Abstract
Hereditary Polyposis Syndromes are a group of rare, inherited syndromes characterized by the presence of histopathologically specific or numerous intestinal polyps and an increased risk of cancer. Some polyposis syndromes have been known for decades, but the development in genetic technologies has allowed the identification of new syndromes.. The diagnosis entails surveillance from an early age, but universal guideline on how to manage and surveille these new syndromes are lacking. This paper represents a condensed version of the recent guideline (2020) from a working group appointed by the Danish Society of Medical Genetics and the Danish Society of Surgery on recommendations for the surveillance of patients with hereditary polyposis syndromes, including rare polyposis syndromes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13053-021-00197-8.
Keywords: Cancer, Polyposis, Genetics, Hereditary, Surveillance, Management, Guideline
Background
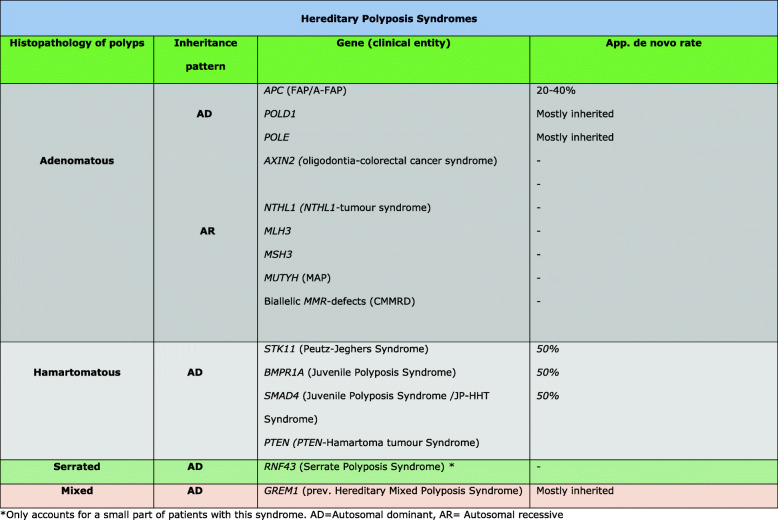
Hereditary Polyposis Syndromes (HPSs) are a group of rare, inherited syndromes characterized by the presence of histopathologically specific or numerous intestinal polyps and sometimes extra-intestinal manifestations. HPSs is associated with an increased risk of cancer in and outside the gastrointestinal (GI)- tract -tract and timely diagnosis is important in order to offer specific organ-targeted surveillance programs with the purpose of reducing morbidity and mortality. The classification of HPS has traditionally been based on the histopathology of the removed polyps as presented in Fig. 1.
Fig. 1.
Classification of Hereditary Polyposis Syndromes. *Only accounts for a small part of patients with this syndrome. AD = Autosomal dominant, AR = Autosomal recessive
Some HPSs have been known for decades, but the possibility of sequencing many genes in a very short time (Next Generation Sequencing (NGS)), has revealed several genes now known to be associated with HPSs, and genetic testing is therefore a part of the diagnostic pipeline for patients with (or suspected of having) a HPS. Both autosomal dominant and autosomal recessive inheritance is seen.
Genetics diagnostics and genetic counselling
Genetic testing includes gene-panel screening using NGS with genes known to be related to polyposis syndromes. As of 2020, the panel should include the genes listed in Fig. 1. The finding of causative monoallelic (autosomal dominant) or biallelic (autosomal recessive) germline pathogenic variations (PVs) is crucial in order to make an accurate diagnosis which in turn is the prerequisite for tailoring the optimal surveillance program for each patient. Additionally, detecting a genetic cause also makes prenatal diagnosis, including preimplantation diagnosis (PGT), possible in some cases. Somatic mosaicism should be considered in patients with a clinically convincing HPS, where standard genetic analyses of blood does not identify the cause.
How to manage HPS?
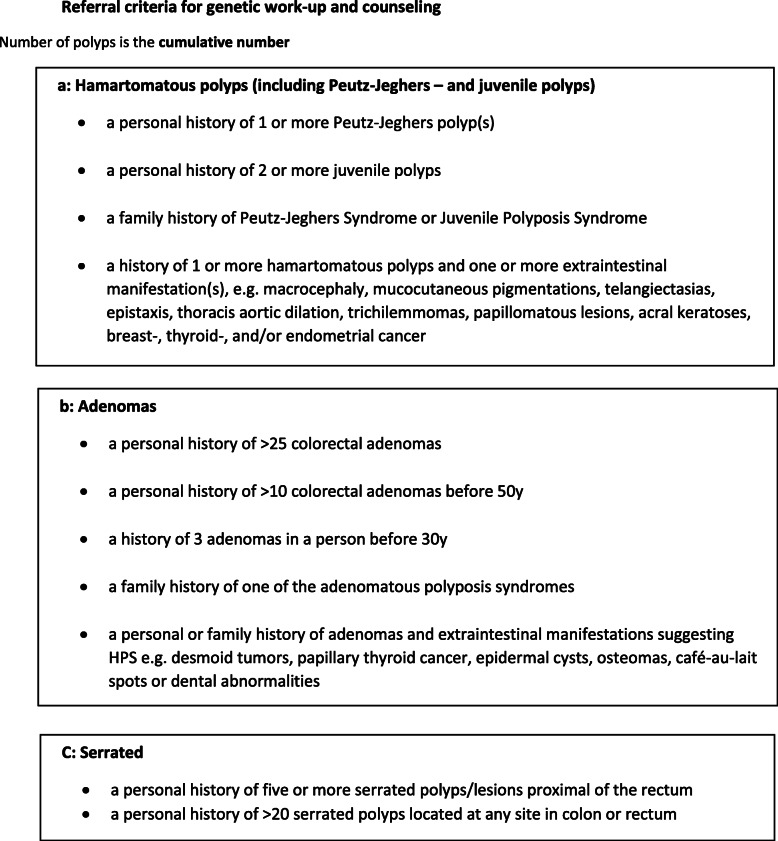
There is a high demand for guidelines addressing questions like: how many and what types of polyps should cause concern? When should a patient be referred for genetic counselling? How should we manage patients and their families when detecting (or not detecting) a PV in an HPS gene? In order to address these questions, the Danish Society of Medical Genetics and the Danish Society of Surgery appointed a group of experts in the field in 2017. This paper is a summarized version of this work and guidelines, approved by the two societies in 2020. The guideline points out referral criteria for genetic work-up and counselling (Fig. 2) and suggests surveillance programs for HPS-patients with or without a known genetic etiology. APC-associated polyposis and PTEN-hamartoma-tumor syndrome are not included in the work. The working group agreed on general recommendations (Suppl. Table 1) and more specific surveillance for each HPS (Suppl. Table 2).
Fig. 2.
Referral criteria for genetic work-up and counseling. Number of polyps is the cumulative number
General considerations of surveillance and prophylactic GI-operations
Endoscopic investigations are the core of surveillance. There is no evidence for recommending prophylactic intestinal resections in any of the HPSs, which are described here, but some patients may have a massive polyp burden in part(s) of the GI-tract, making endoscopic surveillance challenging; gastrointestinal resection is indicated in some patients. In the case of colorectal cancer (CRC), a subtotal or total colectomy should be considered, but taking the polyp burden, age and co-morbidity into consideration. If (large) polyps causes complications such as invagination and/or bleeding, segmental resections with or without peroperative enteroscopy should be performed. After surgery surveillance must be resumed.
HAMARTOMATOUS polyposis syndromes
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome (PJS) is characterized by the presence of hamartomatous Peutz-Jeghers polyps in the GI-tract and mucocutaneous pigmentations (MPs) especially on the lips and buccal mucosa. MPs typically presents in childhood and tend to fade after puberty. The polyps are mainly found in the small intestines and 50–75% of patients experience GI symptoms before 20 years of age, with invagination as the most common complication [1]. PJS is inherited in an autosomal dominant manner and STK11 is the only gene known to be associated with the condition . An age dependent increased risk of cancer in the GI-tract as well as various extra-intestinal cancers are well documented (Table 1) [9]. Surveillance is comprehensive and should start in childhood (see Supp Table 2) [10].
Table 1 .
Estimated cancer risk
| Syndrome | Site of cancer | Cumulative lifetime risk or frequency among carriers | Age of debut |
|---|---|---|---|
| Peutz-Jeghers Syndrome [2] | Colon/rectum | 39% | 42-46y |
| Stomach | 29% | 30-40y | |
| Small bowel | 13% | 37-42y | |
| Breast | 32–54% | 37-59y | |
| Ovarian | 21% | 28y | |
| Cervix (adenoma malignum) | 10% | 34-40y | |
| Uterus | 9% | 43y | |
| Pancreas | 11–38% | 41–52y | |
| Testicular (sertoli cell tumour) | 9% | 6-9y | |
| Lung | 7–17% | 47y | |
| Juvenile Polyposis Syndrome | Colon/rectum | 38% [3] | 36.0 (48) (median) |
| Gastric | 21% [2] | 44.0 (48) | |
| POLE-associated polyposis [4] | Colon/rectum | 28% (M), 21%(F) For p.Leu424VAL: 97% (M), 92% (F) | 50.2 (49) (mean) |
| Uterus | ? | ||
| Ovaries | ? | ||
| Pancreas | ? | ||
| Malignant melanoma | ? | ||
| POLD1-associated polyposis [4] | Colon/rectum | 90% (M), 82% (F) | 39.7 (49) (median) |
| Uterus | ? | ? | |
| Breast cancer | ? | ? | |
| Ovarian | ? | ? | |
| Lymphoma | ? | ? | |
| Bladder | ? | ? | |
| AXIN2-associated polyposis | Colon/rectum | 13/35 individuals | 36–80+ |
| MUTYH- associated polyposis [5] | Colon/rectum | 80–90% | 48.0 (median) |
| Duodenum | 4% | 61.0 | |
| Ovaries | 6–14% | 51.0 | |
| Bladder | 6–8% (females), 6–25% males) | 61.0 | |
| Melanoma | ? | ? | |
| Breast | ? | ? | |
| Uterus | 3% | 51.0 | |
| NTHL1-associated polyposis [6] | Colon/rectum | 16/29 individuals | 61.0 median, (33-73y) |
| Breast | 9/15 female individuals | 48.5 (38-63y) | |
| Uterus | 5/15 Uterus (precancerous and cancerous) | 57.0 (6–74 y). | |
| Duodenum | ? | ||
| CMMRD [7] | Colon/rectum | 59/146 individuals | 8-48y |
| Duodenum | 18/149 individuals | 11-42y | |
| Hematologic malignancies | ? | ¿ | |
| brain tumors | ¿ | ||
| GREM1-associated mixed polyposis | CRC | ? | ? |
| Serrated Polyposis Syndrome [8] | CRC | 15–35%. | 53.9 (median) |
Table 2.
Diagnostic criteria
| Syndrome | Diagnostic criteria |
|---|---|
| Peutz-Jeghers Syndrome |
(1) Two or more histologically confirmed PJS-type hamartomatous polyps, or (2) Any number of PJS-type polyps detected in an individual, who has a family history of PJS in (a) close relative(s) or (3) Characteristic mucocutaneous pigmentations in an individual, who has a family history of PJS in a close relative(s) or (4) Any number of PJS-type polyps in an individual who also has characteristic mucocutaneous pigmentations |
| Juvenile Polyposis Syndrome |
(1) More than five juvenile polyps in the colorectum or (2) Multiple juvenile polyps throughout the GI-tract or (3) Any number of juvenile polyps and a family history of JPS |
| POLE-associated polyposis | Heterozygosity for a pathogenic missense variant in the exonuclease domain (exon 9–14) of POLE, especially p.Leu424Val (Biallelic truncating/splice variants is associated with IMAGe syndrome (MIM: 614732)) and FILS syndrome (MIM: 615139)) |
| POLD1-associated polyposis | Heterozygosity for a pathogenic missense variant in the exonuclease domain (exons 6–12) of POLD1 |
| AXIN2-associated polyposis | Heterozygosity for a pathogenic variant in AXIN2, especially in exon 8 |
| MUTYH- NTHL1-MSH3- or MLH3-associated polyposis | Biallelic pathogenic variants in the relevant gene (MUTYH, NTHL1, MSH3, MLH3,) |
| Constitutional mismatch repair deficiency syndrome | Biallelic pathogenic variants in an MMR gene (MLH1, MSH2, MSH6, and PMS2, mainly PMS2. Biallelic carriers of highly penetrant variants are not viable and will die before birth |
| GREM1-associated mixed polyposis | Heterozygosity for a duplication upstream of GREM1 |
| Serrated Polyposis Syndrome |
(1) At least five serrated lesions/polyps proximal to the rectum, all being 5 or more mm in size with two or more being at least 10 mm in size. (2) More than 20 serrated lesions/polyps of any size distributed throughout the large bowel, with at least five being proximal to the rectum. |
| Polyposis with unknown etiology |
(1) At least one family member has had from 20 to 30 (dependent on age) to 99* colorectal polyps, and (2) screening of the relevant genes has not detected a pathogenic variant that can explain the family history, and (3) no other etiology to the gastrointestinal polyps/cancers observed in the family (e.g. genetic syndromes, inflammatory bowel disease etc.) is likely Furthermore, a family history with a significant occurrence of colorectal adenomas/polyps in two or more relatives can indicate a clinically significant predisposition. *If the patient or a relative has had over 100 polyps, the guidelines for FAP should be used |
Management of patients with a solitary Peutz-Jeghers-polyp or isolated MPs
Patients with a solitary Peutz-Jeghers polyp should be referred to a clinical genetics department for STK11 analysis. Endoscopy with gastroscopy, colonoscopy and video capsule enteroscopy could be performed in order to rule out PJS. If both genetic and endoscopic work-up is negative, PJS is unlikely and the patient (and family members) should not be subjected to further investigations or follow-up. Isolated MPs suggestive of PJS should be managed as described by Latchford et al. [11]
Juvenile polyposis syndrome
Juvenile Polyposis Syndrome (JPS) is characterized by the presence of few to over a 100 hamartomatous juvenile polyps in the GI-tract, mostly in the large intestine and stomach. A subgroup of patients with JPS and a PV in SMAD4 may have symptoms of hereditary hemorrhagic telangiectasia (HHT) as well as an increased risk of aortic aneurisms [12]. The phenotypic spectrum is broad and there is significant intra – and interfamilial variability in expressivity. JPS is inherited in an autosomal dominant manner. The distinction between patients – especially children – with solitary or few juvenile polyps from juvenile polyposis can be difficult, but for patients with only one juvenile polyp the risk for having JPS is low [13]. The risk of CRC and gastric cancer is increased with the risk of gastric polyposis, gastric cancer being highest in SMAD4 carriers [14].
The clinical approach may vary depending on the clinical picture. For SMAD4 carriers surveillance for HHT should start at 12 years, while the starting point for screening for aortopathy is less clear.
Autosomal dominant adenomatous polyposis syndromes
POLE-associated polyposis
PVs in the exonuclease domains of POLE (exon 9–14) were described in adult patients with colonic polyps and/or early-onset CRC in 2013 [15, 16]. Since then, additional cases have been reported [17, 18]. Still, data regarding the phenotypic characteristics, penetrance and estimation of cancer risk are limited. Café-au-lait pigmentations may be part of the phenotypic spectrum and are important to discover, as this manifestation can be suggestive of a more aggressive phenotype.
Several PVs have been reported with c.1270C > G, p.Leu424Val, (NM_006231.3) as the most frequent. Other pathogenic missense variants seem to be associated with a more severe phenotype with cancer including medulloblastoma) and CRC [19, 20]. There is an increased risk of CRC, and a high frequency of extraintestinal cancer has been reported. There is no evidence that truncating, loss-of-function variants cause POLE-associated polyposis.
The genotype should guide surveillance strategies: p.Leu424Val results in a more Lynch-like phenotype with adult-onset cancer, while other pathogenic missense variants (often de novo) result in a more severe phenotype including childhood cancer and skin pigmentations (see Suppl. Table 2). For carriers of other pathogenic missense variants, surveillance programs should be tailored case by case, but in general beginning in childhood.
POLD1-associated polyposis
PVs in the exonuclease domains of POLD1 (exon 6–12) were first described in patients with multiple adenomas and CRCs [21, 22]. Knowledge about phenotype, penetrance and risk of extracolonic cancers is limited, but polyposis and cancer develops in adulthood.
As with POLE, PVs are missense variants and there is no evidence that truncating, loss-of-functions variants cause polyposis and/or cancer.
AXIN2-associated polyposis
AXIN2-associated polyposis (also referred to as oligodontia-colorectal cancer syndrome) is a rare autosomal dominant syndrome, characterized by adenomatous colonic polyps varying in number from 0 to > 100. In most families, affected patients also have oligodontia and/or other aspects of ectodermal dysplasia. There is an increased risk of CRC, which is most often diagnosed in adulthood. Other variants in the gene seem to cause oligodontia/ectodermal dysplasia only [21, 22].
Autosomal recessive adenomatous polyposis syndromes
MUTYH- associated polyposis
MUTYH-associated polyposis (MAP) is characterized by multiple colorectal adenomas. The phenotypic spectrum is wide, and the number of polyps varies from few to over a 100. Approximately 2% of patients with MAP develop CRC without polyps. The lifetime risk of CRC in patients with MAP is well-documented and high (43–100%). Duodenal adenomas and gastric polyps are also found in a significant part of patients. MAP is caused by biallelic PVs in MUTYH thus the inheritance pattern is autosomal recessive. The carrier frequency in the Northern European population is estimated to be 1–2%. Two-point mutations, p.Y179C and p.G396D (NM_001128425), account for approximately. 85% of all PVs in individuals with Northern European ancestry. A high carrier frequency is also found in the Northern African population as well as in the non-Ashkenazi Jewish population.
Risk of cancer in heterozygotes with pathogenic MUTYH variants
The risk for colorectal adenomas in monoallelic carriers of pathogenic MUTYH-variants has been investigated in a prospective study from 2019 [23] which found no evidence of an adenomatous polyposis phenotype in monoallelic carriers. Carriers of a PV who have a first-degree relative with MAP have up to a 5-fold increased risk of CRC [24], while carriers in general have an over 3-fold increased risk. It is debated whether the finding of one PV in an individual should result in genetic testing of relatives (cascade-testing) as recommendations are contradictive [2, 25]. It is recommended that siblings of a patient with MAP are tested for the PV(s) in the family. Spouses of patients with MAP and spouses of patients, who are heterozygous carriers of a PV should be offered genetic screening of MUTYH.
NTHL1-associated polyposis
NTHL1-associated polyposis (or NTHL1-tumour syndrome) was described for the first time by Weren et al. in 2015 in patients with adenomatous polyposis in the lower GI-tract [26]. As of January 2020, reports of 34 patients with NTHL1-associated polyposis have been published [6, 26–32]. Development of NTHL1-associated polyposis is caused by biallelic PVs in NTHL1 and the inheritance pattern is autosomal recessive. Most patients are homozygous for the recurrent PV, NTHL1, c.268C > T, p.Gln90* (NM_002528).
There is a high frequency of CRC in the published cases, but also of breast- and duodenal cancer suggesting a broader cancer predisposition syndrome [32–39]. Thus, the phenotypic spectrum of this syndrome is still emerging.
Constitutional mismatch repair deficiency syndrome
Constitutional mismatch repair deficiency syndrome (CMMRD) is a distinct childhood cancer predisposition syndrome characterized by an increased risk of a broad spectrum of malignancies, and GI-polyposis in both the upper and lower GI-tract. Often café-au-lait spots and other findings that mimic neurofibromatosis type 1 are detected. The patients carry biallelic PVs in the MMR genes (MLH1, MSH2, MSH6 and PMS2) and thus the inheritance pattern is autosomal recessive. More than half of the patients known with CMMRD have bi-allelic PVs in PMS2 [33]. Recommendations for GI-surveillance are listed in Suppl.Table 2. Suggested surveillance for other malignancies is described by the European Consortium of CMMRD [34].
MSH3- and MLH3-associated polyposis
MSH3: As of January 2020, a total of four individuals from two families have been reported with biallelic PVs in MSH3 [35]. The inheritance pattern is autosomal recessive and the associated phenotype is characterized by the presence of colorectal adenomatous polyposis. Polyposis was accompanied by benign and malignant lesions in the GI- tract and extracolonic manifestations such as duodenal adenomas, thyroid adenomas, gastric cancer and astrocytoma.
MLH3: Olkinuora et al. [36] reported five patients from four families to be homozygous for PVs in MLH3,. The patients had 50–200 adenomatous polyps (age range 48–52 years). One of three female patients had breast cancer at age 52, and the male patient had CRC at age 48.
Other polyposis syndromes
GREM1-associated mixed polyposis
GREM1-associated Mixed Polyposis (previously Hereditary Mixed Polyposis syndrome) is an extremely rare condition with an unknown incidence. The condition was first described by Whitelaw et al. in 1997 [37] in an Ashkenazi Jewish family with mixed GI-polyposis and CRC. A genetic cause was reported in 2012 by Jaeger et al, who detected a 40 kb duplication upstream of GREM1 [38]. Since then other duplications have been reported [21, 39, 40]. The mode of inheritance is autosomal dominant and the condition is caused by upstream GREM1 duplications [40]. The histopathology of the polyps is variable and includes atypical juvenile polyps and/or hyperplastic polyps as well as adenomas and serrated adenomas, and there is a phenotypic overlap with other syndromes, although the phenotypic description is limited. CRC occurs with an increased frequency in adulthood [41].
Serrated polyposis syndrome
Serrated Polyposis Syndrome (SPS) (previously named Hyperplastic Polyposis Syndrome) Is a condition characterized by numerous serrated polyps in the colon. Although the prevalence is unknown, the syndrome is probably more common than anticipated. In fecal occult blood test-based screening cohorts 1:111–1:238 individuals were diagnosed [42, 43].
SPS is commonly grouped with the HPSs but does not appear to be inherited in a simple Mendelian fashion. Some studies link PVs in RNF43 to SPS; however, studies of larger cohorts suggest that RNF43 only explains a small proportion of cases [44, 45]. Individuals with SPS have an increased risk of CRC, and relatives have a recognized substantial risk of developing CRC, but the risk is not well defined [46].
Polyposis with unknown etiology
In some patients with a significant number of adenomas in the L-GI-tract, both with and without a family history of polyposis, the etiology is not identified by gene analysis. These patients/families may have the diagnosis of “polyposis of unknown etiology” although there is no clear definition of the term “polyposis”. The definition seen in Table 2 can be used as guidance.
Few publications have focused on this group of patients, and these are likely influenced by selection bias. Cancer occurrence has been reported in relatives, both in the colon and in the U-GI tract, but the inclusion criteria in these studies differ from those listed in Table 2 [47–49]. The National Comprehensive Cancer Network (NCCN) suggests surveillance/management guided by the phenotype of the patient and by the family history [2].
Conclusion
In recent years, HPSs have been identified due to the development in genetic technologies. Patients with these syndromes should be offered surveillance in order to reduce mortality and morbidity, and genetic analysis is crucial in the diagnostic pipeline. Long-term follow-up studies are needed in order to obtain evidence but are complicated by the small number of patients, lack of population-based data and risk of ascertainment bias. The guidelines presented will have to undergo revision as knowledge increases and new polyposis syndromes are identified.
Supplementary Information
Additional file 1: Supplementary Table 1: General recommendations for management of the Hereditary Polyposis Syndromes.
Additional file 2: Supplementary Table 2: Surveillance strategies for each Hereditary Polyposis Syndrome.
Acknowledgements
Not applicable.
Abbreviations
- CMMRD
Congenital mismatch repair deficiency
- CRC
Colorectal cancer
- FAP
Familial adenomatous polyposis
- FDR
First-degree relative
- HHT
Hereditary hemorrhagic telangiectasia
- HPS
Hereditary polyposis syndrome
- MP
Mucocutaneous pigmentations
- JPS
Juvenile polyposis syndrome
- L-GI
Lower gastrointestinal tract
- MAP
MUTYH-associated polyposis
- PJS
Peutz-Jeghers syndrome
- PV
Pathogenic variant
- U-GI
Upper gastrointestinal tract
- SPS
Serrated Polyposis Syndrome
- VCE
Videocapsule endoscopy
Authors’ contributions
John Gasdal Karstensen: literature search on hamartomatous polyposis, discussion of guidelines. Niels Jespersen, literature search on adenomatous polyposis syndromes, discussion of guidelines. Zoreh Ketabi, Literature search on the gynecological aspects of the syndrome, discussion of guidelines. Charlotte Lautrup, literature search on adenomatous polyposis syndromes and serrated polyposis syndromes, discussion of guidelines Karina Rønlund, literature search on adenomatous polyposis syndromes and serrated polyposis syndromes, discussion of guidelines. Lone Sunde, literature search on adenomatous polyposis syndromes, polyposis without known etiology, discussion of guidelines Karin Wadt, literature search on adenomatous polyposis syndromes, discussion of guidelines. Ole Thorlacius-Ussing, literature search on adenomatous polyposis syndromes, discussion of guidelines. Niels Qvist, literature search on hamartomatous polyposis, discussion of guidelines. Anne Marie Jelsig, literature search on hamartomatous polyposis, writing the final draft of the paper, figures and tables. The author(s) read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hinds R, Philp C, Hyer W, Fell JM. Complications of childhood Peutz-Jeghers syndrome: implications for pediatric screening. J Pediatr Gastroenterol Nutr. 2004;39(2):219–220. doi: 10.1097/00005176-200408000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosens LA, van Hattem A, Hylind LM, Iacobuzio-Donahue C, Romans KE, Axilbund J, et al. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56(7):965–967. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan DD, Stewart JR, Clendenning M, Rosty C, Mahmood K, Pope BJ, Jenkins MA, Hopper JL, Southey MC, Macrae FA, Winship IM, Win AK. Risk of colorectal cancer for carriers of a germ-line mutation in POLE or POLD1. Genet Med. 2018;20(8):890–895. doi: 10.1038/gim.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen M, Infante E, Brand R. MUTYH polyposis. 2012 Oct 4 [updated 2019 Oct 10]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK107219/.
- 6.Grolleman JE, de Voer RM, Elsayed FA, Nielsen M, Weren RDA, Palles C, Ligtenberg MJL, Vos JR, ten Broeke SW, de Miranda NFCC, Kuiper RA, Kamping EJ, Jansen EAM, Vink-Börger ME, Popp I, Lang A, Spier I, Hüneburg R, James PA, Li N, Staninova M, Lindsay H, Cockburn D, Spasic-Boskovic O, Clendenning M, Sweet K, Capellá G, Sjursen W, Høberg-Vetti H, Jongmans MC, Neveling K, Geurts van Kessel A, Morreau H, Hes FJ, Sijmons RH, Schackert HK, Ruiz-Ponte C, Dymerska D, Lubinski J, Rivera B, Foulkes WD, Tomlinson IP, Valle L, Buchanan DD, Kenwrick S, Adlard J, Dimovski AJ, Campbell IG, Aretz S, Schindler D, van Wezel T, Hoogerbrugge N, Kuiper RP. Mutational signature analysis reveals NTHL1 deficiency to cause a multi-tumor phenotype. Cancer Cell. 2019;35(2):256–266. doi: 10.1016/j.ccell.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Wimmer K, Kratz CP, Vasen HF, Caron O, Colas C, Entz-Werle N, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium 'care for CMMRD' (C4CMMRD) J Med Genet. 2014;51(6):355–365. doi: 10.1136/jmedgenet-2014-102284. [DOI] [PubMed] [Google Scholar]
- 8.Bleijenberg AG, JE IJ, van Herwaarden YJ, Carballal S, Pellise M, Jung G, et al. Personalised surveillance for serrated polyposis syndrome: results from a prospective 5-year international cohort study. Gut. 2020;69(1):112–121. doi: 10.1136/gutjnl-2018-318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12(10):3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 10.Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the international Cancer of the pancreas screening (CAPS) consortium. Gut. 2020;69(1):7–17. doi: 10.1136/gutjnl-2019-319352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latchford A, Cohen S, Auth M, Scaillon M, Viala J, Daniels R, Talbotec C, Attard T, Durno C, Hyer W. Management of Peutz-Jeghers Syndrome in children and adolescents: a position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr. 2019;68(3):442–452. doi: 10.1097/MPG.0000000000002248. [DOI] [PubMed] [Google Scholar]
- 12.Teekakirikul P, Milewicz DM, Miller DT, Lacro RV, Regalado ES, Rosales AM, Ryan DP, Toler TL, Lin AE. Thoracic aortic disease in two patients with juvenile polyposis syndrome and SMAD4 mutations. Am J Med Genet A. 2013;161A(1):185–191. doi: 10.1002/ajmg.a.35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jelsig AM, Brusgaard K, Hansen TP, Qvist N, Larsen M, Bojesen A, Nielsen CB, Ousager LB. Germline variants in Hamartomatous polyposis syndrome-associated genes from patients with one or few hamartomatous polyps. Scand J Gastroenterol. 2016;51(9):1118–1125. doi: 10.1080/00365521.2016.1174880. [DOI] [PubMed] [Google Scholar]
- 14.Blatter R, Tschupp B, Aretz S, Bernstein I, Colas C, Evans DG, Genuardi M, Hes FJ, Hüneburg R, Järvinen H, Lalloo F, Moeslein G, Renkonen-Sinisalo L, Resta N, Spier I, Varvara D, Vasen H, Latchford AR, Heinimann K. Disease expression in juvenile polyposis syndrome: a retrospective survey on a cohort of 221 European patients and comparison with a literature-derived cohort of 473 SMAD4/BMPR1A pathogenic variant carriers. Genet Med. 2020;22(9):1524–1532. doi: 10.1038/s41436-020-0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45(2):136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valle L, Hernandez-Illan E, Bellido F, Aiza G, Castillejo A, Castillejo MI, et al. New insights into POLE and POLD1 germline mutations in familial colorectal cancer and polyposis. Hum Mol Genet. 2014;23(13):3506–3512. doi: 10.1093/hmg/ddu058. [DOI] [PubMed] [Google Scholar]
- 17.Bellido F, Pineda M, Aiza G, Valdes-Mas R, Navarro M, Puente DA, et al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: review of reported cases and recommendations for genetic testing and surveillance. Genet Med. 2016;18(4):325–332. doi: 10.1038/gim.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosner G, Gluck N, Carmi S, Bercovich D, Fliss-Issakov N, Ben-Yehoyada M, Aharon-Caspi S, Kellerman E, Strul H, Shibolet O, Kariv R. POLD1 and POLE gene mutations in Jewish cohorts of early-onset colorectal Cancer and of multiple colorectal adenomas. Dis Colon Rectum. 2018;61(9):1073–1079. doi: 10.1097/DCR.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay H, Scollon S, Reuther J, Voicu H, Rednam SP, Lin FY, Fisher KE, Chintagumpala M, Adesina AM, Parsons DW, Plon SE, Roy A. Germline POLE mutation in a child with hypermutated medulloblastoma and features of constitutional mismatch repair deficiency. Cold Spring Harbor Mol Case Stud. 2019;5(5):a004499. doi: 10.1101/mcs.a004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wimmer K, Beilken A, Nustede R, Ripperger T, Lamottke B, Ure B, Steinmann D, Reineke-Plaass T, Lehmann U, Zschocke J, Valle L, Fauth C, Kratz CP. A novel germline POLE mutation causes an early onset cancer prone syndrome mimicking constitutional mismatch repair deficiency. Familial Cancer. 2017;16(1):67–71. doi: 10.1007/s10689-016-9925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohlin A, Rambech E, Kvist A, Torngren T, Eiengard F, Lundstam U, et al. Expanding the genotype-phenotype spectrum in hereditary colorectal cancer by gene panel testing. Familial Cancer. 2017;16(2):195–203. doi: 10.1007/s10689-016-9934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera B, Perea J, Sanchez E, Villapun M, Sanchez-Tome E, Mercadillo F, et al. A novel AXIN2 germline variant associated with attenuated FAP without signs of oligondontia or ectodermal dysplasia. Eur J Hum Genet. 2014;22(3):423–426. doi: 10.1038/ejhg.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Hachem N, Abadie C, Longy M, Colas C, Fert-Ferrer S, Leroux D, et al. Endoscopic phenotype of Monoallelic carriers of MUTYH gene mutations in the family of polyposis patients: a prospective study. Dis Colon Rectum. 2019;62(4):470–475. doi: 10.1097/DCR.0000000000001323. [DOI] [PubMed] [Google Scholar]
- 24.Win AK, Dowty JG, Cleary SP, Kim H, Buchanan DD, Young JP, et al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology. 2014;146:1208–1211. doi: 10.1053/j.gastro.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Leerdam ME, Roos VH, van Hooft JE, Dekker E, Jover R, Kaminski MF, Latchford A, Neumann H, Pellisé M, Saurin JC, Tanis PJ, Wagner A, Balaguer F, Ricciardiello L. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51(9):877–895. doi: 10.1055/a-0965-0605. [DOI] [PubMed] [Google Scholar]
- 26.Weren RD, Ligtenberg MJ, Kets CM, de Voer RM, Verwiel ET, Spruijt L, et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet. 2015;47(6):668–671. doi: 10.1038/ng.3287. [DOI] [PubMed] [Google Scholar]
- 27.Rivera B, Castellsague E, Bah I, van Kempen LC, Foulkes WD. Biallelic NTHL1 mutations in a woman with multiple primary tumors. N Engl J Med. 2015;373(20):1985–1986. doi: 10.1056/NEJMc1506878. [DOI] [PubMed] [Google Scholar]
- 28.Belhadj S, Quintana I, Mur P, Munoz-Torres PM, Alonso MH, Navarro M, Terradas M, Piñol V, Brunet J, Moreno V, Lázaro C, Capellá G, Valle L. NTHL1 biallelic mutations seldom cause colorectal cancer, serrated polyposis or a multi-tumor phenotype, in absence of colorectal adenomas. Sci Rep. 2019;9(1):9020. doi: 10.1038/s41598-019-45281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fostira F, Kontopodis E, Apostolou P, Fragkaki M, Androulakis N, Yannoukakos D, Konstantopoulou I, Saloustros E. Extending the clinical phenotype associated with biallelic NTHL1 germline mutations. Clin Genet. 2018;94(6):588–589. doi: 10.1111/cge.13444. [DOI] [PubMed] [Google Scholar]
- 30.Groves A, Gleeson M, Spigelman AD. NTHL1-associate polyposis: first Australian case report. Familial Cancer. 2019;18(2):179–182. doi: 10.1007/s10689-018-0107-1. [DOI] [PubMed] [Google Scholar]
- 31.Broderick P, Dobbins SE, Chubb D, Kinnersley B, Dunlop MG, Tomlinson I, Houlston RS. Validation of recently proposed colorectal Cancer susceptibility gene variants in an analysis of families and patients-a systematic review. Gastroenterology. 2017;152(1):75–77. doi: 10.1053/j.gastro.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altaraihi M, Gerdes AM, Wadt K. A new family with a homozygous nonsense variant in NTHL1 further delineated the clinical phenotype of NTHL1-associated polyposis. Hum Genome Variation. 2019;6(1):46. doi: 10.1038/s41439-019-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakry D, Aronson M, Durno C, Rimawi H, Farah R, Alharbi QK, et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: report from the constitutional mismatch repair deficiency consortium. Eur J Cancer. 2014;50:987–996. doi: 10.1016/j.ejca.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Vasen HF, Ghorbanoghli Z, Bourdeaut F, Cabaret O, Caron O, Duval A, et al. Guidelines for surveillance of individuals with constitutional mismatch repair-deficiency proposed by the European consortium "care for CMMR-D" (C4CMMR-D) J Med Genet. 2014;51(5):283–293. doi: 10.1136/jmedgenet-2013-102238. [DOI] [PubMed] [Google Scholar]
- 35.Adam R, Spier I, Zhao B, Kloth M, Marquez J, Hinrichsen I, Kirfel J, Tafazzoli A, Horpaopan S, Uhlhaas S, Stienen D, Friedrichs N, Altmüller J, Laner A, Holzapfel S, Peters S, Kayser K, Thiele H, Holinski-Feder E, Marra G, Kristiansen G, Nöthen MM, Büttner R, Möslein G, Betz RC, Brieger A, Lifton RP, Aretz S. Exome sequencing identifies Biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am J Hum Genet. 2016;99(2):337–351. doi: 10.1016/j.ajhg.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olkinuora A, Nieminen TT, Martensson E, Rohlin A, Ristimaki A, Koskenvuo L, et al. Biallelic germline nonsense variant of MLH3 underlies polyposis predisposition. Genet Med. 2019;21(8):1868–1873. doi: 10.1038/s41436-018-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitelaw SC, Murday VA, Tomlinson IP, Thomas HJ, Cottrell S, Ginsberg A, Bukofzer S, Hodgson SV, Skudowitz RB, Jass JR, Talbot IC, Northover JM, Bodmer WF, Solomon E. Clinical and molecular features of the hereditary mixed polyposis syndrome. Gastroenterology. 1997;112(2):327–334. doi: 10.1053/gast.1997.v112.pm9024286. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger E, Leedham S, Lewis A, Segditsas S, Becker M, Cuadrado PR, Davis H, Kaur K, Heinimann K, Howarth K, HMPS Collaboration. East J, Taylor J, Thomas H, Tomlinson I. Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet. 2012;44(6):699–703. doi: 10.1038/ng.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna DB, Van Den Akker J, Zhou AY, Ryan L, Leon A, O'Connor R, et al. Identification of a novel GREM1 duplication in a patient with multiple colon polyps. Familial Cancer. 2019;18(1):63–66. doi: 10.1007/s10689-018-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieberman S, Walsh T, Schechter M, Adar T, Goldin E, Beeri R, Sharon N, Baris H, Ben Avi L, Half E, Lerer I, Shirts BH, Pritchard CC, Tomlinson I, King MC, Levy-Lahad E, Peretz T, Goldberg Y. Features of patients with hereditary mixed polyposis syndrome caused by duplication of GREM1 and implications for screening and surveillance. Gastroenterology. 2017;152(8):1876–1880. doi: 10.1053/j.gastro.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson I, Rahman N, Frayling I, Mangion J, Barfoot R, Hamoudi R, Seal S, Northover J, Thomas HJW, Neale K, Hodgson S, Talbot I, Houlston R, Stratton MR. Inherited susceptibility to colorectal adenomas and carcinomas: evidence for a new predisposition gene on 15q14-q22. Gastroenterology. 1999;116(4):789–795. doi: 10.1016/S0016-5085(99)70061-2. [DOI] [PubMed] [Google Scholar]
- 42.Bleijenberg AG, Je IJ, van Herwaarden YJ, Carballal S, Pellise M, Jung G, et al. Personalised surveillance for serrated polyposis syndrome: results from a prospective 5-year international cohort study. Gut. 2020;69(1):112–121. doi: 10.1136/gutjnl-2018-318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeg IJ, Bevan R, Senore C, Kaminski MF, Kuipers EJ, Mroz A, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2017;66(7):1225–1232. doi: 10.1136/gutjnl-2015-310784. [DOI] [PubMed] [Google Scholar]
- 44.Yan HHN, Lai JCW, Ho SL, Leung WK, Law WL, Lee JFY, Chan AKW, Tsui WY, Chan ASY, Lee BCH, Yue SSK, Man AHY, Clevers H, Yuen ST, Leung SY. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut. 2017;66(9):1645–1656. doi: 10.1136/gutjnl-2016-311849. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan DD, Clendenning M, Zhuoer L, Stewart JR, Joseland S, Woodall S, Arnold J, Semotiuk K, Aronson M, Holter S, Gallinger S, Jenkins MA, Sweet K, Macrae FA, Winship IM, Parry S, Rosty C, Genetics of Colonic Polyposis Study Lack of evidence for germline RNF43 mutations in patients with serrated polyposis syndrome from a large multinational study. Gut. 2017;66(6):1170–1172. doi: 10.1136/gutjnl-2016-312773. [DOI] [PubMed] [Google Scholar]
- 46.Win AK, Walters RJ, Buchanan DD, Jenkins MA, Sweet K, Frankel WL, de la Chapelle A, McKeone DM, Walsh MD, Clendenning M, Pearson SA, Pavluk E, Nagler B, Hopper JL, Gattas MR, Goldblatt J, George J, Suthers GK, Phillips KD, Woodall S, Arnold J, Tucker K, Field M, Greening S, Gallinger S, Aronson M, Perrier R, Woods MO, Green JS, Walker N, Rosty C, Parry S, Young JP. Cancer risks for relatives of patients with serrated polyposis. Am J Gastroenterol. 2012;107(5):770–778. doi: 10.1038/ajg.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tieu AH, Edelstein D, Axilbund J, Romans KE, Brosens LA, Wiley E, Hylind L, Giardiello FM. Clinical characteristics of multiple colorectal adenoma patients without germline APC or MYH mutations. J Clin Gastroenterol. 2016;50(7):584–588. doi: 10.1097/MCG.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallenberg FGJ, Latchford A, Lips NC, Aalfs CM, Bastiaansen BAJ, Clark SK, Dekker E. Duodenal adenomas in patients with multiple colorectal adenomas without germline APC or MUTYH mutations. Dis Colon Rectum. 2018;61(1):58–66. doi: 10.1097/DCR.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 49.Giarola M, Stagi L, Presciuttini S, Mondini P, Radice MT, Sala P, et al. Screening for mutations of the APC gene in 66 Italian familial adenomatous polyposis patients: evidence for phenotypic differences in cases with and without identified mutation. Hum Mutat. 1999;13(2):116–23. 10.1002/(SICI)1098-1004(1999)13:2<116::AID-HUMU3>3.0.CO;2-2. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1: General recommendations for management of the Hereditary Polyposis Syndromes.
Additional file 2: Supplementary Table 2: Surveillance strategies for each Hereditary Polyposis Syndrome.
Data Availability Statement
Not applicable.