Abstract
Background
Diabetic foot ulcers characterized by delayed healing are one of the main complications of diabetes. Epidermal keratinocyte dysfunction has been found to play a pivotal role in the poor healing ability of diabetic wounds. In this study, we aimed to explore the relationship between c-Myc and its O-linked N-acetylglucosamine (O-GlcNAc) glycosylation (O-GlcNAcylation) modification and keratinocyte dysfunction in diabetic wounds.
Methods
Clinical wound samples were collected and a full-thickness skin defect wound model of diabetic rats was established. Re-epithelialization of wounds was observed by H&E staining and expressions of proliferating cell nuclear antigen, transglutaminase 1, loricrin, c-Myc and O-GlcNAc were measured by immunohistochemistry. The functional changes of proliferation, migration and differentiation of human immortalized epidermal cells (HaCaT) cells after overexpression or knockdown of c-Myc were observed. O-GlcNAcylation of c-Myc was confirmed using immunoprecipitation and proximity ligation assay. Stability of the c-Myc protein was measured using cycloheximide. Wound healing was observed after topical application of compounds that inhibited c-Myc or O-GlcNAc on diabetic wounds.
Results
Keratinocytes at the diabetic wound margin were characterized by active proliferation and division, slow migration and poor differentiation. Similar phenomena were observed in HaCaT cells cultured in 30 mM glucose and keratinocytes at the wound margin of the diabetic rats. The expression of c-Myc was increased in keratinocytes at the wound margin of diabetic rats, patients, and in HaCaT cells cultured with 30 mM glucose. Increased expression of c-Myc promoted the proliferation while inhibiting the migration and differentiation of the HaCaT cells, and inhibition of c-Myc promoted diabetic wound healing. Increased O-GlcNAcylation of c-Myc with 30 mM glucose stabilized the c-Myc proteins. Inhibition of O-GlcNAc ameliorated keratinocyte dysfunction and promoted diabetic wound healing.
Conclusions
Increased expression of c-Myc promoted abnormal proliferation and inhibited migration and differentiation of keratinocytes at the diabetic wound margin. Increased O-GlcNAcylation of c-Myc with 30 mM glucose stabilized the c-Myc proteins. Inhibition of c-Myc or O-GlcNAc alleviated delayed diabetic wound healing. These findings make c-Myc and O-GlcNAc potential therapeutic targets for diabetic wounds.
Keywords: c-Myc, O-GlcNAc, diabetes, wound healing, keratinocyte, glucose
Highlights
Increased expression of c-Myc promoted abnormal proliferation and inhibited migration and differentiation of keratinocytes at the diabetic wound margin.
Increased O-GlcNAcylation with 30 mM glucose stabilized the c-Myc proteins.
Inhibition of c-Myc or O-GlcNAc alleviated delayed healing of diabetic wounds.
Background
The latest data from the International Diabetes Federation 2019 indicate that there are 463 million people with diabetes worldwide [1]. Diabetic foot ulcers (DFUs) are one of the most serious complications of diabetes and the main cause of amputation in patients with diabetes. The worldwide prevalence of DFUs is 6.3% [1], and treatment is costly with limited efficacy.
The skin barrier helps the body resist external pathogenic microorganisms and maintains a stable internal environment. Re-epithelialization is the main component of skin barrier function reconstruction after injury. Residual epidermal cells at the edge of the wound or basal area undergo complete structural and functional reconstruction through proliferation, migration and differentiation [2]. It has been found that keratinocyte dysfunction plays a vital role in the poor re-epithelialization observed in diabetic wounds [3]. A previous study found that the expression of c-Myc was increased in keratinocytes at the edge of chronic wounds [4]; c-Myc-positive cells at the wound edge is a sign of non-healing, which requires further debridement [5]. The specific mechanism of increased expression of c-Myc and whether it mediates keratinocyte dysfunction remains unclear. The transcription factor c-Myc regulates many genes relevant to migration, proliferation, differentiation and apoptosis [6]. Furthermore, c-Myc has a variety of posttranslational modifications including acetylation, ubiquitination, phosphorylation and O-linked N-acetylglucosamine (O-GlcNAc) glycosylation (O-GlcNAcylation). O-GlcNAcylation occurs when a single O-GlcNAc is linked to the hydroxyl group of serine or threonine of the protein with a β-configuration O-glycosidic bond. O-GlcNAcylation competitively inhibits protein phosphorylation, thereby inhibiting phosphorylation-mediated ubiquitination degradation [7]. O-GlcNAcylation has been confirmed to inhibit phosphorylation-mediated ubiquitination degradation and increase the stability of c-Myc in prostate tumor cells, eventually leading to c-Myc accumulation in the cytoplasm [8]. Uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), produced by the hexosamine biosynthetic pathway (HBP) of glucose, is the donor for O-GlcNAc. Hyperglycemic conditions in diabetes lead to increased HBP flux, resulting in elevated levels of O-GlcNAc [9]. A pair of enzymes, O-GlcNAcase (OGA) and O-GlcNAc transferase (OGT), regulate the O-GlcNAcylation process; OGT acts on proteins O-GlcNAcylation, while OGA removes O-GlcNAc from modified proteins [10].
Thus, we speculated that O-GlcNAcylation of c-Myc played an important role in keratinocyte dysfunction in diabetic wounds. The present study aimed to explore the effects of O-GlcNAcylation of c-Myc on keratinocyte function at diabetic wound margins.
Methods
Cell culture
Human immortalized epidermal cells (HaCaT) were bought from Shanghai Fu Heng Biology. The HaCaT cells were cultured in defined keratinocyte-serum free medium (10744-019, Gibco) with 30 mM glucose or 9 mM glucose for 5 days at 37°C under 5% CO2. Cells were pre-incubated for 24 h with compounds including OGA inhibitor (Thiamet G, HY-12588, MCE) and OGT inhibitor (OSMI-4, HY-114361, MCE) at a concentration of 10 μM. Differentiation of HaCaT cells was induced by increasing the calcium concentration of the medium to 2.8 mM. Subsequently, cells were collected for protein or mRNA extraction.
Lentivirus transduction
Overexpression or knockdown of c-Myc in HaCaT cells was accomplished by lentivirus transduction, which was purchased from Shanghai GeneChem Company. The detailed sequences and vectors information are presented in the Supplementary materials. Briefly, HaCaT cells were seeded into a 6-well plate and cultured for 24 h until the confluence was about 30%. The amount of viruses was adjusted according to the virus titer and cell number (virus amount = multiplicity of infection (MOI) × cell number/virus titer). The virus mixture was prepared according to Table 1. One milliliter of the virus mixture was added to a 6-well plate and cells were incubated for 16 h. Stable cell clones were established using puromycin at a concentration of 2 μg/mL at 3 days postinfection.
Table 1.
Lentiviral transfection
| Group | Virus titer | Virus amount | 25 × HitransG P | Medium volume |
|---|---|---|---|---|
| c-MycOE | 5 × 108 TU/mL | 24 μL | 40 μL | 1 mL |
| OE-NC | 1.5 × 109 TU/mL | 8 μL | 40 μL | 1 mL |
| shMyc | 6 × 108 TU/mL | 20 μL | 40 μL | 1 mL |
| shMyc-NC | 5 × 108 TU/mL | 24 μL | 40 μL | 1 mL |
c-MycOE c-Myc overexpression, OE-NC the negative control of c-Myc overexpression, shMyc c-Myc knockdown, shMyc-NC the negative control of c-Myc knockdown
Cell-counting kit (CCK)-8
Cell proliferation was evaluated by CCK-8. Briefly, cells were seeded in 96-well plates (2000 cells/well, 100 μL). Each group had three replicates. Ten microliters of the CCK-8 solution (C0038, Beyotime) was added to each well at different time points (0, 24, 48, 72, 96 h) and incubated for 1 h at 37°C. The absorbance at 450 nm was measured. The fold of proliferation was determined according to the following formula: Fold = (ODtime-ODblank)/(OD0h-ODblank).
Scratch assay
The treated cells were plated onto a 6-well plate and cultured overnight to form a confluent monolayer. The next day, a scratch was made using a 200 μL pipette tip. After 24, 48, and 72 h, cell migration was recorded under a microscope and analyzed using the Image-Pro Plus software. The cell migration rate was calculated according to the following formula: Cell migration rate = (A0-At)/A0 × 100%. A0 refers to the initial scratch area and At refers to the remaining scratch area at the measurement time point.
Western blotting
Twenty micrograms of the total protein was separated using Biofuraw™ Precast Bis-Tris Gel (180-8008H, Tanon) and then transferred onto PVDF membranes. After being incubated with 5% skim milk in TBST for 1 h, the membranes were incubated with specific primary antibodies including c-Myc (10828-1-AP, Proteintech), loricrin (LOR) (55439-1-AP, Proteintech), transglutaminase 1 (TGM1) (12912-3-AP, Proteintech), O-GlcNAc (MA1-072, Thermo Scientific) or β-actin (66009-1-Ig, Proteintech) overnight at a dilution of 1:1000 at 4°C. Then, appropriate secondary antibodies were chosen for incubation for 1 h at room temperature. Finally, immunoreactive protein bands were imaged using a horseradish peroxidase substrate for enhanced chemiluminescence (WBKLS0050, Millipore). Gray intensity analysis of immunoblots was evaluated by the Image-Pro Plus software.
Immunoprecipitation
The experimental procedure was performed according to the manufacturer’s instructions for the protein A/G magnetic beads for immunoprecipitation (IP) (B23202, Biomake). First, the c-Myc antibody was incubated with the beads for 15 min at room temperature. Then, an equal amount of protein from the HaCaT cells was mixed with the bead-antibody complex at 4°C overnight. Finally, the loading buffer was mixed well with the complex and heated at 95°C for 5 min. The supernatant was collected for Western blot analysis.
Proximity ligation assay
The Duolink® In Situ Red Starter Kit Mouse/Rabbit for proximity ligation assay (PLA) were purchased from Sigma (DUO92101). The PLA was carried out according to the instructions on the kit. The HaCaT cells were seeded in Millicell EZ SLIDE (PEZGS0816, Millipore) and cultured in 30 mM glucose or 9 mM glucose for 5 days. After fixing in 4% paraformaldehyde for 30 min, slides were permeabilized in 0.1% Triton X-100 for 30 min. Then, cells were blocked with blocking solution for 1 h at 37°C and then incubated with primary antibodies against O-GlcNAc and c-Myc overnight at 4°C. On the following day, slides were incubated with PLUS and MINUS PLA probes for 1 h at 37°C and then incubated with ligation solution containing ligase for 30 min at 37°C. Slides were then incubated with amplification buffer containing polymerase for 100 min at 37°C. Finally, slides were incubated with DAPI for 15 min. All images were acquired using Zeiss LSM 880 equipped with a 63× objective lens.
Quantitative PCR
Total RNA of the HaCaT cells was extracted by TRIzol and reverse transcribed using the HiScript® III RT SuperMix kit (R323-01, Vazyme). Quantitative PCR was then performed using ChamQ Universal SYBR qPCR Master Mix (Q711-02, Vazyme). The primer sequences were synthesized by Shanghai Sangon Biotech as follows: GAPDH Forward, GGGAAACTGTGGCGTGAT; GAPDH Reverse, GAGTGGGTGTCGCTGTTGA; c-Myc Forward, CGTCTCCACACATCAGCACAA; c-Myc Reverse, TGTTGGCAGCAGGATAGTCCTT. The relative gene expression was calculated according to the 2-ΔΔCT method.
Protein stability test
We investigated the effect of O-GlcNAc levels on c-Myc protein stability by using cycloheximide (CHX, 40325ES03, Yeasen Biotechnology), which is a protein synthesis inhibitor. Briefly, cells were seeded in 6-well plates and pre-incubated for 24 h with 10 μM Thiamet G or OSMI-4. Subsequently, cells were treated with 50 μM CHX for different time periods (0, 2, 4, 6, 8 h) before harvest. The degradation curve of c-Myc protein was generated by normalizing the relative band intensities at different times compared to that at 0 h.
Animals and wound procedure
Rats were obtained and raised at the Animal Science Center of Shanghai Jiao Tong University School of Medicine (SJTUSM). First, the diabetic rat model was established by high-fat feeding combined with intraperitoneal injection of streptozocin. Diabetic rats were fed a high-fat diet (D12492, Research Diets) for 8 weeks. After fasting for 16 h, diabetic rats were intraperitoneal injected with streptozocin (S0130, Sigma) dissolved in 0.1 mol/L citrate buffer at a dose of 10 mg/kg for 4 consecutive days. The diabetic rats then developed diabetes over 4 weeks with a high-fat diet. Non-diabetic rats were fed a normal diet and intraperitoneally injected with the same volume of 0.1 mol/L citrate buffer. A random blood glucose level > 16.6 mM was considered an indicator of diabetes.
Ten non-diabetic rats and 40 diabetic rats were anesthetized with 3% sodium pentobarbital (60 mg/kg) by intraperitoneal injection. The back hair of the rats was thoroughly removed with a hair removal cream. Four full-thickness skin wounds in the mid-back were created using a 9-mm punch biopsy. Ten non-diabetic rats were treated with 200 μL diluent (10% DMSO+90% corn oil) as the control group (Ctrl). Forty rats with diabetes mellitus (DM) were randomly divided into four groups: (1) DM group [treated with 200 μL diluent (10% DMSO+90% corn oil)]; (2) DM + Thiamet G group (treated with 200 μL Thiamet G dissolved in 10% DMSO+90% corn oil, 10 mg/mL); (3) DM + OSMI-4 group (treated with 200 μL OSMI-4 dissolved in 10% DMSO+90% corn oil, 10 mg/mL); (4) DM + 10 058-F4 (c-Myc inhibitor, S7153, Selleck) group (treated with 200 μL 10 058-F4 dissolved in 10% DMSO+90% corn oil, 6 mg/mL). Rats in all groups were subcutaneously administered the diluent at the edge of the wound for 8 consecutive days. Wound photographs were obtained at days 0, 5, 8, and 11 post-injury. The non-healing rate was calculated as a percentage of the initial wound area using ImageJ software. The wound and an additional 5 mm of the surrounding normal skin tissues were obtained on days 5, 8, and 11 post-injury. Half of the skin tissues was fixed in 4% paraformaldehyde for immunohistochemistry, and the other half was frozen in liquid nitrogen for Western blot analysis. Paraffin sections of the wound tissues were stained with hematoxylin and eosin (H&E).
Human wound specimens
Diabetic wound tissue was obtained from wound edges of patients who underwent debridement or amputation of diabetic foot (n = 6). Non-diabetic wound tissue in the control group was obtained from wound edges of patients who underwent debridement of acute trauma injury or plastic surgery (n = 6). Written informed consent was acquired from all patients. The procedure of collecting wound specimens was approvedby the ethics committee of SJTUSM (Number: 2016-105-T54). The animal experiment was approved by the Institutional Animal Care and Use Committee of SJTUSM.
Immunohistochemical staining
Paraffin sections were deparaffinized in xylene and gradient ethanol, before being immersed in sodium citrate and heated in boiling water for 20 min for antigen retrieval. A solution of 3% hydrogen peroxide was dripped and slides were incubated at room temperature for 30 min to block any endogenous peroxidase. Goat serum was added to slides for blocking. All slides were incubated with primary antibodies overnight at 4°C including c-Myc, LOR, TGM1,O-GlcNAc, and proliferating cell nuclear antigen (PCNA) (ab18197, Abcam) at a dilution of 1:100. The following day, the corresponding secondary antibody was added to slides. Finally, all slides were incubated with DAB. Mean density was calculated as integrated optical density (IOD)/area using Image-Pro Plus.
Statistical analysis
All values are shown as means ± SD and analyzed using SPSS 24 (IBM Corp.). Parametric tests were used for data that conform to the normal distribution (Shapiro–Wilk test). Differences between the two groups were analyzed with Student’s t-test, and differences in multi groups were analyzed with one-way analysis of variance (ANOVA) followed by the Tukey’s post-test. The combination effects of two factors were analyzed with two-way ANOVA followed by the Tukey’s post-test. P values <0.05 were considered statistically significant.
Results
Epidermal keratinocytes at diabetic wound margins were characterized by active proliferation and division, slow migration and poor differentiation
We observed a significant thickening and increase in the epidermal layer adjacent to the wound margin in the skin tissues of the patients with diabetes. However, no epidermal migration was observed (Figure 1a). We also found increased binuclear cells in the stratum basale (Figure 1b) and nucleated cells in the stratum lucidum and the stratum corneum (Figure 1c) in keratinocytes at the diabetic wound margin. The expression of PCNA in keratinocytes at the diabetic wound margin was significantly higher while expressions of TGM1 and LOR were significantly lower than those in the control group (Figure 1d, e). Similar phenomena were observed in HaCaT cells and rats. The proliferation rate of HaCaT cells in the 30 mM glucose group was increased compared with that in the 9 mM glucose group (Figure 2a). The expressions of TGM1 and LOR and the migration rate of HaCaT cells in the 30 mM glucose group were significantly lower than those in the 9 mM glucose group (Figure 2b, c). Similarly, the expression of PCNA at the wound margin of rats in the DM group was increased compared with the Ctrl group (Figure 2d). The epidermal migration of the DM group was slower than that of the Ctrl group (Figure 2e). The expressions of TGM1 and LOR in wound margin tissues on different days in the DM group were decreased compared with those in the Ctrl group (Figure 2f). The above results indicated that 30 mM glucose could promote the proliferation while inhibiting migration and differentiation of keratinocytes.
Figure 1.
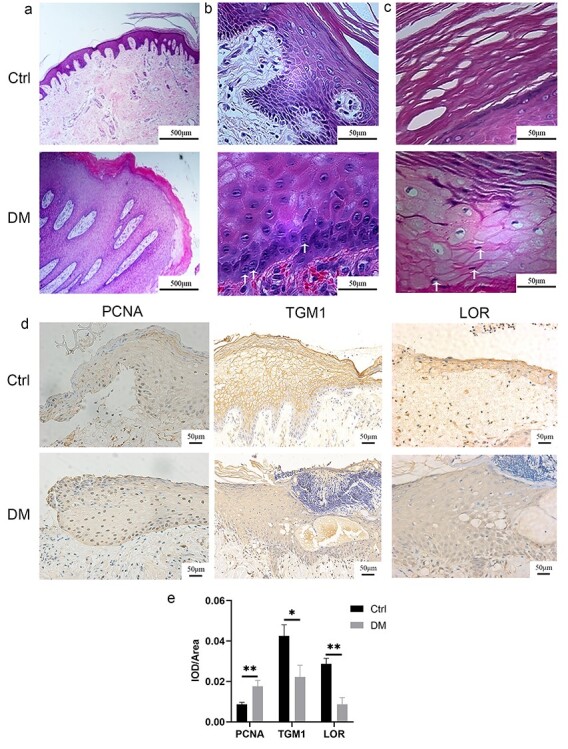
Epidermal keratinocytes at diabetic wound margin of patients were characterized by active proliferation, slow migration and differentiation dysfunction. Keratinocytes at the diabetic wound margin exhibited no migration (a), increased binuclear cells (as pointed out by the white arrow) in the stratum basale (b), and parakeratosis (c), (the white arrow points to the nucleus in the stratum corneum) as demonstrated by H&E. The results of immunohistochemistry (d) and quantitative analysis (e) suggested that keratinocytes at the diabetic wound margin exhibited increased expression of PCNA and decreased expressions of TGM1 and LOR. Scale bar: 500 μm, 50 μm. *p < 0.05, **p < 0.01. Ctrl control, DM diabetes mellitus, IOD integrated optical density, LOR loricrin, PCNA proliferating cell nuclear antigen, TGM1 transglutaminase 1, H&E hematoxylin and eosin staining
Figure 2.
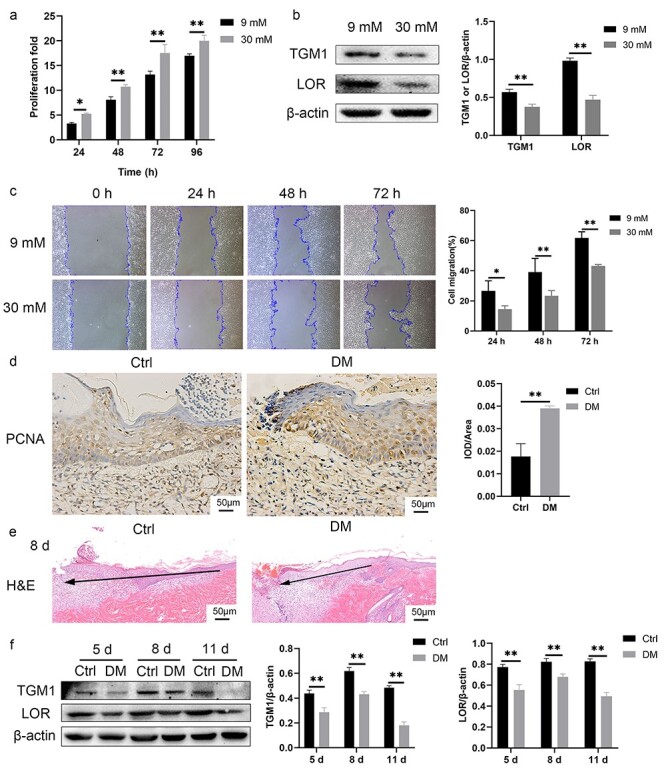
HaCaT cells cultured in 30 mM glucose and keratinocytes at the diabetic wound margin of rats exhibited active proliferation, slow migration and differentiation dysfunction. (a) The proliferation rate measured by CCK-8 of the HaCaT cells in the 30 mM glucose group was significantly higher than that in the 9 mM glucose group. (b) Expressions of TGM1 and LOR in the 30 mM glucose group were lower than those in the 9 mM glucose group. (c) The HaCaT cells cultured in 30 mM glucose exhibited slower migration as assessed by the scratch assay. (d) Keratinocytes at the diabetic wound margin of rats showed increased expression of PCNA observed by immunohistochemistry. Scale bar: 50 μm. (e) Keratinocytes at the diabetic wound margin of rats showed slow migration. The black arrow indicates the direction of epidermal migration. Scale bar: 50 μm. (f) Expressions of TGM1 and LOR in wound margin tissues of the DM group were significantly lower than those in the Ctrl group on different days. *p < 0.05, **p < 0.01. Ctrl control, DM diabetes mellitus, H&E hematoxylin and eosin staining, IOD integrated optical density, LOR loricrin, PCNA proliferating cell nuclear antigen, TGM1 transglutaminase 1
The expression of c-Myc was increased in the pathophysiological state of diabetes or with 30 mM glucose
The HaCaT cells cultured in 30 mM glucose showed increased expression of c-Myc (Figure 3a). Similarly, the expression of c-Myc in the wound margin of diabetic rats or patients was higher than that in the Ctrl groups (Figure 3b, c).
Figure 3.
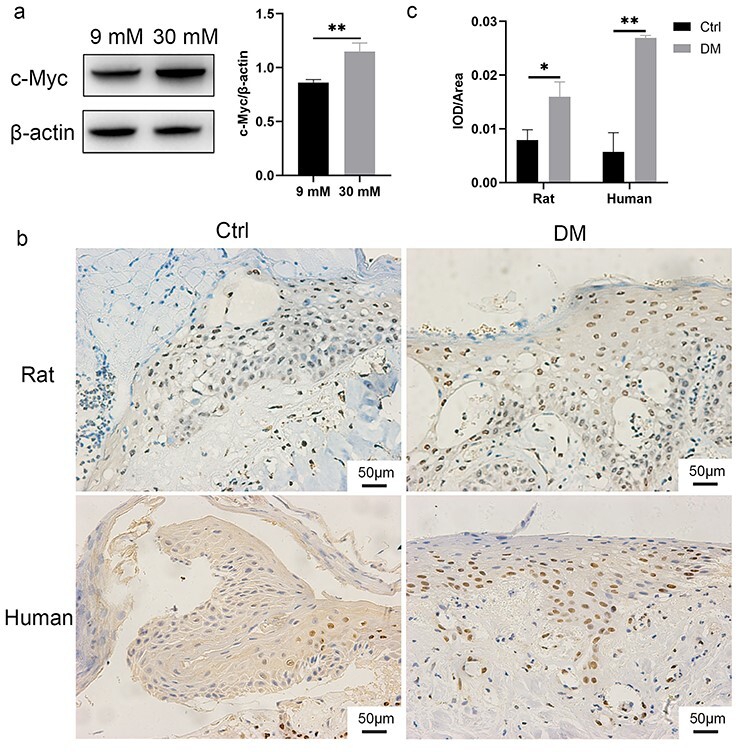
The expression of c-Myc was increased in the HaCaT cells cultured in 30 mM glucose and keratinocytes at diabetic wound margin. (a) The HaCaT cells in 30 mM glucose group showed high expression of c-Myc. (b–c) The results of immunohistochemistry (b) and quantitative analysis (c) suggested that keratinocytes at the diabetic wound margin showed high expression of c-Myc. Scale bar: 50 μm. *p < 0.05, **p < 0.01. Ctrl control, DM diabetes mellitus, IOD integrated optical density
C-Myc regulated the proliferation, differentiation and migration of HaCaT cells and inhibition of c-Myc promoted the healing of diabetic wounds
In order to confirm the relationship between c-Myc and the proliferation, differentiation and migration of the HaCaT cells, we constructed HaCaT cells with c-Myc overexpression or knockdown. The overexpression (Figure 4a) or knockdown (Figure 4b) of c-Myc was confirmed by Western blotting. The proliferation rate of c-Myc overexpressing cells was significantly higher than that of control cells (Figure 4c). The expressions of TGM1 and LOR in c-Myc overexpressing cells were significantly lower than those in control cells (Figure 4d). The migration rate of cells overexpressing c-Myc was significantly lower than that of control cells (Figure 4e). In contrast, knockdown of c-Myc abolished the high proliferation rate (Figure 4f), decreased expressions of TGM1 and LOR (Figure 4g) and slow migration (Figure 4h) caused by 30 mM glucose. Next, we verified whether the topical use of c-Myc inhibitor on the wounds of diabetic rats could promote wound healing. 10058-F4 is a c-Myc inhibitor that can inhibit the transcriptional activation of c-Myc target genes [11]. Additionally, the protein expression of c-Myc is also inhibited in a dose-dependent manner by 10058-F4 [12,13]. 10058-F4 inhibited slow migration, increased expression of PCNA, and decreased expressions of TGM1 and LOR in keratinocytes at the diabetic wound margin (Figure 5a, b). The non-healing rate of wounds in the DM group on day 5, 8, and 11 was significantly higher than that of the Ctrl group at the corresponding time points, while the non-healing rate of the wound in DM + 10058-F4 group was significantly lower than that in the DM group (Figure 5c, d). These results confirmed that increased expression of c-Myc promoted the proliferation while inhibiting the migration and differentiation of HaCaT cells, and inhibition of c-Myc promoted the healing of diabetic wounds.
Figure 4.
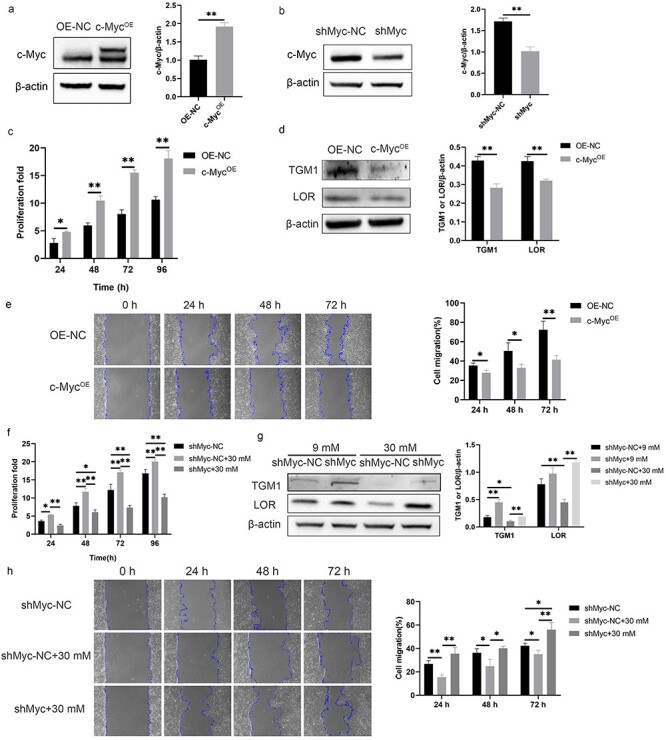
c-Myc promoted proliferation and inhibited migration and differentiation of HaCaT cells. (a, b) Overexpression (a) and knockdown (b) of c-Myc were confirmed by Western blotting. (c) HaCaT cells overexpressing c-Myc showed higher proliferation. (d) HaCaT cells overexpressing c-Myc showed decreased expressions of TGM1 and LOR. (e) HaCaT cells overexpressing c-Myc showed slower migration. (f–h) Knockdown of c-Myc abolished the high proliferation rate (f), decreased expressions of TGM1 and LOR (g) and slow migration (h) caused by 30 mM glucose. *p < 0.05, **p < 0.01. c-MycOE c-Myc overexpression, LOR loricrin, OE-NC the negative control of c-Myc overexpression, shMyc c-Myc knockdown, shMyc-NC the negative control of c-Myc knockdown, TGM1 transglutaminase 1
Figure 5.
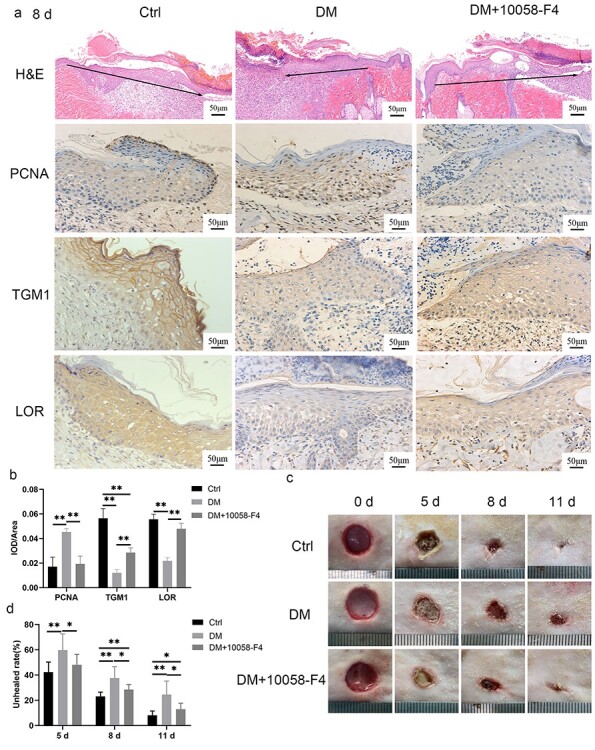
Inhibition of c-Myc promoted diabetic wound healing. (a, b) 10058-F4 (200 μL, 6 mg/mL) was administered subcutaneously at the edge of the wound for 8 consecutive days in the DM + 10058-F4 group. The results of H&E, immunohistochemical staining (a) and quantitative analysis (b) confirmed that 10058-F4 could abolish the slow migration, increased expression of PCNA, and decreased expressions of TGM1 and LOR in keratinocytes at the diabetic wound margin. The black arrow indicates the direction of epidermal migration. (c, d) Representative pictures of wound healing in rats (c) and quantitative statistics of the non-healing rate (d) indicated that inhibition of c-Myc (10058-F4) promoted the healing of diabetic wounds. Scale bar: 50 μm. *p < 0.05, **p < 0.01. Ctrl control, DM diabetes mellitus, H&E hematoxylin and eosin staining, IOD integrated optical density, LOR loricrin, PCNA proliferating cell nuclear antigen, TGM1 transglutaminase 1
Increased O-GlcNAcylation of c-Myc with 30 mM glucose stabilized c-Myc proteins
The HaCaT cells cultured in 30 mM glucose showed increased modification of O-GlcNAc (Figure 6a). Additionally, the O-GlcNAc level in the wound margin of rats or patients with diabetes was higher than that in the control group (Figure 6b). Increased O-GlcNAcylation of c-Myc with 30 mM glucose was confirmed using direct IP with anti-c-Myc followed by immunoblotting with an antibody against O-GlcNAc (Figure 6c). Results of the PLA indicated that the fluorescence signal in the 30 mM glucose group was significantly higher than that in the 9 mM glucose group (Figure 6d). In order to confirm whether the increased O-GlcNAcylation of c-Myc caused by 30 mM glucose affects the expression of total c-Myc protein, we used a pair of inhibitors to regulate the overall O-GlcNAc level. Thiamet G is an effective and selective OGA inhibitor, which increases O-GlcNAc levels [14], while OSMI-4 is an inhibitor of OGT, which reduces O-GlcNAc levels [15]. The expression of c-Myc was increased in the HaCaT cells treated with Thiamet G and decreased in HaCaT cells treated with OSMI-4 (Figure 6e), while neither Thiamet G nor OSMI-4 affected c-Myc mRNA expression (Figure 6f). We investigated the effect of O-GlcNAc levels on the degradation of the c-Myc protein using CHX, which inhibits protein synthesis. The expression of the c-Myc protein gradually decreased with time after CHX treatment. Thiamet G delayed the degradation rate of c-Myc protein compared with that in the Ctrl group, while OSMI-4 accelerated the degradation rate of the c-Myc protein (Figure 6g). The above results indicated that the increased O-GlcNAcylation of c-Myc caused by 30 mM glucose stabilized c-Myc protein.
Figure 6.
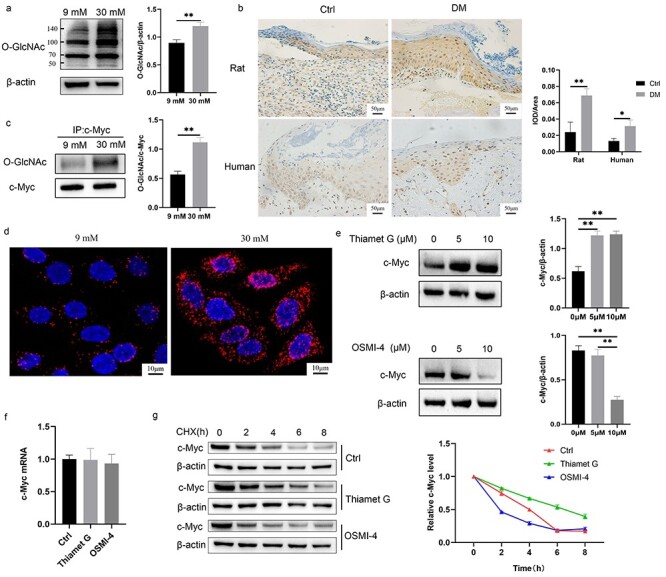
O-GlcNAcylation of c-Myc made c-Myc proteins more stable. (a, b) HaCaT cells (a) cultured with 30 mM glucose and keratinocytes (b) at the diabetic wound margin showed higher level of O-GlcNAc. (c, d) Increased O-GlcNAcylation of c-Myc with 30 mM glucose was confirmed using immunoprecipitation (c) and proximity ligation assay (d). (e, f) The protein (e) and mRNA (f) expression of c-Myc was detected after treatment with 10 μM Thiamet G or OSMI-4 for 24 h. (g) c-Myc protein stability was measured using CHX (a protein synthesis inhibitor). Cells were pre-incubated for 24 h with 10 μM Thiamet G or OSMI-4 and subsequently treated with 50 μM CHX for different times (0, 2, 4, 6, 8 h) before harvesting. Compared with the Ctrl group, Thiamet G delayed the degradation rate of c-Myc protein, while OSMI-4 accelerated the degradation rate of c-Myc protein. Scale bar: 50 μm, 10 μm. *p < 0.05, **p < 0.01. CHX cycloheximide, Ctrl control, DM diabetes mellitus, IOD integrated optical density, O-GlcNAc O-linked N-acetylglucosamine
Inhibition of O-GlcNAc ameliorated keratinocyte dysfunction and promoted diabetic wound healing
Finally, we verified whether the proliferation, migration and differentiation of keratinocytes and wound healing could be affected by regulating O-GlcNAc. OSMI-4 inhibited the increased proliferation rate of HaCaT cells in the 30 mM glucose and 30 mM + Thiamet G group at 48, 72, and 96 h. Although there were no statistical differences among the groups at 24 h, the trend of the proliferation rate was consistent with those from other time points (Figure 7a). The expressions of TGM1 and LOR were decreased in the 30 mM glucose and 30 mM + Thiamet G group compared with those in the 9 mM glucose group. OSMI-4 abolished decreased expressions of TGM1 and LOR caused by 30 mM glucose (Figure 7b). OSMI-4 improved the slow migration caused by 30 mM glucose, while Thiamet G exacerbated the slow migration (Figure 7c). These results were also verified in rats. Re-epithelialization in the DM + OSMI-4 group was significantly better than that in the DM and DM + Thiamet G group on day 8 after wound formation, even though it was still worse than that in the Ctrl group. The expression of PCNA was increased in the DM and DM + Thiamet G group compared with that in the Ctrl group, while it was decreased in the DM + OSMI-4 group compared with that in the DM and DM + Thiamet G group. The expressions of LOR and TGM1 were decreased in the DM and DM + Thiamet G group compared with those in the Ctrl group, while they were increased in DM + OSMI-4 group compared with those in the DM and DM + Thiamet G group (Figure 7d). Topical application of OSMI-4 in diabetic wounds could alleviate delayed wound healing (Figure 7e).
Figure 7.
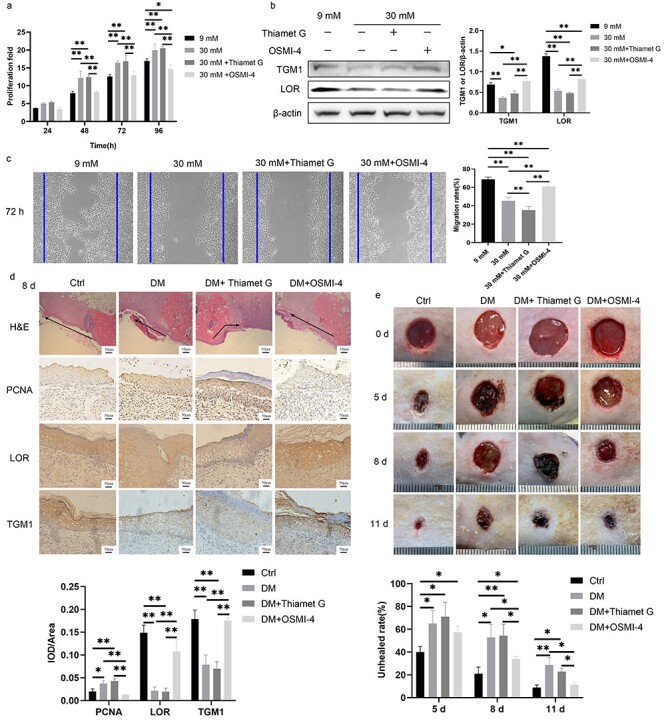
Inhibition of O-GlcNAcylation promoted diabetic wound healing. (a) The proliferation of HaCaT cells treated with 9 mM glucose, 30 mM glucose, 30 mM + Thiamet G and 30 mM + OSMI-4 was demonstrated by CCK-8 analysis. (b) Expressions of TGM1 and LOR of HaCaT cells treated with 9 mM glucose, 30 mM glucose, 30 mM + Thiamet G and 30 mM + OSMI-4 were measured by Western blotting. (c) The migration of HaCaT cells treated with 9 mM glucose, 30 mM glucose, 30 mM + Thiamet G and 30 mM + OSMI-4 was detected by the scratch assay at 72 h. (d) Thiamet G (200 μL, 10 mg/mL) or OSMI-4 (200 μL, 10 mg/mL) was administered subcutaneously at the edge of the wound for 8 consecutive days in the DM + Thiamet G group or DM + OSMI-4 group, respectively. Migration, proliferation (PCNA), and differentiation (LOR and TGM1) of keratinocytes at rat wound margin of different groups were measured. The black arrow indicates the direction of epidermal migration. (e) Representative pictures of wound healing in rats from different groups and quantitative statistics of non-healing rate are shown. Scale bar: 10 μm, 50 μm. *p < 0.05, **p < 0.01. Ctrl control, DM diabetes mellitus, H&E hematoxylin and eosin staining, IOD integrated optical density, LOR loricrin, PCNA proliferating cell nuclear antigen, TGM1 transglutaminase 1
Discussion
While up to 25% of patients with diabetes are reported to develop DFU [16], effective treatment options remain limited. More than 100 known pathophysiological factors lead to the delayed healing of diabetic wounds [5]. Therefore, it is desired to identify precise targets and new therapies for healing diabetic wounds. Normal wound healing involves a variety of different cells, which release growth factors and cytokines in a coordinated manner [17]. This study focused on keratinocyte dysfunction in diabetic wounds, as keratinocytes are the main cell type found in the skin. The expression of PCNA was measured as a proliferation marker of keratinocytes [18], and expressions of TGM1 and LOR were measured as late differentiation markers of keratinocytes [19]. In addition, we chose HaCaT cells for our experiments because these cells have similar characteristics to epidermal stem cells [20] and are widely used in the study of the pathophysiology of diabetic foot ulcers [21]. We found that keratinocytes at the diabetic wound margin were characterized by active proliferation, slow migration and poor differentiation. These functional changes of keratinocytes are detrimental to the re-epithelialization of wounds [22].
Increased expression of c-Myc was found in the HaCaT cells cultured in 30 mM glucose and in the keratinocytes at the diabetic wound margin. Our results confirmed that increased expression of c-Myc could promote the proliferation while inhibiting the migration and differentiation of HaCaT cells. c-Myc is considered a molecular marker of delayed healing in diabetic wounds [4]. Basal keratinocytes overexpressing c-Myc show impaired migration and reduced epidermal stem cells, leading to inhibition of wound healing [23]. Activated c-Myc in chronic wounds leads to impaired migration and over-proliferation of epidermal keratinocytes, causing the cells to fail to respond to damage [24]. 10054-F4 can specifically inhibit the interaction between c-Myc and Max, thereby inhibiting transcriptional activation of numerous c-Myc target genes [11]. We confirmed that the topical application of c-Myc inhibitor 10054-F4 on diabetic wounds could promote healing. However, the tissue context specificity should also be considered. It has been reported that c-Myc is also involved in the protective impact of resveratrol on vascular endothelial cells and promotes angiogenesis [25]. In addition, elevated c-Myc expression induced by Wnt signaling can stimulate intestinal epithelial cell repair [26]. The main reason for this variance might be the use of different cell types. The exact mechanism of c-Myc causing delayed healing of diabetic wound is unknown. It has been reported that siRNA-mediated loss of c-Myc can downregulate the proliferation-related gene network and upregulate the migration and adhesion-related gene network, thereby affecting the function of keratinocytes [27]. The target genes of c-Myc that regulate the proliferation, migration and differentiation of keratinocytes need to be further explored and verified in future studies.
Next, we explored the mechanism of increased expression of c-Myc in diabetic wounds. We found that modification of O-GlcNAc was increased in the wound margins of rats or patients with diabetes and in the HaCaT cells cultured with 30 mM glucose. O-GlcNAc plays a vital role in the pathophysiology of diabetes, including glucose toxicity and insulin resistance [28]. More than 4000 proteins can be modified with O-GlcNAc. The O-GlcNAcylation site of c-Myc is located in or near the N-terminal transcriptional activation domain [29], specifically at threonine 58 [30]. The c-Myc protein can be competitively modified by O-GlcNAcylation or phosphorylation at the same site [31]. In our study, increased O-GlcNAcylation of c-Myc with 30 mM glucose was confirmed using IP and PLA. PLA allows in situ detection of protein–protein interactions or protein modifications. A fluorescence signal can only be generated when the distance of two antibodies targeted proteins is less than 40 nm [32]. Our results confirmed that the protein (not mRNA) expression of c-Myc was regulated by Thiamet G or OSMI-4, indicating a posttranslational modification. Furthermore, increased O-GlcNAcylation of c-Myc was confirmed to stabilize the c-Myc protein. Similarly, c-Myc has also been confirmed to be more stable after O-GlcNAcylation in other cells, such as human prostate cancer cells [8], pre-B cells [33], and human lung carcinoma cells [34]. After O-GlcNAcylation, c-Myc is prevented from being phosphorylated and further degraded by ubiquitination [35], leading to the accumulation of c-Myc, which is closely related to keratinocyte dysfunction in diabetic wounds. Since O-GlcNAcylation of c-Myc could affect its stability, regulating the O-GlcNAc level should be able to regulate keratinocyte function by affecting the expression of c-Myc. Our results also showed that OSMI-4 inhibited the high proliferation, impaired migration and differentiation dysfunction caused by 30 mM glucose in the HaCaT cells. Similar results were observed in keratinocytes at wound margins of rats. A study reported that O-GlcNAcylation of c-Myc played an important role in the process of megakaryocyte differentiation; inhibition of OGT disrupted O-GlcNAcylation of c-Myc, leading to c-Myc degradation, thereby promoting megakaryocyte differentiation and platelet production [36]. Although we found that 10058-F4 or OSMI-4 promoted wound healing in diabetic rats, there is still a long way to go before their clinical application due to the lack of cell selectivity [37].
Conclusions
Our study firstly evaluated that the regulation of c-Myc O-GlcNAcylation could affect the healing of diabetic wounds. Increased expression of c-Myc promoted abnormal proliferation and inhibited migration or differentiation of keratinocytes at diabetic wound margins. Increased O-GlcNAcylation of c-Myc in the high-glucose diabetes state stabilized the c-Myc proteins. Inhibition of c-Myc or O-GlcNAc alleviated delayed diabetic wound healing. These findings make c-Myc and O-GlcNAc potential therapeutic targets for diabetic wounds.
Authors’ contributions
JZ implemented the research and wrote the manuscript; PLY and DL participated in the research design and implementation; MG, JZW and TYY helped perform the research; XZ provided statistical analysis and guided the project implementation; YL provided technical and material support and revised the manuscript. All authors read and approved the final paper.
Acknowledgments
We gratefully acknowledge the assistance of Min Yao for providing ethical and experimental technical guidance.
Abbreviations
- CHX: cycloheximide; DFU: diabetic foot ulcer; h: hour; H & E: hematoxylin and eosin; IP: immunoprecipitation; LOR: loricrin; O-GlcNAc: O-linked N-acetylglucosamine; OGA: O-GlcNAcase; OGT: O-GlcNAc transferase; PCNA: proliferating cell nuclear antigen; PLA: proximity ligation assay; TGM1: transglutaminase 1; PVDF: polyvinylidene fluoride; TBST: tris buffered saline with tween.
Contributor Information
Jie Zhang, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Peilang Yang, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Dan Liu, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Min Gao, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Jizhuang Wang, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Tianyi Yu, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Xiong Zhang, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Yan Liu, Department of Burn, Ruijin Hospital Affliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Ethics approval and consent to participate
The procedure of collecting wound specimens was approved by the ethics committee of SJTUSM (Number: 2016-105-T54). The animal experiment was approved by the Institutional Animal Care and Use Committee of SJTUSM.
Funding
This work was supported by National Natural Science Foundation of China (No. 81871564 and 82072173), Shanghai Committee of Science and Technology (18ZR1423800) and Shanghai Municipal Key Clinical Specialty (shslczdzk02302).
Conflicts of interest
The authors declare that they have no conflict of interest.
Availability of data and materials
The data used to support the findings of the present study are available from the corresponding author upon request.
References
- [1]. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- [2]. Rousselle P, Braye F. Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–65. [DOI] [PubMed] [Google Scholar]
- [3]. Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci. 2016;84:121–7. [DOI] [PubMed] [Google Scholar]
- [4]. Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, et al. . Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-Myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Brem H, Tomic-CANIC M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Huang H, Weng H, Zhou H, Qu L. Attacking c-Myc: targeted and combined therapies for cancer. Curr Pharm Des. 2014;20:6543–54. [DOI] [PubMed] [Google Scholar]
- [7]. Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18:452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, et al. . O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73:5277–87. [DOI] [PubMed] [Google Scholar]
- [9]. Marsh SA, Dell'Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Ferron M, Denis M, Persello A, Rathagirishnan R, Lauzier B. Protein O-GlcNAcylation in cardiac pathologies: past, present, future. Front Endocrinol (Lausanne). 2018;9:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Huang MJ, Cheng YC, Liu CR, Lin S, Liu HE. A small-molecule c-Myc inhibitor, 10058-F4, induces cell-cycle arrest, apoptosis, and myeloid differentiation of human acute myeloid leukemia. Exp Hematol. 2006;34:1480–9. [DOI] [PubMed] [Google Scholar]
- [12]. Tan Y, Sementino E, Pei J, Kadariya Y, Ito TK, Testa JR. Co-targeting of Akt and Myc inhibits viability of lymphoma cells from Lck-Dlx5 mice. Cancer Biol Ther. 2015;16:580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Wang J, Ma X, Jones H M, Chan L L, Song F, Zhang W, et al. . Evaluation of the antitumor effects of c-Myc-Max heterodimerization inhibitor 100258-F4 in ovarian cancer cells. J Transl Med. 2014;12:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Yuzwa S A, Macauley M S, Heinonen J E, Shan X, Dennis R J, He Y, et al. . A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–90. [DOI] [PubMed] [Google Scholar]
- [15]. Martin SE S, Tan Z W, Itkonen H M, Duveau D Y, Paulo J A, Janetzko J, et al. . Structure-based evolution of low nanomolar O-GlcNAc transferase inhibitors. J Am Chem Soc. 2018;140:13542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. . Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Camacho Leal M, Costamagna A, Tassone B, Saoncella S, Simoni M, Natalini D, et al. . Conditional ablation of p130Cas/BCAR1 adaptor protein impairs epidermal homeostasis by altering cell adhesion and differentiation. Cell Commun Signal. 2018;16:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Miyai M, Hamada M, Moriguchi T, Hiruma J, Kamitani-KAWAMOTO A, Watanabe H, et al. . Transcription factor MafB coordinates epidermal keratinocyte differentiation. J Invest Dermatol. 2016;136:1848–57. [DOI] [PubMed] [Google Scholar]
- [20]. Colombo I, Sangiovanni E, Maggio R, Mattozzi C, Zava S, Corbett Y, et al. . HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediators Inflamm. 2017;2017:7435621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Ochoa-Gonzalez F, Cervantes-Villagrana AR, Fernandez-Ruiz JC, Nava-Ramirez HS, Hernandez-Correa AC, Enciso-Moreno JA, et al. . Metformin induces cell cycle arrest, reduced proliferation, wound healing impairment in vivo and is associated to clinical outcomes in diabetic foot ulcer patients. PLoS One. 2016;11:e0150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Moura L I, Cruz M T, Carvalho E. The effect of neurotensin in human keratinocytes--implication on impaired wound healing in diabetes. Exp Biol Med (Maywood). 2014;239:6–12. [DOI] [PubMed] [Google Scholar]
- [23]. Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–8. [DOI] [PubMed] [Google Scholar]
- [24]. Sawaya AP, Pastar I, Stojadinovic O, Lazovic S, Davis SC, Gil J, et al. . Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5. J Biol Chem. 2018;293:1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Huang X, Sun J, Chen G, Niu C, Wang Y, Zhao C, et al. . Resveratrol promotes diabetic wound healing via SIRT1-FOXO1-c-Myc signaling pathway-mediated angiogenesis. Front Pharmacol. 2019;10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Liu L, Rao JN, Zou T, Xiao L, Smith A, Zhuang R, et al. . Activation of Wnt3a signaling stimulates intestinal epithelial repair by promoting c-Myc-regulated gene expression. Am J Physiol Cell Physiol. 2012;302:C277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Wu N, Rollin J, Masse I, Lamartine J, Gidrol X. p63 regulates human keratinocyte proliferation via MYC-regulated gene network and differentiation commitment through cell adhesion-related gene network. J Biol Chem. 2012;287:5627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Peterson SB, Hart GW. New insights: a role for O-GlcNAcylation in diabetic complications. Crit Rev Biochem Mol Biol. 2016;51:150–61. [DOI] [PubMed] [Google Scholar]
- [29]. Chou TY, Dang CV, Hart GW. Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci U S A. 1995;92:4417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Chou TY, Hart GW, Dang CV. C-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–5. [DOI] [PubMed] [Google Scholar]
- [31]. Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem. 2002;277:19229–35. [DOI] [PubMed] [Google Scholar]
- [32]. Soldatova I, Prilepskaja T, Abrahamyan L, ForstovÁ J, HuÉRFANO S. Interaction of the mouse polyomavirus capsid proteins with importins is required for efficient import of viral DNA into the cell nucleus. Viruses. 2018;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Park S-K. O-GlcNAcylation of c-Myc dynamically regulates pre-B cell proliferation. J Immunol. 2019;202:123.13. [Google Scholar]
- [34]. Luanpitpong S, Angsutararux P, Samart P, Chanthra N, Chanvorachote P, Issaragrisil S. Hyper-O-GlcNAcylation induces cisplatin resistance via regulation of p53 and c-Myc in human lung carcinoma. Sci Rep. 2017;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Sekine H, Motohashi H. Roles of CNC transcription factors NRF1 and NRF2 in cancer. Cancer. 2021;13:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Luanpitpong S, Poohadsuan J, Klaihmon P, Kang X, Tangkiettrakul K, Issaragrisil S. Metabolic sensor O-GlcNAcylation regulates megakaryopoiesis and thrombopoiesis through c-Myc stabilization and integrin perturbation. Stem Cells. 2021;39:787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Carabet LA, Rennie PS, Cherkasov A. Therapeutic inhibition of Myc in cancer. Structural bases and computer-aided drug discovery approaches. Int J Mol Sci. 2018;20:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of the present study are available from the corresponding author upon request.


