-2. [DOI] [PubMed] [Google Scholar]
- 18.Luz K G, DaSilva V O, Gomes E M, Machado F C S, Araujo M A F, Fonseca H E M, Freire T C, Dalmeida J B, Palatnik M, Palatnik de Sousa C B. Prevalence of anti-Leishmania donovani antibody among Brazilian blood donors and multiply transfused hemodialysis patients. Am J Trop Med Hyg. 1997;57:168–171. doi: 10.4269/ajtmh.1997.57.168. [DOI] [PubMed] [Google Scholar]
- 19.Martinez J E, Arias A L, Escobar M A, Saravia N G. Haemoculture of Leishmania (Viannia) braziliensis from two cases of mucosal leishmaniasis: re-examination of haematogenous dissemination. Trans R Soc Trop Med Hyg. 1992;86:392–394. doi: 10.1016/0035-9203(92)90233-3. [DOI] [PubMed] [Google Scholar]
- 20.Marty P, Le Fichoux Y, Pratlong F, Gari-Toussaint M. Human visceral leishmaniasis in Alpes-Maritimes, France: epidemiological characteristics for the period 1985–1992. Trans R Soc Trop Med Hyg. 1994;88:33–34. doi: 10.1016/0035-9203(94)90485-5. [DOI] [PubMed] [Google Scholar]
- 21.Marty P, Lelièvre A, Quaranta J F, Rahal A, Gari-Toussaint M, Le Fichoux Y. Use of the leishmanin skin test and Western blot analysis for epidemiological studies in visceral leishmaniasis areas: experience in a highly endemic focus in Alpes-Maritimes (France) Trans R Soc Trop Med Hyg. 1994;88:658–659. doi: 10.1016/0035-9203(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 22.Mary C, Lamouroux D, Dunan S, Quilici M. Western blot analysis of antibodies to Leishmania infantum antigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am J Trop Med Hyg. 1992;147:764–771. doi: 10.4269/ajtmh.1992.47.764. [DOI] [PubMed] [Google Scholar]
- 23.Mauny I, Blanchot I, Degeilh B, Dabadie A, Guiguen C, Roussey M. Leishmaniose viscérale chez un nourisson en Bretagne: discussion sur les modes de transmission hors des zones endémiques. Pédiatrie. 1993;48:237–239. [PubMed] [Google Scholar]
- 24.Molina R, Canavate C, Cercenado E, Laguna F, Lopez-Velez R, Alvar J. Indirect xenodiagnosis of visceral leishmaniasis in 10 HIV-infected patients using colonized Phlebotomus perniciosus. AIDS. 1994;8:277–280. doi: 10.1097/00002030-199402000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Molina R, Lohse J M, Pulido F, Laguna F, Lopez-Velez R, Alvar J. Infection of sand flies by humans coinfected with leishmania infantum and human immunodeficiency virus. Am J Trop Med Hyg. 1999;60:51–53. doi: 10.4269/ajtmh.1999.60.51. [DOI] [PubMed] [Google Scholar]
- 26.Pampiglione S, Manson-Bahr P E C, La Placa M, Borgatti M A, Musumeci S. Studies in Mediterranean leishmaniasis. 3. The leishmanin skin test in kala-azar. Trans R Soc Trop Med Hyg. 1975;69:60–68. doi: 10.1016/0035-9203(75)90012-7. [DOI] [PubMed] [Google Scholar]
- 27.Pearson R D, de Queiroz Sousa A. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1995;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Ravel S, Cuny G, Reynes J, Veas F. A highly sensitive and rapid procedure for direct PCR detection of Leishmania infantum within human peripheral blood mononuclear cells. Acta Trop. 1995;59:187–196. doi: 10.1016/0001-706x(95)00079-t. [DOI] [PubMed] [Google Scholar]
- 29.Reed S G, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers M R, Popper S J, Wirth D F. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp Parasitol. 1990;71:267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal R K. Leishmaniasis in the Indian subcontinent. In: Chang K P, Bray R S, editors. Leishmaniasis. Amsterdam, The Netherlands: Elsevier Science Publishers; 1985. pp. 443–467. [Google Scholar]
- 32.Singh S, Chaudhry V P, Wali J P. Transfusion-transmitted kala-azar in India. Transfusion. 1996;36:848–849. doi: 10.1046/j.1537-2995.1996.36996420769.x. [DOI] [PubMed] [Google Scholar]
- 33.Suffia I, Quaranta J F, Eulalio M C M, Ferrua B, Marty P, Le Fichoux Y, Kubar J. Human T-cell activation by 14- and 18-kilodalton nuclear proteins of Leishmania infantum. Infect Immun. 1995;63:3765–3771. doi: 10.1128/iai.63.10.3765-3771.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization and Joint United Nations Programme on HIV/AIDS. Leishmania and HIV in gridlock. Geneva, Switzerland: CTD/LEISH/98.9; 1998. [Google Scholar]