Abstract
Purpose
Sperm function tests do not adequately assess fertilization potential, and new indices are required. We have previously reported that human testis‐specific actin capping proteins may be involved in both sperm morphology and function. This study aimed to determine whether testis‐specific actin capping proteins can be a predictive marker of IVF success.
Methods
Ninety‐seven infertile couples who underwent IVF at an infertility clinic were included. Sperm were immunohistochemically stained to evaluate capping protein expression, and the percentage of sperms with normal staining was calculated. The relationship between actin capping protein expression and IVF outcomes was examined.
Results
The couples were divided into four groups according to the percentage of normally expressing actin capping protein as follows: ≥90% Group Ⅰ, 80%–90% Group Ⅱ, 70%–80% Group Ⅲ, and <70% Group Ⅳ. Multiple regression analysis showed a significant trend in fertilization rates across the 4 groups (p for trend =0.008).
There was no significant trend in pregnancy rates (p for trend =0.276).
Conclusion
The human testis‐specific actin capping protein may be a marker of male contributing factors that predict IVF outcomes.
Keywords: actin capping protein, in vitro fertilization, male infertility, predictive marker, sperm function
This study aimed to determine whether testis‐specific actin capping proteins can be a predictive marker of IVF success.Multiple linear regression showed a significant trend in the fertilization rates of IVF across the 4 groups. The human testis‐specific actin capping protein may be a marker of male contributing factors that predicts IVF outcomes.
![]()
1. INTRODUCTION
Up to 15% of couples wishing to conceive are infertile. 1 The popularity of assisted reproductive technologies, such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), has increased as more of these techniques have been developed. Approximately 50% of the causes of infertility are on the male side. The majority of male infertility cases are caused by spermatogenesis dysfunction, although the reasons for the dysfunction are only known in half of the cases. 2 Tests for male infertility include ultrasonography to determine the testicular volume and the presence of varicocele, endocrine evaluation by blood tests to determine levels of luteinizing hormone (LH), follicle‐stimulating hormone (FSH), estradiol, and testosterone, chromosome testing, anti‐sperm antibody testing, and semen examination. In addition to these common assays, sperm function tests offer more direct ways to assess sperm function as it affects fertility, such as the Acrobeads test, hypo‐osmotic swelling test (HOST), sperm survival test (SST), and zona‐free hamster egg sperm penetration test. 3
While there have been some reports indicating that the results of these tests are associated with fertilization rates, 4 , 6 they have proven to be inadequate as markers for predicting the fertilization rate of assisted reproductive approaches such as IVF. 6 , 9 As such, there is a need to develop new methods to measure sperm function as it relates to fertilization potential.
In order to develop a treatment for male infertility, our approach has been to understand the underlying causes by investigating genes specific to germ cells. We have specifically focused on elucidating the details of various testis‐specific genes. One example is human testis‐specific actin capping protein (hCP), for which we have cloned the capα3 and capβ3 genes, and analyzed their expression. 10 , 11 Our data revealed that human testis‐specific actin capping proteins α3 (hCPα3) and β3 (hCPβ3) are dynamically expressed during the various stages of spermatogenesis, suggesting that hCP may be involved in sperm morphogenesis. Interestingly, the expression patterns of hCPα3 and hCPβ3 were abnormal in infertile men with oligozoospermia and/or asthenozoospermia. Furthermore, even when only morphologically normal sperm were examined, the expression pattern of hCP was still abnormal, suggesting a possible association with male infertility. Thus, hCP may be involved not only in sperm morphogenesis, but also in sperm function specifically related to fertilization and embryonic development. 11 We hypothesized that hCP may be a new biomarker for predicting sperm fertility, and we therefore investigated the association between hCP expression and IVF treatment outcomes in male infertile patients.
2. MATERIALS AND METHODS
2.1. Semen collection and analysis
A total of 97 infertile couples who underwent IVF at Okamoto Clinic between September 2016 and July 2019 were included in this study. In all cases, IVF was performed using fresh sperm. Semen samples are the same semen used for IVF and were diluted with freezing medium from FUJIFILM Wako Pure Chemical Corporation, stored at −80°C, and thawed at 37°C for use in subsequent analyses. Semen analysis was performed according to the World Health Organization (WHO) manual (WHO, 2010).
2.2. Immunohistochemical analysis
The expression of hCPα3 and β3 in spermatozoa was assessed by immunohistochemical staining. Sperm samples were stained using previously described procedures, 11 with some modifications. Briefly, sperm samples were diluted to 5–10×106 cells/ml and smeared on MAS‐coated glass slides (Matsunami Glass Ind., Ltd.), followed by fixation with 4% paraformaldehyde on ice for 15 min. After permeabilization with 0.5% Triton X‐100 in PBS at 22°C for 15 min, the smears were blocked with Blocking One (Nacalai Tesque) for 1 h at 22°C and probed with the primary antibodies [rabbit anti‐CPα3 polyclonal antibody (H00093601‐D01) (Abnova) and guinea pig anti‐CPβ3 polyclonal antibody (GP‐SH5) (PROGEN)] diluted in PBS‐T (1:100) at 4°C overnight. After a wash with PBS‐T, the slides were incubated with the appropriate secondary antibody [Alexa Fluor 488 goat anti‐guinea pig secondary antibody ((A‐11073) (Life Technologies)) and Alexa Fluor 568 goat anti‐rabbit secondary antibody ((A‐11011) (Thermo Fisher Scientific)] diluted in PBS‐T (1:500) for 1 h at 22°C. Finally, the slides were washed in PBS‐T 10 times at 22°C and then counterstained with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). Stained spermatozoa were examined using a Keyence BZ‐X700 microscope (Keyence, Co).
The staining patterns were categorized according to previously reported criteria. 11
Post‐acrosomal regions of sperm heads stained with both hCPα3 and hCPβ3 were classified as normal staining. “Missing staining” or “abnormal localization” of either or both hCPα3 or hCPβ3 were classified as abnormal staining (Figure 1). “Missing staining” was defined as the presence of regions where the staining signal was reduced by more than 50% compared with the fully stained samples, and “abnormal localization” was defined as staining of regions other than the post‐acrosomal region. Spermatozoa with normal morphology were selected according to David's classification criteria. 12 At least 100 normal morphology spermatozoa were counted, and the percentage of normal staining sperm was calculated (referred to as the normal staining proportion).
FIGURE 1.
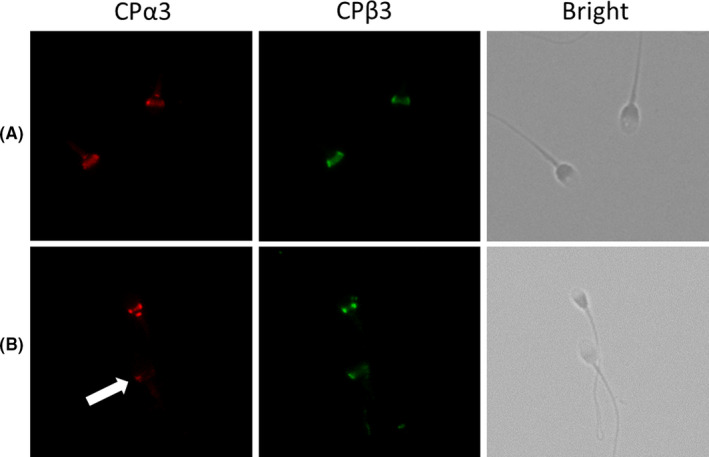
Immunocytochemistry showing human capping proteinα3 (hCPα3) and human capping proteinβ3 (hCPβ3) labeling in human ejaculated sperm. hCPα3 and hCPβ3 are localized at the post‐acrosomal region of the sperm head. (A) Normal staining, (B) abnormal staining, heterogeneous staining for CPα3 (arrow) [Colour figure can be viewed at wileyonlinelibrary.com]
2.3. Statistical analysis
Values are presented as mean ± SD. Statistical analysis was performed using JMP® Pro (version 14.1.0) and R (version 4.0.3.). The one‐way analysis of variance (ANOVA) was used to assess the difference among the four groups. Simple and multiple linear regression were used to examine the association of sperm staining status with fertilization rates, adjusting for covariates such as ages of males and females, sperm concentration, motility, abnormality rates, and white blood cells in semen. Tests for linear trend of fertilization rates, good quality blastocyst ratio, and pregnancy rates were performed by modeling sperm staining status as a categorical variable. A p‐value of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Background characteristics
A total of 97 couples was included in the present study. Patient backgrounds were as follows: The mean male age was 37.9 years old, sperm concentration was 126.7 × 106 cells/ml, sperm motility was 59.8%, abnormal morphology sperm proportion was 48.2%, and female age was 36.7 years old (Table 1). The mean fertilization rate of IVF was 59.2%, and the median normal staining proportion of hCP was 81.1%.
TABLE 1.
Background characteristics of all couples
| All patients (n = 97) | hCP normal stain proportion | |||||
|---|---|---|---|---|---|---|
| Group Ⅰ (n = 12) | Group Ⅱ (n = 44) | Group Ⅲ (n = 26) | Group Ⅳ (n = 15) | p‐value a | ||
| Male age (years) | 37.9 ± 5.1 | 38.7 ± 1.5 | 37.3 ± 0.78 | 38.5 ± 1.0 | 38.1 ± 1.3 | 0.750 |
| Female age (years) | 36.7 ± 4.3 | 37.8 ± 1.2 | 36.5 ± 0.65 | 36.4 ± 0.85 | 37.2 ± 1.1 | 0.764 |
| Sperm concentration (106ml−1) | 126.7 ± 71.3 | 138.8 ± 20.3 | 140.6 ± 10.6 | 116.3 ± 13.8 | 94.3 ± 18.2 | 0.128 |
| Sperm motility (%) | 59.8 ± 16.7 | 57.9 ± 4.8 | 60.3 ± 2.5 | 62.8 ± 3.3 | 54.5 ± 4.3 | 0.480 |
| Abnormal morphology (%) | 48.2 ± 15.9 | 47.3 ± 4.6 | 45.7 ± 2.4 | 48.9 ± 3.1 | 54.8 ± 4.1 | 0.294 |
| White blood cell in semen (/ml) | 23.0 ± 25.7 | 16.1 ± 7.4 | 25.9 ± 3.9 | 17.7 ± 5.0 | 29.3 ± 6.6 | 0.331 |
Figure shows as mean ± SD.
One‐way ANOVA.
3.2. Grouping by hCP expression in spermatozoa
The couples were divided into four groups according to the normal staining proportion of hCP as follows: ≥90% Group Ⅰ, 80%–90% Group Ⅱ, 70%–80% Group Ⅲ, and <70% Group Ⅳ. Patient backgrounds for each group are summarized in Table 1. In all couples, there was no significant difference in the mean male age, sperm concentration, sperm motility, abnormal morphology sperm proportion, white blood cell in semen, or female age among the four groups.
3.3. Sperm hCP expression and IVF outcomes
In simple linear regression analysis, the mean fertilization rates of IVF were 73%, 67%, 49%, and 44% in Group Ⅰ, Group Ⅱ, Group Ⅲ, and Group Ⅳ, respectively, and significantly lower in Group Ⅲ and Group Ⅳ than in Group Ⅰ (p = .040, 0.027). There was a trend for the fertilization rates to increase across Group Ⅳ to Group Ⅰ (p for trend =0.010). In multiple linear regression analysis, the mean fertilization rates of IVF were 70%, 64%, 48%, and 39% in Group Ⅰ, Group Ⅱ, Group Ⅲ, and Group Ⅳ, respectively, and significantly lower in Group Ⅳ than in Group Ⅰ (p = 0.019). There was a trend for the fertilization rates to increase across Group Ⅳ to Group Ⅰ (p for trend =0.008). There were no significant differences among the four groups and no trends across the four groups in the good quality blastocyst ratio and the pregnancy rates (Table 2, Figure 2).
TABLE 2.
Comparison of IVF outcomes
| hCP normal stain proportion | |||||
|---|---|---|---|---|---|
| Group Ⅰ (≥90%) | Group Ⅱ (80–90%) | Group Ⅲ (70–80%) | Group Ⅳ (<70%) | p for trend | |
| Fertility rate | |||||
| Crude | 0.73 | 0.64 | 0.53 a | 0.42 a | .010 |
| (0.54–0.91) | (0.57–0.76) | (0.36–0.62) | (0.28–0.61) | ||
| Adjusted b | 0.70 | 0.64 | 0.48 | 0.39 a | .008 |
| (0.51–0.89) | (0.53–0.75) | (0.35–0.61) | (0.22–0.57) | ||
| Good quality blastocyst ratio | |||||
| Crude | 0.23 | 0.24 | 0.20 | 0.29 | .729 |
| (0.03–0.43) | (0.15–0.34) | (0.07–0.34) | (0.12–0.47) | ||
| Adjusted b | 0.21 | 0.19 | 0.17 | 0.26 | .766 |
| (0.02–0.40) | (0.08–0.29) | (0.04–0.30) | (0.08–0.44) | ||
| Pregnancy rate | |||||
| Crude | 0.33 | 0.29 | 0.40 | 0.20 | .619 |
| (0.06–0.61) | (0.16–0.43) | (0.22–0.58) | (−0.06–0.46) | ||
| Adjusted b | 0.37 | 0.19 | 0.35 | 0.11 | .276 |
| (0.11–0.62) | (0.04–0.34) | (0.17–0.52) | (−0.15–0.36) | ||
Figures show mean (95%CI).
p < 0.05 vs Group.
Adjusted for ages of patients and patients’ wives, sperm concentration, motility, abnormality rates, and white blood cells in semen.
FIGURE 2.
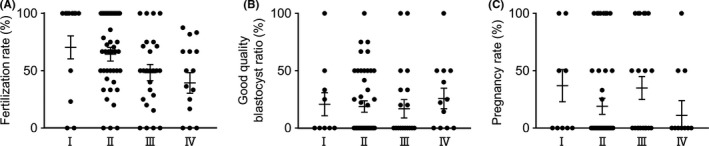
Comparison of IVF outcomes In simple linear regression analysis, the mean fertilization rates of IVF were significantly lower in Group Ⅲ and Group Ⅳ than in Group Ⅰ (p = 0.040, 0.027), and there was a trend for the fertilization rates to increase across Group Ⅳ to Group Ⅰ (p for trend =0.010). In multiple linear regression analysis, the mean fertilization rates of IVF were significantly lower in Group Ⅳ than in Group Ⅰ (p = 0.019) and there was a trend for the fertilization rates to increase across Group Ⅳ to Group Ⅰ (p for trend =0.008). There were no significant differences among the four groups and no trends across the four groups in the good quality blastocyst ratio and the pregnancy rates (p for trend =0.766, 0.276)
4. DISCUSSION
In this study, we investigated the relationship between the expression of hCP in sperm with normal morphologies from male infertility patients and the treatment outcomes of IVF. The results showed that even when sperm morphology was normal, there was a trend toward a significant decrease in the fertilization rate of IVF with a lower proportion of hCP staining. The results were similar when adjusted for other factors that could affect the fertilization rate, such as female age and abnormal sperm rate. 13
Germ cells differentiate in a completely different manner compared to somatic cells. In males, spermatocytes divide meiotically into haploid spermatids, which differentiate into mature sperm that exhibit marked changes in cell morphology, including the formation of flagella, condensation of the nucleus, and removal of excess cytoplasm. This process is a spermatozoon‐specific morphological and functional change, and a large number of sperm‐specific genes are involved in each process. 14
To date, we and our collaborators have discovered several testis‐specific genes in our investigations into the causes of male infertility 15 and we have observed that some of these genes are clinically associated with male infertility.
The cpα3 and cpβ3 genes investigated in this study are among the testis‐specific genes that we have discovered. 16 , 17 Actin capping protein is one of the important regulatory proteins of actin, with isoforms α1, α2, β1, and β2 present in somatic cells, and α3 and β3 expressed in germ cells. Although much work has been done to reveal the importance of hCP in somatic cells, it has not been elucidated in germ cells. 18 , 23 We have cloned and demonstrated the expression of the human cpα3 gene in our 2002 study 10 and, more recently, we identified the human cpβ3 gene, which was thought to form a dimer with hCPα3. 11 Both genes were specifically expressed in the testes. We found that the localization of hCPα3 and hCPβ3 matched perfectly and that they dynamically change their sites of expression during spermatogenesis, aggregating from the cytoplasm to the acrosomal cap, acrosome, and finally to the post‐acrosomal region. 11 This suggests that hCP may be involved in morphogenesis during spermatogenesis. A comparison of hCPα3 and hCPβ3 expression in sperm from infertile patients with azoospermia and oligozoospermia with sperm from healthy volunteers showed that the expression of hCPα3 and hCPβ3 was significantly reduced in infertile patients. Since reduced expression was more common in sperm with abnormal morphologies, hCP expression was evaluated in sperm with normal morphology from infertile patients compared to sperm from healthy volunteers. The results showed that hCP expression was significantly reduced in infertile patients. This suggests that hCP may be involved not only in sperm morphogenesis, but also in sperm function. 11
Azpiazu et al. assessed protein expression in normal morphology sperm from patients who underwent IVF and found that 31 proteins were down‐regulated in the group that did not get pregnant, including hCPα3. 24 This supports our hypothesis that hCP may be involved in sperm function, such as fertilizing capacity.
The results of the present study demonstrate that reduced hCP expression, even when sperm morphology is normal, significantly reduces the fertilization rate of IVF. We believe that hCP may be a novel biomarker of the sperm fertility in IVF.
Various sperm function tests have been developed as more detailed methods of evaluating sperm function, compared with a semen analysis. The Acrobeads test, HOST, SST, and zona‐free hamster egg sperm penetration test are used to evaluate fertilization and other factors. Although there have been some reports that sperm function tests can predict fertilization rate of IVF, each test alone is not sufficient to assess sperm fertilization potential, and more accurate test methods are necessary. 4 , 6 , 25
The measurement of hCP expression is a simple method of assessment, in which collected sperm are immunostained and the proportion of normally stained sperm is calculated. The ease of use of this assay is one of the features recommending hCP expression as an excellent marker to predict sperm function.
This study has several limitations. First, the lack of association between hCP expression and the good quality blastocyst ratio or the pregnancy rate suggests that hCP alone may not be enough to predict male infertility in this context. There are several possible causes. It has been reported that some sperm genes play an important role in the process of the acrosome reaction, sperm‐oocyte fusion, and activation of the oocyte. 26 , 27 And it has been reported that maternal gene expression may have a significant impact on the process of embryonic development, implantation, and pregnancy. 28 , 29 The expression status of hCP can be a marker for the structural and functional evaluation of sperm, but it is difficult to evaluate maternal factors, and may need to be combined with other markers to predict good blastocyst ratio and pregnancy rates. Recently, it has been reported that increased oxidative stress and inflammatory factors in patients with metabolic syndrome are a cause of implantation failure. 30 This study did not examine whether the females had metabolic syndrome, which may have also influenced the results. The traditional ranking criteria may not be able to select the best embryo for transfer. 31 Second, it is not clear what role hCP plays in sperm function. Further research into this question could indicate whether hCP expression will become a more useful predictive marker in combination with other tests.
The expression of hCP in sperm with normal morphologies is related to the fertilization rate of IVF in male infertile patients. hCP may be a marker of male contributing factors that predict the outcomes of IVF.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
ACKNOWLEDGMENTS
The authors deeply appreciate M. Tsuchiya and A. Yasumoto for technical assistance with the experiments. This research was supported by Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number: 18K16733).
Inagaki Y, Fukuhara S, Kuribayashi S, et al. The expression of human testis‐specific actin capping protein predicts in vitro fertilization outcomes: A novel biomarker of sperm function for assisted reproductive technology. Reprod Med Biol. 2021;20:537–542. 10.1002/rmb2.12407
REFERENCES
- 1. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith RP, Coward RM, Lipshultz LI. The office visit. Urologic Clinics of North America. 2014;41(1):19–37. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Standard procedures. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010:7–113. [Google Scholar]
- 4. Ohashi K, Saji F, Kato M, Okabe M, Mimura T, Tanizawa O. Evaluation of acrosomal status using MH61‐beads test and its clinical application. Fertil Steril. 1992;58:803‐808. [PubMed] [Google Scholar]
- 5. Coccia ME, Becattini C, Criscuoli L, Fuzzi B, Scarselli G. A sperm survival test and in‐vitro fertilization outcome in the presence of male factor infertility. Hum Reprod. 1997;12:1969‐1973. [DOI] [PubMed] [Google Scholar]
- 6. Margalioth EJ, Navot D, Laufer N, Lewin A, Rabinowitz R, Schenker JG. Correlation between the zona‐free hamster egg sperm penetration assay and human in vitro fertilization. Fertil Steril. 1986;45:665‐670. [DOI] [PubMed] [Google Scholar]
- 7. Vazquez‐Levin M, Kaplan P, Sandler B, Garrisi GJ, Gordon J, Navot D. The predictive value of zona‐free hamster egg sperm penetration assay for failure of human in vitro fertilization and subsequent successful zona drilling. Fertil Steril. 1990;53:1055‐1059. [DOI] [PubMed] [Google Scholar]
- 8. Hershlag A, Paine T, Scholl GM, et al. Acrobeads test as a predictor of fertilization in vitro. Am J Reprod Immunol. 1997;37:291‐299. [DOI] [PubMed] [Google Scholar]
- 9. Xu F, Guo G, Zhu W, Fan L. Human sperm acrosome function assays are predictive of fertilization rate in vitro: a retrospective cohort study and meta‐analysis. Reprod Biol Endocrinol. 2018;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyagawa Y, Tanaka H, Iguchi N, et al. Molecular cloning and characterization of the human orthologue of male germ cell‐specific actin capping protein α3 (cpα3). Mol Hum Reprod. 2002;8:531‐539. [DOI] [PubMed] [Google Scholar]
- 11. Soda T, Miyagawa Y, Ueda N, et al. Systematic characterization of human testis‐specific actin capping protein β3 as a possible biomarker for male infertility. Hum Reprod. 2017;32:514‐522. [DOI] [PubMed] [Google Scholar]
- 12. David G, Bisson JP, Czyglik F, Jouannet P, Gernigon C. Anomalies morphologiques du spermatozoïde humain, propositions pour un système de classification. J Gynecol Obs Biol Reprod. 1975;4:17‐36. [Google Scholar]
- 13. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee . Female age‐related fertility decline. committee opinion No. 589. Fertil Steril. 2014;101:633‐634. [DOI] [PubMed] [Google Scholar]
- 14. Okuda H, Tsujimura A, Yamamoto K, et al. Morphologic and mitochondrial characterization of human spermatogenic cells dispersed in wet preparation for testicular sperm extraction: establishment of a microscopic diagram of developing human spermatogenic cells. Fertil Steril. 2011;95:2665‐2668. [DOI] [PubMed] [Google Scholar]
- 15. Nishimune Y, Tanaka H. Infertility caused by polymorphisms or mutations in spermatogenesis‐specific genes. J Androl. 2006;27:326‐334. [DOI] [PubMed] [Google Scholar]
- 16. Okuda H, Tsujimura A, Irie S, et al. A single nucleotide polymorphism within the novel sex‐linked testis‐specific retrotransposed PGAM4 gene influences human male fertility. PLoS One. 2012;7:e35195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanaka H, Kohroki J, Iguchi N, Onishi M, Nishimune Y. Cloning and characterization of a human orthologue of testis‐specific succinyl CoA: 3‐oxo acid CoA transferase (Scot‐t) cDNA. Mol Hum Reprod. 2002;8:16‐23. [DOI] [PubMed] [Google Scholar]
- 18. Hart MC, Cooper JA. Vertebrate isoforms of actin Capping protein β have distinct functions in vivo. Journal of Cell Biology. 1999;147:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. Capping protein regulators fine‐tune actin assembly dynamics. Nat Rev Mol Cell Biol. 2014;15:677‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schafer DA, Hug C, Cooper JA. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol. 1995;128:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ydenberg CA, Smith BA, Breitsprecher D, Gelles J, Goode BL. Cease‐fire at the leading edge: new perspectives on actin filament branching, debranching, and cross‐linking. Cytoskeleton. 2011;68:596‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyoshi T, Tsuji T, Higashida C, et al. Actin turnover‐dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing. J Cell Biol. 2006;175:947‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinnar SA, Antoku S, Saffin JM, Cooper JA, Halpain S. Capping protein is essential for cell migration in vivo and for filopodial morphology and dynamics. Mol Biol Cell. 2014;25:2152‐2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azpiazu R, Amaral A, Castillo J, et al. High‐throughput sperm differential proteomics suggests that epigenetic alterations contribute to failed assisted reproduction. Hum Reprod. 2014;29:1225‐1237. [DOI] [PubMed] [Google Scholar]
- 25. Fuse H, Kazama T, Katayama T. Relationship between hypoosmotic swelling test, semen analysis, and zona‐free hamster ovum test. Arch Androl. 1991;27:73‐78. [DOI] [PubMed] [Google Scholar]
- 26. Inoue F, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234‐238. [DOI] [PubMed] [Google Scholar]
- 27. Noda T, Lu Y, Fujihara Y, et al. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm−oocyte fusion in mice. Proc Natl Acad Sci U S A. 2020;117:11493‐11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukui Y, Hirota Y, Matsuo M, et al. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod Med Biol. 2019;18:234‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Byun H, Kwon S, Wagner KU, Shin H, Lim HJ. Tumor susceptibility gene 101 is required for the maintenance of uterine epithelial cells during embryo implantation. Reprod Biol Endocrinol. 2021;19(112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheikhansari G, Soltani‐Zangbar MS, Pourmoghadam Z, et al. Oxidative stress, inflammatory settings, and microRNA regulation in the recurrent implantation failure patients with metabolic syndrome. Am J Reprod Immunol. 2019;82:e13170. [DOI] [PubMed] [Google Scholar]
- 31. Scott RT Jr, Upham KM, Forman EJ, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697‐703. [DOI] [PubMed] [Google Scholar]


