Abstract
Purpose:
Immune checkpoint inhibition (ICI) alone is not active in mismatch repair-proficient (MMR-P) metastatic colorectal cancer (mCRC), nor does radiotherapy (RT) alone result in objective systemic benefit. However, combined RT plus ICI can induce systemic anti-tumor immunity in pre-clinical and clinical models.
Experimental Design:
In this single-center, phase II study, patients with chemotherapy-refractory MMR-P mCRC received durvalumab 1500 mg plus tremelimumab 75 mg every 4 weeks plus RT. The primary endpoint was objective response rate (ORR) in non-irradiated lesions. Treatment and efficacy were correlated with peripheral immune cell profiles.
Results:
We enrolled 24 patients, and report outcomes after a median follow up of 21.8 (range: 15.9 to 26.3) months. The ORR was 8.3% (2 patients) (95% confidence interval [CI], 1.0% to 27.0%). The median progression-free survival was 1.8 (95% CI, 1.7 to 1.9) months, median overall survival was 11.4 (95% CI, 10.1 to 17.4) months. Twenty five percent of patients (n=6) had treatment-related grade 3–4 adverse events. We observed increased circulating CD8+ T lymphocyte activation, differentiation, and proliferation in patients with objective response.
Conclusion:
This combination of RT plus ICI study did not meet the prespecified end point criteria to be considered worthwhile for further study. However, rare instances of systemic immune augmentation and regression in non-irradiated lesions were observed (an abscopal response). Combination durvalumab and tremelimumab plus RT is feasible in MMR-P mCRC with a manageable safety profile. Further studies of novel immunotherapy combinations, and identification of biomarkers predictive of abscopal response are warranted.
Introduction
While immunotherapy with immune checkpoint inhibition (ICI), using monoclonal antibodies targeting programmed cell death 1 (PD-1), PD-1 ligand (PDL-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4), may lead to durable benefit in patients with several tumor types, patients with mismatch repair-proficient (MMR-P) metastatic CRC have limited benefit (1–3). Novel strategies to augment immunity are thus needed in this population.
Radiotherapy (RT) is a commonly used treatment modality for all stages of CRC and may augment anti-tumor activity when combined with immunotherapy by synergistically priming and sustaining the immune response. Initial preclinical and clinical studies suggested increased tumor antigen release; immunogenic tumor cell death with release of endogenous danger signals, such as HMGB1 and ATP; up-regulation of pro-inflammatory cytokines, such as TNF-α, and IL-1α; recruitment and activation of dendritic cells; improved antigen presentation; and, increased T lymphocyte infiltration (4–14).
Combining RT with ICI is therefore compelling, with reported observations of regression in distant non-irradiated tumors, in several types of malignancies, including melanoma, lung cancer, and pancreatic ductal adenocarcinoma (15–17). We have previously described a case of tumor regression of non-irradiated distant tumors in a patient with metastatic MMR-P CRC following RT plus pembrolizumab (18).
In the current study, we aimed to determine whether the combination of tremelimumab, a selective human IgG2 monoclonal antibody inhibitor of CTLA-4 (19), and durvalumab, a selective, high-affinity, engineered human IgG1 monoclonal antibody that blocks PD-L1 binding to PD-1 (20), can lead to enhanced immunity with shrinkage of non-irradiated tumors (an abscopal effect) in patients with metastatic MMR-P CRC.
Methods
Patients and Treatment:
Patients aged 18 years or older with a histologic or cytologic diagnosis of metastatic CRC, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and who had received at least two prior therapies were eligible for the study. Patients had at least one lesion for which palliative RT was considered appropriate therapy, and at least one other index lesion that is outside of the RT field and measurable based on the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) (21). All patients had adequate organ and bone marrow function. Standard key exclusion criteria applied. The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (MSKCC) and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent before study enrollment. ClinicalTrials.gov number, NCT03122509.
This was an investigator-initiated, single-center, non-randomized phase II study. Patients received durvalumab 1500 mg and tremelimumab 75 mg intravenously every 4 weeks during the first 4 cycles followed by durvalumab 1500 mg alone, beginning within one week prior to starting RT (Supplemental Figure 1).The RT dose and schedule was not pre-specified, and was determined per standard care. Treatment was continued until progression of disease, initiation of alternative cancer therapy, or unacceptable toxicity. Patients who had a prior response, and who did not discontinue tremelimumab due to toxicity, could resume combination therapy with tremelimumab plus durvalumab for 4 doses upon disease progression, followed by durvalumab alone. No dose reductions were permitted, but dose interruption and repeat palliative RT were allowed. Patients could continue to receive treatment beyond radiographic progression in the absence of clinical deterioration.
Biopsies for research purposes were performed pre-treatment, 1 week after completing RT (of the irradiated lesion) and 4 weeks after starting ICI (of a non-irradiated lesion). Peripheral blood samples were obtained at baseline, then at week 2, week 4, and week 8 after starting ICI.
Safety:
Safety was assessed for all patients by physical exam, vital signs, and routine blood tests every 2 to 4 weeks. Adverse events (AEs) were monitored throughout treatment and for 28 days thereafter. Serious AEs were collected for 90 days after the end of treatment and were graded in severity according to the Common Terminology Criteria for Adverse Events version 4.03. Treatment-related AEs (TRAEs) were defined as any AE possibly, probably, or definitely related to study drug.
Efficacy:
The primary end point was objective response rate (ORR) in a non-irradiated lesion according to RECIST v1.1. ORR was defined as the percentage of patients achieving either a partial response (PR) or a complete response (CR) to treatment. Tumor response was assessed by means of computed tomography and/or magnetic resonance imaging performed at baseline and every 8 weeks thereafter. The secondary end points were safety and tolerability, overall survival (OS), and progression free survival (PFS). All patients were followed up to 2 years for survival or until death, consent withdrawal, or study closure.
A two-stage Simon optimal design was employed to test the null hypothesis that the ORR was ≤ 5% versus the alternative hypothesis that the ORR was at least 25% with type I and II error rates of 10% each. In the first stage, 9 patients were enrolled, with the study expanded to the second stage to enroll an additional 15 patients if at least 1 out of 9 patients achieved a PR or CR. It was pre-defined that further investigation of the treatment would be considered worthwhile if ≥ 3 of 24 objective responses were observed by RECIST v1.1. Accrual time was estimated to be 2 years.
Genomic Correlates:
Immunohistochemical staining (IHC) for mismatch repair (MMR) protein expression was performed using standard procedures (22). Microsatellite instability (MSI) was assessed from Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) via MSIsensor. MSI-high (MSI-H) status was defined as an MSIsensor score ≥ 10 or an MSIsensor score ≥ 3 with tumor mutation burden (TMB) > 10 mutations/ megabase (mut/Mb) (23). MSK-IMPACT is approved by the NYS Department of Health for clinical use and authorized by the FDA for clinical reporting of somatic mutations, indels, rearrangements, and MSI calculated from the microsatellite regions covered by the assay (24, 25).
Immune Correlates:
Peripheral blood mononuclear cells (PBMC) were isolated and cryopreserved from patient whole blood samples. Flow cytometry was then performed as previously described (26), on batch thawed PBMC samples in the Immune Monitoring Facility at MSKCC to examine T cell phenotypic markers. Samples were stained with a fixable viability dye (FVS510, BD Biosciences) and a cocktail of antibodies to the following surface markers: CD45RA-BUV395 (BD, HI100), CD4-BUV496 (BD, SK3), ICOS-BUV563 (BD, DX29), CD25-BUV615 (BD, 2A3), TIM-3-BUV661 (BD, 7D3), CD27-BUV737 (BD, L128), CD8-BUV805 (BD, SK1), CD57-BV421 (BD, NK-1), CXCR5-BV480 (BD, RF8B2), CD14-BV570 (BioLegend, M5E2), CD19-BV570 (BioLegend, HIB19), CCR4-BV605 (BioLegend, L291H4), CCR7-SB645 (eBioscience, 3D12) HLA-DR-BV711 (BD, G46–6), CD3-BV750 (BD, SK7), CD28-BV786 (BD, CD28.2), PD-1-BB515 (BD, MIH4), CD127-BB700 (BD, HIL-7R-M21), CD38-BB790 (BD, HIT2), TIGIT-PE (eBioscience, MBSA43), and GITR-PE-Cy7 (eBioscience, eBioAITR), in the presence of Brilliant Stain Buffer Plus (BD). Cells were next fixed and permeabilized with the FoxP3/Ki-67 Fixation/Permeabilization Concentrate and Diluent (eBioscience), and subsequently stained intracellularly with antibodies to LAG-3-BB660 (BD, T47–530), Ki-67-AlexaFluor700 (BD, B56), FoxP3-PE-Cy5.5 (eBioscience, PCH101), CTLA-4-PE-Cy5 (BD, BNI3), Eomes-PE-eFluor610 (eBioscience, WD1928), T-bet-APC (eBioscience, ebio4B10), and Granzyme B-APC-Fire750 (BioLegend, QA16A02), in the presence of Brilliant Stain Buffer Plus (BD). Stained cells were acquired on a BD Biosciences FACSymphony and analyzed using FlowJo software (FlowJo, LLC).
Statistical methods:
Patient demographic and disease characteristics were summarized using frequency for categorical and median (range) for continuous characteristics. ORR and 95% CI were estimated using a binomial distribution. Kaplan-Meier methods were used to evaluate PFS and OS. PFS was measured from the start of treatment with ICI until the documentation of disease progression, or death due to any cause, whichever occurs first. Patients who came off study due to clinical progression were included as progression of disease at the off-study date. OS was determined as the time from the start of treatment with ICI until death. For patients who are alive at the end of study or lost to follow-up, OS was censored on the last date when patients were known to be alive. The data-lock date was 09/03/2019. The data-lock for survival endpoints was 05/22/2020.
Immuno-biomarkers at baseline, week 2 and week 8 after treatment were summarized using median and range. Wilcoxon Sign-Rank test was used to evaluate the difference in median of biomarkers from baseline to week 2, as well as from baseline to week 8. False discovery rate approach was applied to control the Type I errors (27). Two-sided p-values less than 0.05 were considered statistically significant. All analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) or SAS 9.3 (The SAS Institute, Cary, NC).
Results
Patients and Treatment:
24 patients were enrolled between May 2017 and February 2019, and received at least one dose of durvalumab plus tremelimumab (durva/treme). Median RT dose delivered was 4,500 cGy (range: 2,000 cGy to 7,000 cGY) over 5 Fractions (Fx) (range: 3 Fx to 30 Fx). Most patients received RT to tumor located within liver (29%), colorectum (17%), lung (13%), or bone (13%). The demographic and patient characteristics of the patients at baseline are shown in Table 1. Radiation details are provided in Supplemental Table 1. MMR status was confirmed proficient on IHC or determined to be MSS on MSK-IMPACT in 23 patients (96%), with results unavailable for 1 patient (a non-responder). Patients had received a median of 2 prior treatments (range: 1–6). All patients were included in the analyses of the primary and secondary end points.
Table 1:
Baseline Patient and Disease Characteristics
| Characteristic | Patients |
|---|---|
| No of pts | 24 |
| Age, years, median (range) | 55 (26–78) |
| Sex, male | 13 (54%) |
| Race | |
| White | 19 (79%) |
| Asian | 3 (13%) |
| Black | 1 (4%) |
| Other | 1 (4%) |
| ECOG PS | |
| 0 | 6 (25%) |
| 1 | 18 (75%) |
| RT site | |
| Liver | 7 (29%) |
| Colorectum | 4 (17%) |
| Lung | 3 (13%) |
| Bone | 3 (13%) |
| Lymph node | 2 (8%) |
| Peritoneum/ mesentery | 2 (8%) |
| Soft tissue | 2 (8%) |
| Spleen | 1 (4%) |
| Confirmed MMR-P/ MSS | 23 (96%) |
| Prior systemic therapy, median (range) | 2 (1–6) |
Safety:
TRAEs occurring in >10 patients, or any grade 3–4 TRAE are summarized in Table 2 (complete list of TRAE in Supplemental Table 2). TRAEs of any grade occurred in 21/24 patients (88%). Grade 3/4 TRAEs occurred in 6/24 patients (25%); one grade 4 TRAE of hyperglycemia. The most common immune-mediated AEs were pruritis (33%), skin rash (25%), diarrhea (21%), colitis (13%), and arthralgia (13%).
Table 2:
Treatment-related adverse events (>10%, or any grade 3–4)
| Adverse event | All Grades (1–4),*
No. patients (%) |
All Grades 3–4,*
No. patients (%) |
|---|---|---|
| Total No. of treatment-related adverse events | 21 (88) | 6 (25) |
| GI disorders | ||
| Diarrhea | 5 (21) | 3 (13) |
| Colitis | 3 (13) | 2 (8) |
| Anorexia | 3 (13) | 1 (4) |
| Vomiting | 2 (8) | 1 (4) |
| Gastroenteritis | 1 (4) | 1 (4) |
| Rectal pain | 1 (4) | 1 (4) |
| Enteritis | 1 (4) | 1 (4) |
| Abdominal pain | 1 (4) | 1 (4) |
| Dry mouth | 4 (17) | |
| Nausea | 4 (17) | |
| Laboratory investigations | ||
| Hyperglycemia | 2 (8) | 2 (8) |
| Alanine aminotransferase increased | 1 (4) | 1 (4) |
| Aspartate aminotransferase increased | 1 (4) | 1 (4) |
| Endocrine disorders | ||
| Diabetic Ketoacidosis | 1 (4) | 1 (4) |
| Skin disorders | ||
| Pruritus | 8 (33) | |
| Rash maculo-papular | 6 (25) | |
| Musculoskeletal disorders | ||
| Arthralgia | 3 (13) | |
| General disorders | ||
| Fatigue | 7 (29) | |
| Chills | 5 (21) | |
| Malaise | 3 (13) | |
| Fever | 3 (13) |
A patient that experienced multiple occurrences of an adverse event was counted once at the maximum recorded grade.
The most common grade 3–4 immune-mediated AEs were diarrhea (13%), colitis (8%) and hyperglycemia (8%). Four (16%) patients developed grade 3 gastrointestinal toxicity (colitis, enteritis or gastroenteritis), having received RT to pelvis/colorectum (n=2), mesentery (n=1) or liver (n=1), respectively. Two patients (8%) developed diabetes mellitus, having received RT to liver, one in the setting of pancreatic metastases. Among patients who experienced TRAEs, 7 (29%) received systemic corticosteroids, and 7 (29%) experienced treatment interruption. No patient fully discontinued treatment on study due to toxicity. Three (13%) patients discontinued tremelimumab due to toxicity.
Efficacy:
Two patients of the 24 treated had an objective response in non-irradiated tumors, with an ORR of 8.3% (95% CI, 1.0% to 27%). One patient progressed 3.4 months after achieving PR, the other patient remains progression-free at 12 months following PR. Three patients (12%) experienced stable disease as best response, no SD of 4 months or more was observed. The characteristics of response to durva/treme are shown in Table 3 and Figure 1. The median follow-up at the time of data-lock was 21.8 (range: 15.9 to 26.3) months amongst survivors. Twenty-one deaths were observed at analysis. No deaths were attributed to treatment. Median PFS was 1.8 (95% CI, 1.7 to 1.9) months. The median OS was 11.4 (95% CI, 10.1 to 17.4) months.
Table 3:
Objective Response According to RECIST v1.1 Criteria
| Type of response | Patients |
|---|---|
| (n=24) | |
| Complete response, No. (%) | 0 |
| Partial response, No. (%) | 2 (8.3) |
| Stable disease, No. (%) | 3 (12) |
| Progressive disease, No. (%)† | 19 (79.2) |
| Objective response rate, % (95% CI) | 8.3 (1 to 27) |
| Time to response, months, median (range) | 3.5 (3.48–3.58) |
Includes patients who not undergo a scan due to clinical progression
Figure 1:
Waterfall plot showing target lesion change
Maximum percentage change from baseline in the size of tumors in all patients treated with durva/treme and RT (progression >100% was cut off at 100%). Two patients (right bars) achieved an objective response, one patient remains on treatment.
Notable findings in the responder patients:
Two patients demonstrated a PR in terms of overall non-irradiated tumor burden (outside of the RT field). Patient 1 received RT (5,000 cGy over 5 Fx) to a liver metastasis 7 days after starting durva/treme. The tumor was MMR-P on IHC and MSS on MSK-IMPACT (MSISensor score 0.77), TMB was 10.8 mt/Mb and 11 gene mutations were identified (Supplemental Table 3). For comparison, median TMB available for 18 non-responders was 5.9 mt/Mb (range: 3.0 to 8.8 mt/Mb). The primary tumor was located in the distal transverse colon. The second follow up scan (week 15) showed PR in lung metastases (−46% according to RECIST v1.1) that was subsequently confirmed (Figure 2). Duration of response was 3.4 months. The patient experienced grade 2 colitis, with improvement on high dose steroids. Prior therapies included FOLFOX, floxuridine and mitomycin via hepatic arterial infusion pump (HAIP), FOLFIRI and panitumumab.
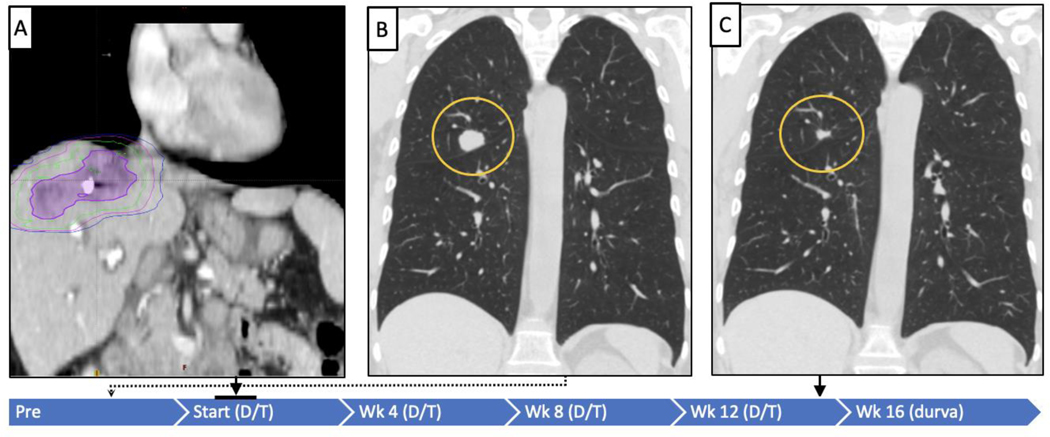
Figure 2:
Treatment and response timeline for responding patient 1
The blue bar indicates study time line, and treatment with durva/treme (D/T) or durva alone (D). Panel A illustrates the RT field targeting a liver tumor, the purple outline is tumor. The green is the line corresponding to 100% of radiation dose (5,000 cGy/5 Fx), magenta is 50% and blue is 30% of the dose. Panels B and C demonstrate baseline imaging and representative lesion of RECIST-defined objective response in a non-radiated tumor at week 16 after starting durva/ treme (−45.8%).
Patient 2 underwent RT (2,000 cGy over 5 Fx) to a left retroperitoneal mass 3 days after starting durva/treme. The tumor was MMR-P on IHC and MSS on MSK-IMPACT (MSISensor score 1.1), TMB was 5.3 mt/Mb and 6 gene mutations were identified (Supplemental Table 3). The primary tumor was located in the left-sided colon. The first follow up scan (week 8) showed a mixed response in liver metastases, with areas of pseudoprogression. Imaging at week 16 showed PR overall in non-irradiated tumor burden (−52% according to RECIST v1.1) that was subsequently confirmed. Tumor biopsies revealed necrosis and an immune infiltrate (Figure 3). The patient continues on treatment. At data-lock, duration of response was 12 months. Prior systemic therapies included CAPEOX/ bevacizumab, IFL/ bevacizumab, regorafenib, followed by TAS-102.
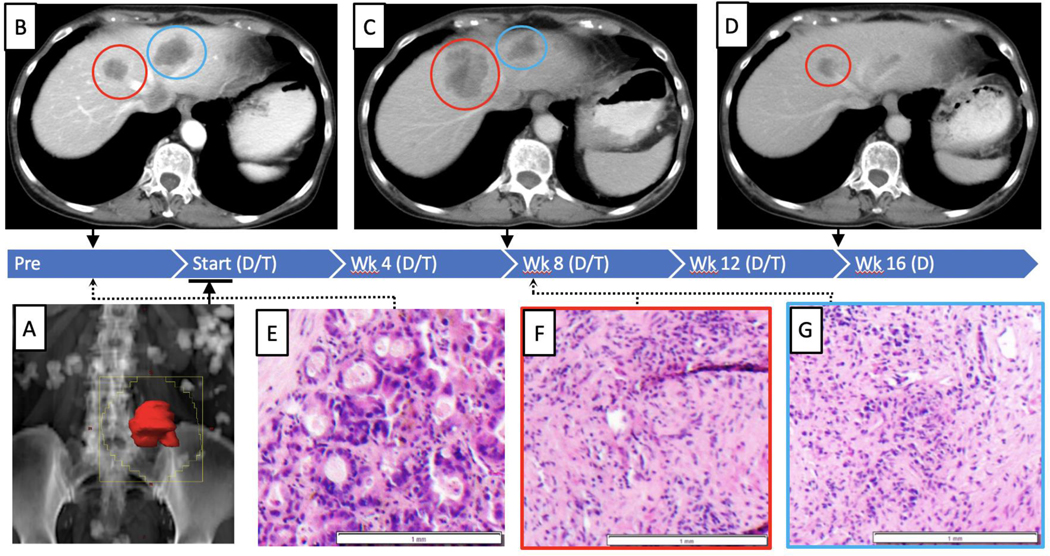
Figure 3:
Treatment and response timeline for responding patient 2
Mixed response and pseudoprogression in a patient achieving an objective response. The blue bar indicates study time line, and treatment with durva/treme (D/T) or durvalumab alone (D). Panel A illustrates the RT field (i.e. 2,000 cGy/5 Fx), targeting a retroperitoneal tumor, the tumor is in red, and the portal is the jagged yellow outline. Upper panels demonstrate radiographic change on treatment. Panel B illustrates two liver metastases observed on baseline imaging. Panel C illustrates response in a non-radiated liver lesion (blue circle) and pseudoprogression in a separate non-radiated lesion (red circle) at week 8 on treatment. Panel D illustrates a subsequent RECIST-defined objective response in both lesions at week 16 (−49.4%). Lower panels illustrate histopathology obtained pre-treatment (panel E), and 9 weeks post-treatment of the pseudoprogression lesion (panel F) and the responding lesion (panel G). Both lesions demonstrated replacement by necrosis and marked inflammatory infiltrate at week 9.
Immune Correlates:
Flow cytometry was performed on PBMCs obtained prior to treatment (n=21 patients), and then at 2, 4, and 8 weeks after starting durva/treme. Since RT started after durva/treme, the on-treatment time points corresponded to a median of approximately 11 days, 3.5 weeks, and 7.6 weeks after starting RT.
Exploratory flow analysis of the patient aggregate data showed a slight decrease in the frequency of CD3+ T lymphocytes as a percentage of all live cells at week 2 (0.8-fold of baseline [padj 0.013], median of 56% CD3+ vs. 44% CD3+)), although the proportion of CD8+ and CD4+ T lymphocytes was unchanged. At 2–4 weeks after initial treatment, both CD4+ and CD8+ T cell populations showed increased markers of T cell activation and exhaustion, such as Ki-67, CD38, HLA-DR, ICOS, TIM-3, LAG-3, and PD-1. The population of Ki-67+ proliferating CD8+ and CD4+ T lymphocytes peaked at week 2 (2.5-fold increase over baseline for CD8 [padj <0.001], 3.5-fold increase over baseline for CD4 [padj <0.001]), and then declined by week 8. T regulatory cells (FoxP3+CD4+ Treg) increased in frequency by week 2 (1.5-fold of baseline [padj 0.001]) (Supplemental Table 4).
Among the two responders, flow cytometry data revealed a relatively higher percentage of CD25+CD4+ T lymphocytes compared to non-responders at baseline and on treatment, indicative of increased frequencies of activated CD4+ T lymphocytes in these patients. Amongst the responders’ CD8+ T lymphocytes, there were more sustained increases in HLA-DR, Ki-67, and PD-1, along with increases in effector memory CD8+ T lymphocytes, indicating more pronounced CD8+ T lymphocyte activation, differentiation, and proliferation compared to non-responders. Moreover, there was increased co-expression of Ki-67, ICOS, and PD-1 in responders that may indicate reinvigoration of exhausted cells, compared to non-responders. There appeared to be a more sustained increase in effector memory CD8+ T lymphocytes (CCR7-CD45RA-) that were actively proliferating as indicated by expression of Ki-67 on effector memory T cells (Tem), compared to non-responders (Figure 4 and representative flow dot plots for Ki-67 and PD-1 in Supplemental Figure 2).
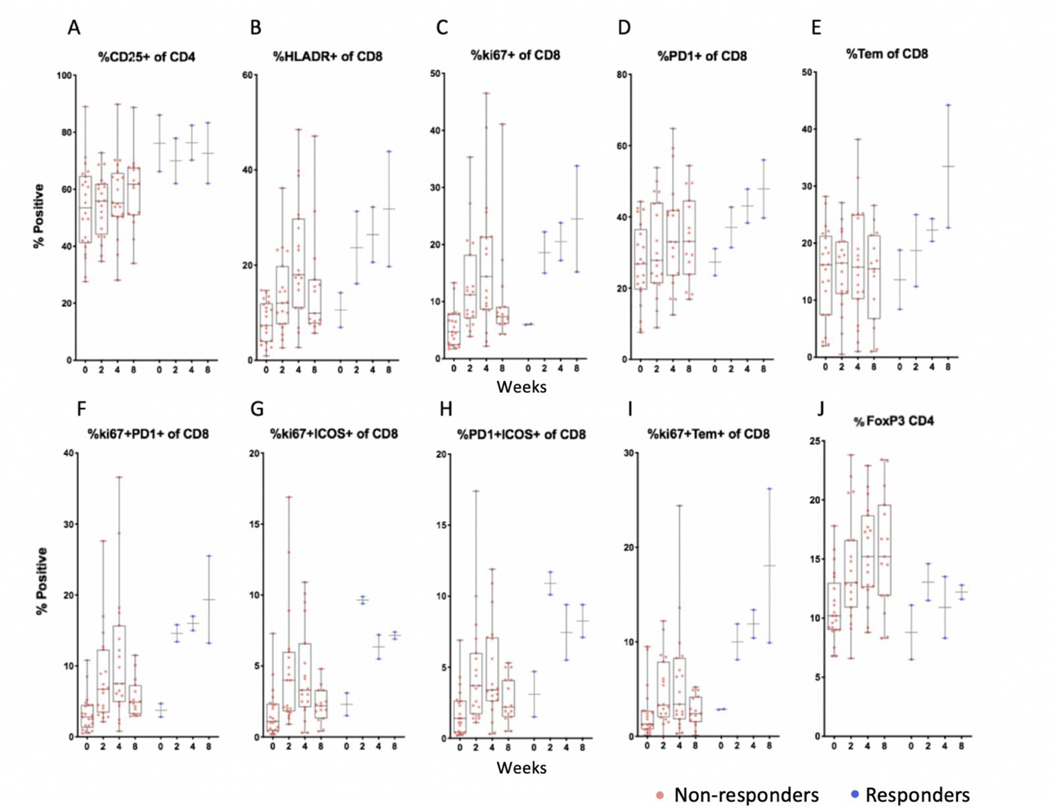
Figure 4:
Peripheral blood flow cytometry showing population % positive and median values among responders and non-responders
Flow cytometry analysis showing the percentage (y-axis) of CD4+ or CD8+ T cells positive for the indicated immune markers at pre-treatment baseline and at weeks 2, 4 and 8 after starting treatment. Symbols reflect unique patients. Two responding patients (blue) compared to non-responders (brown) showed higher frequencies of CD25+CD4+ T lymphocytes relative to most non-responders. Within the CD8+ T lymphocyte population, relatively higher increases in HLA-DR, Ki-67, PD-1, and effector memory cells were observed (L to R, panels A-E) along with more sustained increases in CD8 T cells co-expressing Ki-67, PD-1 and ICOS (panels F-H). More sustained increases were seen in proliferating Ki67+ Tem effector memory (CCR7-CD45RA-) CD8 T lymphocytes (panel I). T regulatory cells (FoxP3+CD4+ Treg) increased in frequency by week 2 in responders and non-responders, but were overall lower in frequency relative to most non-responders (panel J).
Discussion
We report the safety and efficacy of RT plus durvalumab and tremelimumab in metastatic CRC. The study did not meet the pre-defined endpoint for further study (defined as ≥ 3 of 24 objective responses by RECIST v1.1), yet the results did demonstrate that RT in combination with durvalumab and tremelimumab can lead to shrinkage of distant, non-irradiated tumors in MMR-P metastatic CRC, with manageable safety. To our knowledge, this is the first prospective report of safety and efficacy of combination ICI plus RT in CRC.
Our observations are supported by two recent studies. We previously described that RT plus pembrolizumab, an anti-PD-1 monoclonal antibody, led to shrinkage of non-irradiated distant tumors in a patient with metastatic MMR-P CRC, with an ORR of 4.8% (1/24) (18). Parikh and colleagues described that RT plus ipilimumab, an anti-CTLA-4 monoclonal antibody, and nivolumab, an anti-PD-1 monoclonal antibody, led to shrinkage of non-irradiated distant tumors in patients with metastatic MMR-P CRC with an ORR of 15% (4/27) (28). Combined, these studies suggest abscopal immunity in 7 out of 75 patients with metastatic MMR-P CRC following RT plus ICI.
This finding is significant because neither RT or ICI alone is expected to result in clinically meaningful distant tumor regression in patients with MMR-P CRC (1–3). As previously reported, MMR-P CRC has been exceedingly challenging to treat with ICI, and responses are rare and anecdotal, including with the combination of durvalumab plus tremelimumab that resulted in one response out of 118 patients (<1% ORR) (29).
Our observations in CRC provide further support for synergy between ICI and RT with shrinkage in non-radiated lesions, as reported in other tumor types, including melanoma, Hodgkin’s lymphoma, lung cancer, and pancreatic ductal adenocarcinoma (15–17, 30, 31).
We observed 2 confirmed objective responses (8.3%) outside of the radiation field, and an overall median survival of 11.4 months. Both patients were confirmed MMR-P on IHC and MSS on MSK-IMPACT. TMB in the 2 responders was 10.8 mt/Mb and 5.3 mt/Mb, respectively. For comparison, the median TMB among 18 non-responders was 5.9 mt/Mb (range: 3.0 to 8.8 mt/Mb). These data do not support a hypothesis that an abscopal effect is more likely in tumors with higher TMB. Of note, the proportion of MSS CRC estimated to be TMB high (≥11.7 mut/Mb) is approximately 2.9% (32), and the recent study of durva/treme in CRC patients identified a possible correlation between TMB (measured in cell free DNA) and improved outcome, but not between TMB and response rate (29).
The combination of durva/treme and radiation overall appeared tolerable with manageable safety. The rate of grade 3–4 TRAE (25%) was consistent with prior reports of the combination of CTLA-4 and PD-1/PDL-1 blockade (29, 33–35), and no new safety signals were identified. Four (16%) patients experienced grade 3 gastrointestinal TRAE, following RT to the liver, mesentery or pelvis, that improved with standard immunosuppressive treatment. The most common toxicities observed, regardless of grade, were pruritus, fatigue, rash, diarrhea, chills, dry mouth and nausea.
To identify additional biomarkers of immune activation and response to combination ICI plus RT, we performed an exploratory analysis using PBMC samples obtained from patients prior to starting treatment and on treatment. We observed increased proliferation and activation of CD4+ and CD8+ T lymphocytes after the combination ICI and RT. In general, changes were seen early, by the first analysis at week 2, and were variable by week 8, with slowing of CD8+ and CD4+ T lymphocyte proliferation. Although we had only 2 responders, we observed an association between clinical benefit and increased CD8+ T lymphocyte activation and proliferation, along with increased effector memory phenotype CD8+ T lymphocytes and reinvigoration of exhausted cells as indicated by increased Ki-67+ proliferating CD8+ T lymphocytes that were PD-1+. These phenotypic changes suggest enhanced CD8+ adaptive immunity in the two patients who derived clinical benefit. Studies of archival tumor samples collected before and during treatment are ongoing.
This study is limited by the small number of patients evaluated. It also leaves several questions unanswered in general, and in CRC in particular, about the optimal use of radiation plus ICI to prime immunity and lead to an abscopal response. These include, the optimal immune checkpoint combination, sequencing the treatments, understanding the optimal RT dose and schedule, and understanding differences in host organ metastatic site for radiation. The current trial was not designed or powered to answer these questions.
In summary, although infrequent antitumor activity in non-irradiated lesions was observed, the results did not meet the study’s prespecified end point criteria to be considered worthwhile. However, we observed that RT can lead to immunomodulation in metastatic MMR-P CRC, and together with combination ICI, lead to systemic immunity. Further studies to identify predictive biomarkers to allow for a priori identification of appropriate subgroups more likely to achieve abscopal immunity are needed. In addition, further studies of RT plus novel immunotherapy combinations are warranted.
Supplementary Material
Statement of Translational Relevance.
Immune checkpoint inhibitors have shown limited efficacy in mismatch repair-proficient (MMR-P) colorectal cancer. Preclinical and clinical models of immune checkpoint inhibition in combination with radiotherapy have shown immune augmentation, and enhanced tumor regression in several tumor types. The current study reports the safety, efficacy, and immunologic correlates of combining CTLA-4 and PD-L1 blockade with local radiotherapy, to enhance systemic immunity, in patients with metastatic MMR-P colorectal cancer.
Acknowledgments.
We acknowledge the support of the Immune Monitoring Facility, Ludwig Center for Cancer Immunotherapy, MSKCC. This work was supported by Astra Zeneca, and the MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
Abbreviations:
- MMR-P
Mismatch Repair Proficient
- CRC
Colorectal Cancer
- RT
Radiotherapy
- durva
durvalumab
- treme
tremelimumab
- ICI
Immune Checkpoint Inhibition
- ECOG
Eastern Cooperative Oncology Group
- MSS
Microsatellite stable
- MSI-H
Microsatellite high
- MMR-P
mismatch repair proficient
- RECIST
Response Evaluation Criteria in Solid Tumors
- mAb
monoclonal antibody
- PD-1
programmed cell death 1
- PDL-1
programmed cell death-1 ligand
- CTLA-4
cytotoxic T lymphocyte antigen-4
- MSK-IMPACT
Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets
References
- 1.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England journal of medicine. 2015;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. The lancet oncology. 2019;20(6):849–61. [DOI] [PubMed] [Google Scholar]
- 3.O’Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PloS one. 2017;12(12):e0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Bode B, Studer G, Moch H, Okoniewski M, Knuth A, et al. Radiotherapy of human sarcoma promotes an intratumoral immune effector signature. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(17):4843–53. [DOI] [PubMed] [Google Scholar]
- 6.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer research. 2011;71(7):2488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23. [DOI] [PubMed] [Google Scholar]
- 9.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–70. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124(2):687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature Communications. 2017;8(1):15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Bode B, Studer G, Moch H, Okoniewski M, Knuth A, et al. Radiotherapy of Human Sarcoma Promotes an Intratumoral Immune Effector Signature. 2013;19(17):4843–53. [DOI] [PubMed] [Google Scholar]
- 14.Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(16):1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(2 Pt 1):728–34. [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366(10):925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal NH, Kemeny NE, Cercek A, Reidy DL, Raasch PJ, Warren P, et al. Non-randomized phase II study to assess the efficacy of pembrolizumab (Pem) plus radiotherapy (RT) or ablation in mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) patients. Journal of Clinical Oncology. 2016;34(15_suppl):3539. [Google Scholar]
- 19.Tarhini AA. Tremelimumab: a review of development to date in solid tumors. Immunotherapy. 2013;5(3):215–29. [DOI] [PubMed] [Google Scholar]
- 20.Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. Journal of Clinical Oncology. 2014;32(15_suppl):3002. [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Stadler ZK, Zhang L, Weiser MR, Basturk O, Hechtman JF, et al. Immunohistochemical null-phenotype for mismatch repair proteins in colonic carcinoma associated with concurrent MLH1 hypermethylation and MSH2 somatic mutations. Fam Cancer. 2018;17(2):225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middha S, Zhang L, Nafa K, Jayakumaran G, Wong D, Kim HR, et al. Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics (Oxford, England). 2014;30(7):1015–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wargo J, Andrews M, Duong C, Gopalakrishnan V, Iebba V, Chen W-S, et al. DOI:1021203/rs3rs-119925/v1 (https://wwwresearchsquarecom/article/rs-119925/v1).
- 27.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–88. [Google Scholar]
- 28.Parikh AR, Clark JW, Wo JY-L, Yeap BY, Allen JN, Blaszkowsky LS, et al. A phase II study of ipilimumab and nivolumab with radiation in microsatellite stable (MSS) metastatic colorectal adenocarcinoma (mCRC). Journal of Clinical Oncology. 2019;37(15_suppl):3514. [Google Scholar]
- 29.Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michot JM, Mazeron R, Dercle L, Ammari S, Canova C, Marabelle A, et al. Abscopal effect in a Hodgkin lymphoma patient treated by an anti-programmed death 1 antibody. Eur J Cancer. 2016;66:91–4. [DOI] [PubMed] [Google Scholar]
- 31.Xie C, Duffy AG, Brar G, Fioravanti S, Mabry-Hrones D, Walker M, et al. Immune Checkpoint Blockade in Combination with Stereotactic Body Radiotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma. Clinical Cancer Research. 2020;26(10):2318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabrizio DA, George TJ Jr., Dunne RF, Frampton G, Sun J, Gowen K, et al. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol. 2018;9(4):610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2017;377(14):1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postow MA, Knox SJ, Goldman DA, Elhanati Y, Mavinkurve V, Wong P, et al. A Prospective, Phase 1 Trial of Nivolumab, Ipilimumab, and Radiotherapy in Patients with Advanced Melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018:JCO2017769901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.