Abstract
During mitosis the Xenopus polo-like kinase 1 (Plx1) plays key roles in the activation of Cdc25C, in spindle assembly, and in cyclin B degradation. Previous work has shown that the activation of Plx1 requires phosphorylation on serine and threonine residues. In the present work, we demonstrate that replacement of Ser-128 or Thr-201 with a negatively charged aspartic acid residue (S128D or T201D) elevates Plx1 activity severalfold and that replacement of both Ser-128 and Thr-201 with Asp residues (S128D/T201D) increases Plx1 activity approximately 40-fold. Microinjection of mRNA encoding S128D/T201D Plx1 into Xenopus oocytes induced directly the activation of both Cdc25C and cyclin B-Cdc2. In egg extracts T201D Plx1 delayed the timing of deactivation of Cdc25C during exit from M phase and accelerated Cdc25C activation during entry into M phase. This supports the concept that Plx1 is a “trigger” kinase for the activation of Cdc25C during the G2/M transition. In addition, during anaphase T201D Plx1 reduced preferentially the degradation of cyclin B2 and delayed the reduction in Cdc2 histone H1 kinase activity. In early embryos S128D/T201D Plx1 resulted in arrest of cleavage and formation of multiple interphase nuclei. Consistent with these results, Plx1 was found to be localized on centrosomes at prophase, on spindles at metaphase, and at the midbody during cytokinesis. These results demonstrate that in Xenopus laevis activation of Plx1 is sufficient for the activation of Cdc25C at the initiation of mitosis and that inactivation of Plx1 is required for complete degradation of cyclin B2 after anaphase and completion of cytokinesis.
Progression through the eucaryotic cell cycle is controlled through the periodic activation or inactivation of various cyclin-dependent protein kinases (cdk’s) at specific points in the cycle (26). The fidelity of events in a given cell cycle phase is monitored by checkpoints, which control a signaling system that can delay cell cycle progression and changes in cdk activity (5, 9). One of the best-understood checkpoints blocks activation of the cyclin B-Cdc2 complex in G2 phase if DNA replication is incomplete (see reference 27 for a review). This block to mitotic entry requires that the phosphatase Cdc25C remain inactive. Throughout late S and early G2 phases, cyclin B is synthesized and immediately complexes with Cdc2, which is kept catalytically inactive by phosphorylation of Thr-14 and Tyr-15 in the ATP-binding site. This phosphorylation and inactivation are catalyzed by the protein kinases Wee1 and Myt1 (22, 29), and dephosphorylation and activation of cyclin B-Cdc2 are catalyzed by the phosphatase Cdc25C (6, 14, 21). Activation of Cdc25C requires phosphorylation on specific serine and threonine sites, which fails to occur if DNA synthesis is incomplete and the replication checkpoint is activated (13, 16). These considerations have focused attention on the phosphorylation pathway by which Cdc25C becomes activated at the G2/M transition. Cyclin B-Cdc2 can phosphorylate and activate Cdc25C, forming a positive feedback loop that contributes to the abrupt transition from G2 into M phase (10, 12). However, a variety of evidence indicates that in Xenopus initial phosphorylation and activation of Cdc25C result from activation of the polo-like kinase (plk) Plx1. Plx1 can phosphorylate and activate Cdc25C in vitro (15), and in vivo activation of Plx1 is concurrent with the activation of Cdc25C (30). Moreover, inhibition of Plx1 delays the activation of Cdc25C, and an elevated level of Plx1 accelerates the rate of Cdc25C activation (30). Plx1 is also activated by phosphorylation, and the newly identified Xenopus polo-like kinase kinase 1 (xPlkk1) is able to phosphorylate and activate Plx1 in vitro (31). The xPlkk1 protein itself is also activated by phosphorylation, and in vivo activation of xPlkk1 coincides with the activation of Plx1. Moreover, an elevated level of xPlkk1 accelerates the timing of activation of Plx1 and the transition from the G2 to the M phase of the cell cycle (31). Both Plx1 and xPlkk1 might be subject to inhibition when the DNA replication checkpoint is activated.
In addition to the role for plk’s at the G2/M transition, in a variety of organisms, including Xenopus, plk’s also have other roles in mitosis. One important role in yeast, Drosophila, Xenopus, and mammalian cells is the requirement of plk function for bipolar spindle formation (7, 8, 17, 28, 30, 36). In the absence of plk function, monopolar spindles form, and localization studies show that plk’s can be found on centrosomes and kinetochores at metaphase (30, 35). plk’s appear to continue to function in mitosis even after the metaphase/anaphase transition has been triggered. In Xenopus egg extracts, addition of a catalytically inactive (kinase-dead) form of Plx1 blocks the degradation of B-type cyclins, and the system remains in M phase with high histone H1 kinase activity (4). In budding yeast cells the plk homolog Cdc5p is normally degraded by the anaphase-promoting complex (APC) (3, 33), and in Xenopus Plx1 activity decreases late in mitosis (30). Overexpression of Cdc5p results in increased degradation of certain Clbs, suggesting that degradation of Cdc5p is required to turn off the degradation of B-type cyclins by the APC (3). In both Saccharomyces cerevisiae and Drosophila, plk’s are localized to the midbody and plk function appears to be required for cytokinesis (2, 19, 20). Most of the information obtained to date about the function of plk’s has come from studies with loss-of-function mutants or inhibitory antibodies. To learn more about the function of plk’s it was imperative to generate a constitutively active mutant of Plx1 and determine its effects on mitosis, as reported here.
MATERIALS AND METHODS
Manipulation of oocytes and eggs.
Xenopus laevis was obtained from Xenopus I (Ann Arbor, Mich.). Techniques for dissection and culture of oocytes, in vitro fertilization of eggs, culture of embryos, and microinjection, have been described elsewhere (30).
Mutagenesis.
The S128D, T140D, T201D, T205D, S227D, S128A, and T201V mutants of Plx1 were created by PCR with pairs of oligonucleotides with the following sequences: GAGGAGGGACCTGTTGGAGCTGCACAAGAG and CCAACAGGTCCCTCCTCCTGCACAGCTC, AGCGGTTGACGAGCCAGAAGCTCGCTACT and CTGGCTCGTCAACCGCTTTTCTCCTCTTGTG, CAAAAAGGACCTCTGTGGCACTCCAAA and CACAGAGGTCCTTTTTGCGCTCGCCATC, CTGTGGCGACCCAAACTACATTGCACCTGAG and AGTTTGGGTCGCCACAGAGGGTCTTTTTGC, CATATGGGACATAGGATGCATCATGTACACAC and CATCCTATGTCCCATATGTCCACTTCAAAACTG, GAGGAGGGCTCTGTTGGAGCTGCACAAG and CCAACAGAGCCCTCCTCCTGCACAGCTC, and CAAAAAGGTGCTCTGTGGCACTCCAAAC and CACAGAGCACCTTTTTGCGCTCGCCAGCATC, respectively. The S128D/T201D and S128A/T201V mutants were created by PCR with two pairs of the above oligonucleotides corresponding to S128D and T201D and to S128A and T201V, respectively.
Immunoprecipitation, immunoblotting, and kinase assays.
Stage VI oocytes injected with mRNA encoding Plx1 or the various Plx1 mutants, tagged at the COOH terminus with FLAG, were lysed, the extracts were immunoprecipitated with anti-FLAG M2-agarose beads (Sigma), and Plx1 activity was assayed by phosphorylation of α-casein (30). Characterization of antibodies generated by this laboratory against cyclins, Cdc25C, and Plx1 and used for immunoblotting has been detailed (13, 30, 32). Anti-phospho-mitogen-activated protein (MAP) kinase (9101) was from New England Biolabs, and anti-Mos (C237) was from Santa Cruz Biotechnology, Inc. Immunoblots were developed with the appropriate peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) and enhanced chemiluminescence (Amersham). Histone H1 kinase activity was quantified as described previously (30). Peptides encompassing Ser-128 (Plx1, 119 to 136; VVLELCRRRSLLELHKRRRK) and Thr-201 (Plx1, 191 to 204; KKVEYDGERKKTLCG) were synthesized by the HHMI Protein Chemistry Facility at the University of California, San Francisco. Phosphorylation of these peptides was assayed in 30 μl of kinase buffer (31) containing 100 μM [γ-32P]ATP, 1 mM peptide, and 50 ng of active xPlkk1, or the equivalent volume of kinase-dead xPlkk1 (K65M), purified from okadaic acid-treated Sf9 cells (31). Reactions were incubated at 30°C for 15 min, and phosphorylation was quantified by the P81 paper assay.
CSF extract preparation and manipulation.
Metaphase II-arrested (cytostatic factor [CSF]) extracts were prepared as described previously (13). These extracts are arrested at meiosis II with a high level of cyclin B-Cdc2 activity due to the activity of CSF. Addition of calcium causes CSF release, and as these extracts exit metaphase the cyclins are destroyed and Cdc25C is deactivated. Subsequently, upon reentry into M phase, cyclins reaccumulate and Cdc25C is reactivated. An individual reaction was made by transfer of 70 μl of extract into a 1.5-ml microcentrifuge tube on ice and the addition of 5 μl of buffer or recombinant Plx1 proteins (1.5 mg/ml), as indicated. After incubation on ice for 20 min, release from CSF arrest was initiated by the addition of 1 μl of 30 mM CaCl2 and incubation at 21°C. Samples of 2.5 μl were removed at the indicated times, mixed with 20 μl of extraction buffer (30), frozen on dry ice, and stored at −80°C until further analysis. For immunocomplex kinase assays of Plx1 activity, 2.5 μl of each diluted sample and 0.75 μg of anti-Plx1 antibodies were used, and assays were performed as described previously (30). One microliter of each diluted sample was assayed for histone H1 kinase activity, and 2 μl was used for immunoblotting. Recombinant T201D and N172A Plx1 proteins used in these experiments were produced in Sf9 cells and purified with Talon resin, hydroxyapatite, and Mono S chromatography as described previously (31). The yield of S128D/T201D Plx1 or S128D Plx1 in Sf9 cells was too low for further purification.
Microinjection and immunofluorescence of Xenopus embryos.
Embryos were microinjected at the two-cell stage with 20 nl of mRNA encoding FLAG-tagged Plx1 proteins (1 mg/ml), cultured in 0.1× MMR for 4 to 5 h, fixed in methanol, sectioned, and stained with SYTOX Green (Molecular Probes). Embryos were photographed with a Wild Heerbrugg dissecting microscope equipped with a 35-mm camera. For immunolocalization of Plx1, cells in late-blastula-stage embryos were fixed in methanol, and immunofluorescence staining was performed as previously described (30). DNA was stained with SYTOX Green, α-tubulin was detected with an anti-α-tubulin monoclonal antibody (Sigma) and visualized by Cy3-conjugated donkey anti-mouse immunoglobulin G (IgG) antibodies (Jackson ImmunoResearch), and Plx1 was detected with anti-Plx1 antibodies and visualized by Cy2- or Cy3-conjugated donkey anti-rabbit IgG antibodies (Jackson ImmunoResearch). Confocal microscopy was performed with an MRC-600 microscope (Bio-Rad).
RESULTS
In vitro kinase activity of Plx1 mutants expressed in oocytes.
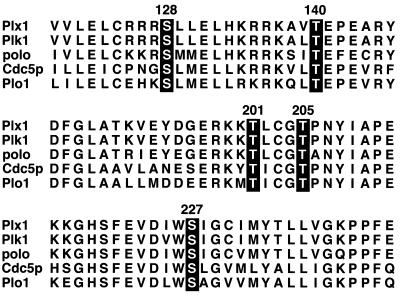
The plk’s are highly conserved among different species, and their activity can be regulated by transcription, biosynthesis, and degradation, intracellular localization, and phosphorylation (8, 18, 30, 36). During meiotic maturation (the G2/M transition) in Xenopus oocytes Plx1 is activated by phosphorylation on both serine and threonine residues (30). Because plk’s from several different organisms are activated by phosphorylation, it is plausible that the phosphorylation sites essential for their activation are also conserved. When the amino-terminal catalytic domain of Plx1 is compared with those of mammalian Plk, Drosophila polo, Saccharomyces cerevisiae Cdc5p, and Schizosaccharomyces pombe plo1, several conserved serine and threonine residues are evident (Fig. 1). In many protein kinases substitution of a phosphorylated residue with a negatively charged aspartic acid residue can mimic the effect of phosphorylation on enzyme activity (11, 23). Therefore, five of these conserved residues were individually mutated to an aspartic acid residue, and the effect on Plx1 activity was assessed.
FIG. 1.
Sequence alignment of selected regions of indicated polo family members. Numbering of the amino acids is based on the sequence of Plx1, and serine and threonine residues that were mutated to aspartic acid, alanine, or valine residues in this study are highlighted.
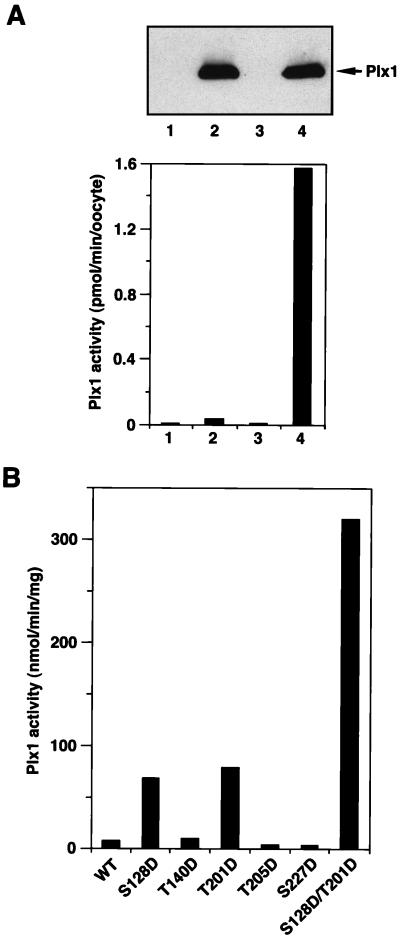
To analyze the activity of ectopically expressed Plx1 and its mutants, stage VI oocytes were microinjected with the various FLAG-tagged Plx1 mRNAs, and anti-FLAG immunoprecipitates were used for casein kinase assays. Immunoprecipitates prepared from stage VI (G2 phase) oocytes expressing wild-type (WT) FLAG-tagged Plx1 had very low casein kinase activity, whereas immunoprecipitates prepared from the corresponding mature oocytes (M phase) had at least a 40-fold increase in kinase activity toward casein. No casein kinase activity was observed in immunoprecipitates prepared from either stage VI or mature oocytes injected with buffer (Fig. 2A). Casein kinase activity in the immunoprecipitates was linear with time and amount of extract (data not shown). These results indicate that the kinase assay with anti-FLAG immunoprecipitates is specific for the FLAG-tagged ectopically expressed Plx1 protein.
FIG. 2.
Protein kinase activity of various Plx1 mutants. (A) Wild-type Plx1 is not activated in the absence of progesterone treatment. Stage VI oocytes were microinjected with buffer or 40 ng of mRNA encoding FLAG-tagged WT Plx1, either treated or untreated with progesterone, and harvested 15 min after the progesterone-treated oocytes underwent GVBD. Extracts were prepared, and samples equivalent to one oocyte were immunoprecipitated with anti-FLAG M2 agarose beads and washed; the immunocomplexes were then assayed for phosphorylation of α-casein (lower panel) or immunoblotted with anti-Plx1 antibody (upper panel). Lane 1, stage VI oocytes, buffer injection; lane 2, stage VI oocytes, Plx1 mRNA injection; lane 3, GVBD oocytes, buffer injection; lane 4, GVBD oocytes, Plx1 mRNA injection. (B) Specific acidic mutations activate Plx1 in the absence of progesterone treatment. Stage VI oocytes were microinjected with 40 ng of mRNA encoding the various FLAG-tagged Plx1 mutants, as indicated, and harvested 3 h later. At this time the oocytes expressing mutant Plx1 were still in stage VI, based on the histone H1 kinase activity (data not shown). Extracts were prepared, and immunoprecipitates were assayed for phosphorylation of α-casein. The amount of Plx1 protein in the immunocomplexes was determined by immunoblotting, with purified recombinant Plx1 as a standard.
Mutation of Ser-128 to Asp (S128D) or of Thr-201 to Asp (T201D) increased substantially the kinase activity of Plx1 toward casein, whereas mutation of Thr-140, Thr-205, or Ser-227 to Asp (T140D, T205D, and S227D) did not alter Plx1 activity significantly (Fig. 2B). Constitutive activity after mutation of Thr-201 to Asp in Plx1 is consistent with a previous report that mammalian plk with a Thr-Asp mutation at the equivalent site has elevated activity (19). This residue is in the activation loop between protein kinase subdomains VII and VIII, and phosphorylation within this loop results in the activation of several protein kinases including Cdc2 and Mek1 (11, 23, 25). To generate an even more active Plx1 mutant, both Ser-128 and Thr-201 were substituted with Asp (S128D/T201D), mRNA was injected, and Plx1 activity was assayed. The S128D/T201D double mutant displayed 40-fold increased casein kinase activity (Fig. 2B).
Thr-201 is required for activation of Plx1.
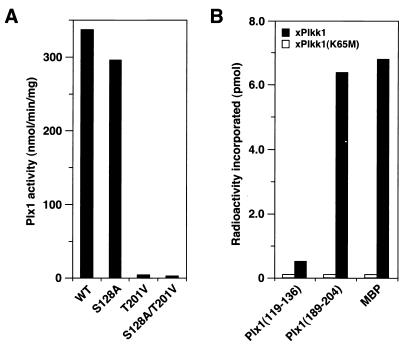
The results above suggested that phosphorylation of both T201 and S128 might be important for activation of Plx1 in vivo. To assess this possibility, mRNAs encoding T201V Plx1 or S128A Plx1 were injected into resting oocytes. After 2 h of incubation, progesterone was added to trigger the G2/M transition. After nuclear (germinal vesicle) breakdown, recombinant Plx1 was recovered on anti-FLAG beads and kinase activity was assessed (Fig. 3A). The T201V mutant exhibited essentially no activation in response to progesterone, whereas the S128A mutant was activated substantially. We have recently purified and cloned a protein kinase, termed xPlkk1, that is able to phosphorylate and activate Plx1 in vitro and that accelerates the activation of Plx1 in vivo (31). Both T201 and S128 are preceded by three basic residues and followed by a hydrophobic residue (Fig. 1). Therefore, synthetic peptides encompassing these residues were synthesized, and their ability to serve as substrates for xPlkk1 in vitro was evaluated in comparison with myelin basic protein (MBP) (Fig. 3B). The T201 peptide was phosphorylated almost as well as MBP, whereas the S128 peptide was not significantly phosphorylated, even though the sequences surrounding these residues are highly conserved. These results suggest that phosphorylation of T201, but not S128, by xPlkk1 is required for activation of Plx1.
FIG. 3.
Threonine-201 is required for activation of Plx1. (A) Activation studies in vivo. mRNAs encoding WT or mutant forms of FLAG-tagged Plx1 (S128A, T201V, and S128A/T201V) were injected into oocytes. Two hours later progesterone was added, and the oocytes were harvested 15 min after undergoing GVBD. The specific activities of the mutant forms of Plx1 were quantified by anti-FLAG immunocomplex kinase assays and immunoblotting. (B) T201 is a substrate for xPlkk1. Either MBP or synthetic peptides encompassing S128 or T201 (Plx1 residues 119 to 136 and Plx1 residues 191 to 204, respectively) were used as substrates in kinase assays in vitro with active recombinant xPlkk1, or with an equal volume of catalytically inactive xPlkk1 (K65M), purified from okadaic acid-treated Sf9 cells (31).
Constitutively active Plx1 is sufficient to initiate the G2/M transition.
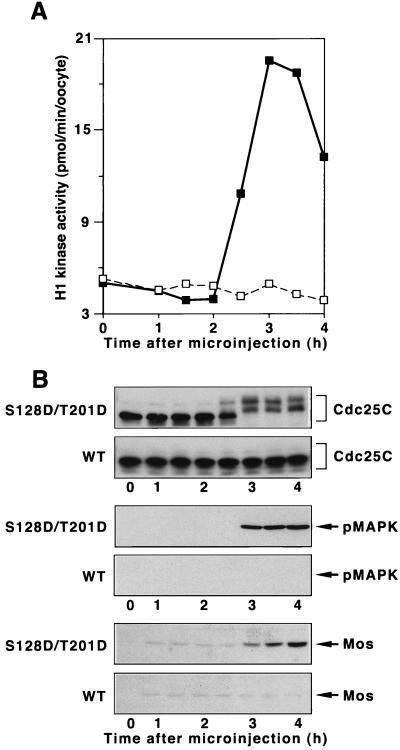
Inhibiting the activation of Plx1 significantly delays the activation of both Cdc25C and cyclin B-Cdc2 in progesterone-treated oocytes, and an elevated level of activated WT Plx1 accelerates the activation of Cdc25C and cyclin B-Cdc2 and oocyte maturation (30). The inability of an elevated level of WT enzyme to effect directly the activation of Cdc25C may be due to the fact that it cannot be phosphorylated and/or maintained in a phosphorylated state in the G2 environment of a stage VI oocyte. To determine whether constitutively active Plx1 is sufficient to initiate the G2/M transition, mRNAs encoding either WT Plx1 or S128D/T201D Plx1 were microinjected into stage VI oocytes, and both cyclin B-Cdc2 and Cdc25C activities were monitored. As shown in Fig. 4, S128D/T201D Plx1, but not WT Plx1, induced the activation of both Cdc25C and cyclin B-Cdc2, indicating that Plx1 is a “trigger” kinase for mitotic entry. In addition, at the time of germinal vesicle breakdown (GVBD) S128D/T201D Plx1 also induced the synthesis of Mos and activation of MAP kinase (Fig. 4B), further supporting the existence of a positive feedback loop between cyclin B-Cdc2 and MAP kinase and the synthesis of Mos (24, 32). In similar experiments neither S128D Plx1 nor T201D Plx1 caused entry into M phase (data not shown). Previous results (30) have shown that endogenous Plx1 remains highly activated after GVBD throughout both meiosis I and II in Xenopus cells. However, whether meiotic events beyond GVBD are normal in the presence of constitutively active Plx1 remains to be determined.
FIG. 4.
Induction of oocyte maturation by S128D/T201D Plx1. Stage VI oocytes were microinjected with 40 ng of mRNA encoding either WT Plx1 or S128D/T201D Plx1, and groups of six oocytes were frozen at the indicated times. (A) Extracts were prepared, and histone H1 kinase activity was determined. Symbols: □, WT Plx1; ■, S128D/T201D Plx1. (B) Samples of extracts were subjected to immunoblotting with anti-Cdc25C, anti-phospho-MAP kinase, and anti-Mos antibodies as indicated. Upper panel of each pair, S128D/T201D Plx1 (mRNA injected); lower panel of each pair, WT Plx1 (mRNA injected).
Constitutively active Plx1 attenuates the degradation of cyclin B2.
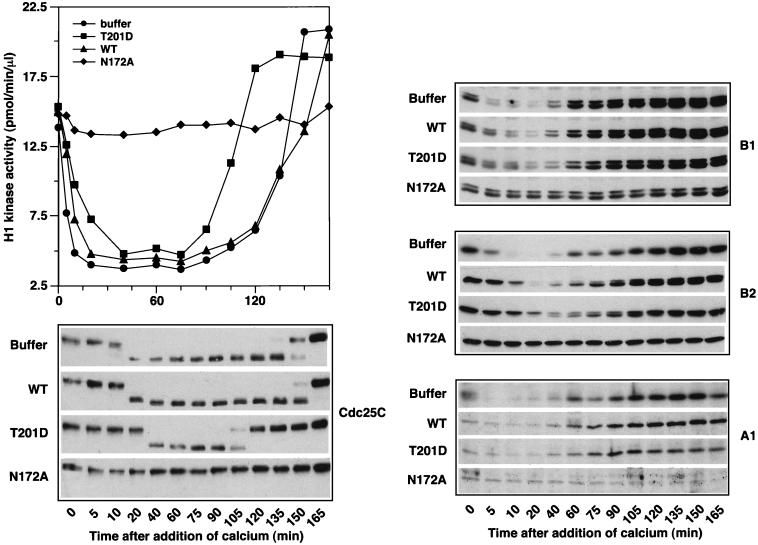
In S. cerevisiae the activity of plk’s is required for activation of the APC, which degrades cyclins and other proteins to drive the metaphase/anaphase transition and exit from mitosis (3, 33). This function of plk is likely to be conserved, inasmuch as addition of kinase-dead Plx1 to Xenopus egg extracts blocks degradation of cyclins and exit from M phase (4). In S. cerevisiae, the plk homolog Cdc5p is itself degraded by the APC during exit from mitosis (3, 33). Accumulation of particular B-type cyclins is reduced when Cdc5p activity is increased, and this effect is blocked by certain mutations in the APC (3). To determine whether Plx1 also affects cyclin reaccumulation after exit from M phase, metaphase-arrested CSF extracts were supplemented with T201D Plx1 purified from baculovirus-infected Sf9 cells. After addition of Ca2+ to trigger the metaphase/anaphase transition and exit from M phase, the levels of the cyclins A1, B1, and B2, the histone H1 kinase activity, and the activity of Cdc25C, as judged by its electrophoretic mobility, were monitored (Fig. 5). The extract containing constitutively active Plx1 had approximately fivefold-higher Plx1 activity than the endogenous level, and this level of activity remained constant throughout the experiment (data not shown). There was no effect of activated Plx1 on the rate of reaccumulation of any of the cyclins. However, constitutively active Plx1 delayed slightly the inactivation of Cdc25C during exit from mitosis and caused a much earlier activation of Cdc25C during the next mitosis. These effects were mirrored by changes in histone H1 kinase activity of Cdc2 and support the idea that Plx1 functions as a “trigger” kinase for the activation of Cdc25C during entry into mitosis. T201D Plx1 had a slight effect on the degradation of cyclin A1 and B1 (Fig. 5); however, the degradation of cyclin B2 was significantly attenuated and a larger fraction of the protein was in a shifted, phosphorylated form. This effect of activated Plx1 was consistently seen in five independent experiments. In agreement with the report of Descombes and Nigg (4), kinase-dead Plx1 blocked degradation of all three cyclins.
FIG. 5.
Effect of constitutively active Plx1 on M phase in egg extracts. Metaphase-arrested Xenopus egg extracts are arrested at meiosis II, with a high level of cyclin B-Cdc2 kinase activity due to the activity of CSF. Addition of calcium causes CSF release, and these extracts complete the metaphase/anaphase transition, exit metaphase, and enter S phase. Upon CSF release B-type cyclins are destroyed by the APC, histone H1 kinase activity is reduced, and Cdc25C is deactivated. Subsequently, cyclins reaccumulate, Cdc25C becomes reactivated, and the extracts enter M phase. To assess the effect of constitutively active Plx1 on the APC in this system, purified recombinant Plx1 proteins (100 μg/ml, final concentration) were added to the metaphase egg extracts. After a 20-min incubation, calcium was added to trigger the metaphase/anaphase transition and exit from M phase. At the times indicated, histone H1 kinase activity was determined, and Cdc25C and cyclins A1, B1, and B2 were monitored by Western blotting. At time zero the extract containing T201D Plx1 had approximately fivefold-higher Plx1 activity than the endogenous level, and this level of activity remained constant throughout the course of the experiment (data not shown). Similar results were obtained in five independent experiments.
Constitutively active Plx1 causes cleavage arrest.
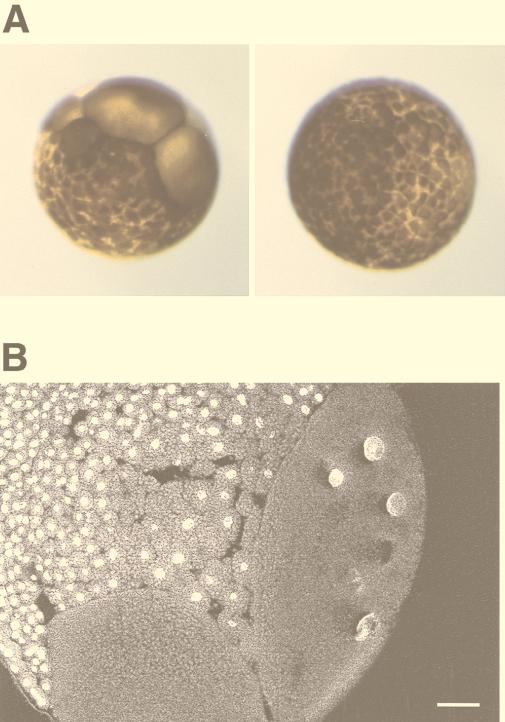
The activity of Plx1 increases dramatically at the G2/M transition, remains high during mitosis, and decreases during cytokinesis due to dephosphorylation (30). In S. cerevisiae, plk activity declines after exit from mitosis due to APC-mediated degradation (3, 33). In both yeast and Drosophila, plk’s play a role in cytokinesis (2, 7, 19, 28), and plk’s have been localized at the midbody in several organisms. If downregulation of the activity of Plx1 in telophase is essential for exit from mitosis, expression of constitutively active Plx1 might result in a defect in cytokinesis. To test this hypothesis, one blastomere of a two-cell embryo was injected with mRNA encoding either WT Plx1 or S128D/T201D Plx1. The uninjected blastomere served as an additional control. Expression of S128D/T201D Plx1 in the embryo caused cleavage arrest, resulting in large cells, whereas overexpression of WT Plx1 had no effect relative to uninjected controls (Fig. 6A). To examine the defects within the embryo, the embryo was sectioned and the DNA was stained with SYTOX Green. As shown in Fig. 6B, the large cells resulting from expression of S128D/T201D Plx1 contain multiple enlarged nuclei. This suggests, but does not prove, that cytokinesis is blocked when the activity of Plx1 remains high.
FIG. 6.
Overexpression of constitutively active Plx1 causes cleavage arrest in early embryos. (A) Cleavage arrest in an injected blastomere. mRNA encoding either WT Plx1 (right) or S128D/T201D Plx1 (left) was microinjected into one blastomere of a two-cell embryo, and embryonic development was monitored with a dissecting microscope. (B) An arrested embryo as shown in panel A (left) was fixed, sectioned, and stained with SYTOX Green as described in Materials and Methods. Bar, 100 μm.
Localization of Plx1 is consistent with its multiple functions during mitosis.
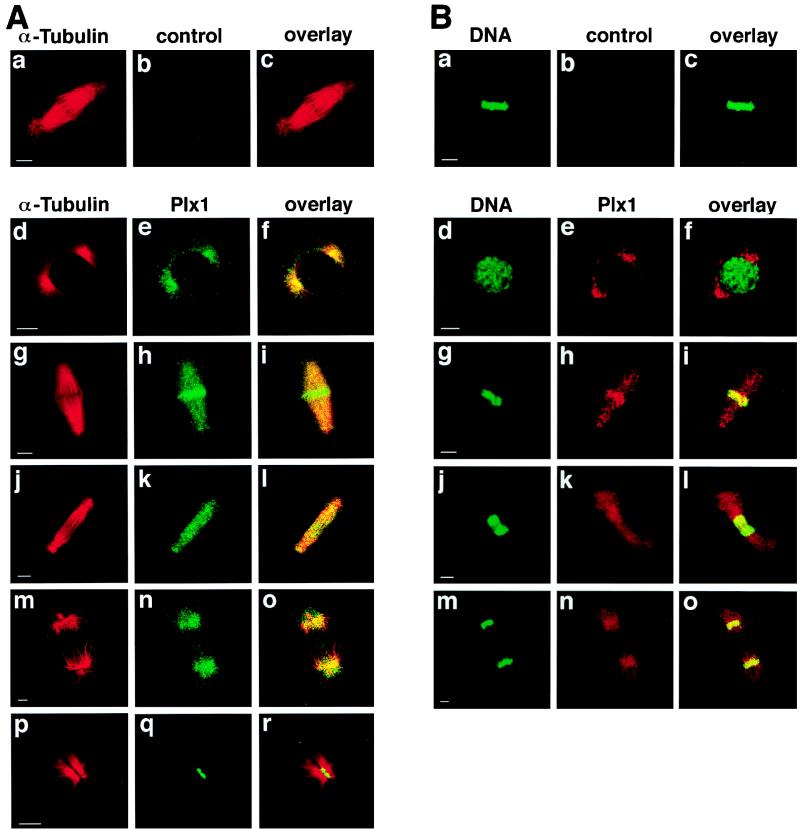
Based on previous studies and the data presented here, Plx1 plays multiple essential roles during mitosis. It activates Cdc25C, leading to cyclin B-Cdc2 activation and mitotic entry (Fig. 4), it is required for organization of bipolar spindles (17, 30, 35), it is necessary for activation of the APC (3, 4, 33), and its persistent activation causes a cleavage arrest (Fig. 6). Entry into mitosis begins with breakdown of the nuclear envelope while anaphase is initiated from the metaphase plate and cytokinesis from the midbody. This suggests that the localization of Plx1 might change during different stages of mitosis. To analyze this directly, subcellular localization of Plx1 during mitosis was examined by confocal microscopy (Fig. 7). Plx1 is localized to centrosomes early in mitosis (Fig. 7d to f), consistent with its role in Cdc25C activation and the organization of bipolar spindle assembly; just before anaphase it is concentrated on the metaphase plate (Fig. 7g to i), consistent with its role in activation of the APC; and late in mitosis it is localized to the midbody (Fig. 7Ap to r), consistent with a role in cytokinesis (Fig. 6).
FIG. 7.
Localization of Plx1 changes during mitosis. (A) Late-blastula-stage embryos were fixed in methanol, and immunofluorescence staining was performed as previously described (30). α-Tubulin was detected with an anti-α-tubulin monoclonal antibody (Sigma) and visualized by Cy3-conjugated donkey anti-mouse IgG antibodies, and Plx1 was detected with anti-Plx1 antibodies and visualized by Cy2-conjugated donkey anti-rabbit IgG antibodies. Confocal microscopy was performed with an MRC-600 microscope (Bio-Rad). Bars, 10 μm. (B) Embryos were fixed as in panel A. Plx1 was visualized by Cy3-conjugated donkey anti-rabbit IgG antibodies and DNA was detected with SYTOX Green. Control IgG from immune sera that had been depleted of all Plx1-specific antibodies was used as a negative control (b).
DISCUSSION
Several lines of evidence support the hypothesis that Plx1 is a trigger kinase that initiates the positive feedback loop between Cdc25C and cyclin B-Cdc2. First, Plx1 is able to bind, phosphorylate, and activate Cdc25C in vitro (reference 15 and our unpublished data). Second, Plx1 is activated with the same kinetics as Cdc25C during oocyte maturation (30). Third, inhibition of Plx1 in vivo with antibodies or by overexpression of kinase-dead Plx1 significantly delays the activation of Cdc25C, and immunodepletion or neutralization of Plx1 with antibodies suppresses the activation of Cdc25C (1, 30). Fourth, Cdc25C itself can overcome the inhibitory effect of Plx1 antibody (30). Finally, as shown here, in resting oocytes constitutively active Plx1 is sufficient to activate Cdc25C and initiate the G2/M transition, and in egg extracts it markedly accelerates Cdc25C activation and entry into M phase.
The results presented here indicate that both Ser-128 and Thr-201 residues play important roles for Plx1 activity. Mutation of Thr-201 to valine (T201V) abolished activation of Plx1, whereas mutation to aspartic acid conferred significant constitutive activity. Moreover, a synthetic peptide encompassing Thr-201 was a good substrate for xPlkk1 in vitro, and Plx1 activated in vivo contains phosphothreonine. T201 is in the activation loop, and phosphorylation of the corresponding residue is associated with activation of many other kinases. These considerations suggest that T201 phosphorylation in vivo is required for Plx1 activation. In contrast, the Ser-128 site was not phosphorylated as a synthetic peptide in vitro nor did a mutant at this site (S128A) suffer any impairment in activation in vivo. Nevertheless, the S128D mutant did display significant constitutive activity and contributed greatly to the high activity of the S128D/T201D double mutant. An understanding of the role of Ser-128 on the activity of Plx1 will require knowledge of the three-dimensional structure of the enzyme.
The work presented here with kinase-dead enzyme confirms the requirement of Plx1 for activation of the APC and exit from mitosis (4). Moreover, new data shows that high T201D Plx1 activity during exit from mitosis and progression into the subsequent mitosis did not affect the reaccumulation of mitotic cyclins, suggesting that, unlike the situation in S. cerevisiae, inactivation of Plx1 is not required for inactivation of the APC in G1. However, the degradation of cyclin B2 was attenuated by constitutively active Plx1 whereas the degradation of cyclin B1 and A1 was much less affected. Previous work has suggested that cyclin B2 degradation in X. laevis is regulated differently from that of cyclin B1 in terms of a requirement for binding to Cdc2 (34), and in S. cerevisiae overexpression of plk affects the degradation of some Clbs but not others (3, 33). Our results suggest that in Xenopus Plx1 regulates degradation of cyclin B2 differently from that of cyclin B1.
In addition to a role for plk’s during entry into mitosis, a variety of evidence points to an essential role for plk’s in cytokinesis. Disruption of the fission yeast polo-like kinase gene, plo1, leads not only to a failure of spindle formation but also to a failure to form either an actin ring or a septum (28). Moreover, overexpression of either plo1 in S. pombe or Plk in S. cerevisiae induces ectopic septal structures (19, 28). Mutations in the Drosophila polo gene also cause defects in the early events of cytokinesis at various stages of spermatogenesis (2). These results indicate that activity of plk’s is essential for the initiation of cytokinesis. Interestingly, in the current studies, expression of constitutively active Plx1 in Xenopus embryos led to a cleavage arrest that could result from defects in cytokinesis. It is likely that the defects observed here occur in the late events of cytokinesis. Consistent with this idea, in Xenopus embryos Plx1 is activated for entry into mitosis, kept high during mitosis, and deactivated after exit from mitosis during completion of cytokinesis (30). Deactivation of Plx1 may be essential for complete exit from mitosis or for completion of cytokinesis. Together, these results suggest that the initiation of cytokinesis requires the activity of plk’s, and the execution or completion of cytokinesis requires their inactivation. Although cleavage arrest is predicted to occur from a failure of cytokinesis, other defects, including a failure of chromosome segregation, could also give a similar phenotype. In addition, nuclei in a common cytoplasm might fuse together and/or continue to undergo rounds of DNA synthesis and division. Further work is needed to fully understand the basis of the cleavage arrest that results from the constitutive activity of Plx1.
In summary, the generation of a constitutively active form of Plx1 has revealed the importance of the enzyme at multiple stages of mitosis. The various functions in mitosis in which plk’s are implicated are correlated with the changes in the subcellular localization of Plx1 (Fig. 7). The domains of plk’s involved in localization changes are not clear, but it has been reported that mutants in the conserved polo box fail to localize to the midbody (20). Together with loss-of-function mutants, such as the N172A enzyme, elucidation of the mitotic functions of plk’s in a variety of organisms should be enhanced with constitutively active Plk.
ACKNOWLEDGMENTS
We thank Andrea Lewellyn for help with sectioning embryos and R. L. Erikson (Harvard University) for helpful discussions early in the course of this work. The baculovirus-infected Sf9 cells were produced in the tissue culture-monoclonal antibody core facility at the University of Colorado Cancer Center (P309CA46934).
This work was supported by a grant from the NIH (GM26743). Y.-W.Q. is an Associate and J.L.M. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Abrieu A, Brassac T, Galas S, Fisher D, Labbé J-C, Dorée M. The polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M phase transition of the cell cycle in Xenopus eggs. J Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- 2.Carmena M, Riparbelli M G, Minestrini G, Tavares A, Adams R, Callaini G, Glover D. Drosophila polo kinase is required for cytokinesis. J Cell Biol. 1998;143:659–671. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charles J, Jaspersen S, Tinker-Kulberg R, Hwang L, Szidon A, Morgan D. The polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- 4.Descombes P, Nigg E. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 6.Gautier J, Solomon M J, Booher R N, Bazan J F, Kirschner M W. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 7.Glover D M, Ohkura H, Tavares A. Polo kinase: the choreographer of the mitotic stage? J Cell Biol. 1996;135:1681–1684. doi: 10.1083/jcb.135.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamanaka R, Smith M R, O’Connor P M, Maloid S, Mihalic K, Spivak J L, Longo D L, Ferris D K. Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J Biol Chem. 1995;270:21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- 9.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman I, Clarke P R, Marcote M J, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Kessler D S, Erikson R L. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi T, Maller J L. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumi T, Walker D H, Maller J L. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumagai A, Dunphy W G. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai A, Dunphy W G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 16.Kumagai A, Dunphy W G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- 17.Lane H A, Nigg E A. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plx1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane H A, Nigg E A. Cell-cycle control: polo-like kinase joins the outer circle. Trends Cell Biol. 1997;7:63–68. doi: 10.1016/S0962-8924(96)10051-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee K S, Erikson R L. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K, Greenfell T, Yarm F, Erikson R. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M S, Ogg S, Xu M, Parker L L, Donoghue D J, Maller J L, Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992;3:73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Stanton J J, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 24.Matten W T, Copeland T D, Ahn N G, Vande Woude G F. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 25.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 26.Nigg E A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 27.Ohi R, Gould K L. Regulating the onset of mitosis. Curr Opin Cell Biol. 1999;11:267–273. doi: 10.1016/s0955-0674(99)80036-2. [DOI] [PubMed] [Google Scholar]
- 28.Ohkura H, Hagan I M, Glover D M. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 29.Parker L L, Atherton-Fessler S, Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci USA. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian Y-W, Erikson E, Li C, Maller J L. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian Y-W, Erikson E, Maller J L. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998;282:1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 32.Roy L M, Haccard O, Izumi T, Lattes B G, Lewellyn A L, Maller J L. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- 33.Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase-promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart E, Kobayashi H, Harrison D, Hunt T. Destruction of Xenopus cyclins A and B2, but not B1, requires binding to p34cdc2. EMBO J. 1994;13:584–594. doi: 10.1002/j.1460-2075.1994.tb06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunkel C E, Glover D M. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 36.Tavares Á, Glover D M, Sunkel C E. The conserved mitotic kinase polo is regulated by phosphorylation and has preferred microtubule-associated substrates in Drosophila embryo extracts. EMBO J. 1996;15:4873–4883. [PMC free article] [PubMed] [Google Scholar]