Abstract:
To examine the accuracy between analyzers, the Terumo CDI 500 and the Spectrum Medical Quantum were compared to each other and to the ABL90 FLEX benchtop blood analyzer. Patients were retrospectively identified who underwent cardiac surgery requiring cardiopulmonary bypass between August 1, 2018 and November 1, 2019. Hemoglobin and venous saturation (SvO2) values from all three analyzers were collected. Measurements from the Quantum and the CDI 500 were averaged over 1 minute to provide a single value for the minute for the given device. Blood analysis on the ABL90 benchtop device was performed at a minimum of every hour during congenital cardiopulmonary bypass (CPB). There were 519 patients included in the analysis. Data points numbering 69,404 and 70,598 were analyzed when comparing the CDI 500 to the Quantum for hemoglobin and SvO2, respectively. Comparison of hemoglobin and SvO2 for the CDI 500 and Quantum versus ABL90 used 2283 and 1414 data points respectively, in each group. The CDI 500 and Quantum reported hemoglobin within 1 g/dL of the ABL90 86.9% and 87.5% of the time, respectively. The CDI 500 and Quantum reported SvO2 within 3% of the ABL90 61.0% and 57.9% of the time, respectively. The mean difference between the CDI 500 and Quantum hemoglobin and SvO2 measurements equaled .194 g/dL (p < .001) and .861% (p < .001), respectively and were both significantly different from zero. All device comparisons were statistically significantly different when compared to zero difference, likely due to the large data set as the magnitudes of these differences are all quite small and may not be clinically significant. However, while the reader should judge for themselves based upon their specific practice, in our opinion, the 95% Limit of Agreement was too large for either the CDI 500 or Quantum hemoglobin and SvO2 values to be substituted for ABL90 values. As recommended by the manufacturers, the CDI 500 and Quantum should only be used as a trending device.
Keywords: cardiopulmonary bypass, blood monitoring, inline monitoring, online monitoring, Terumo CDI 500, spectrum medical quantum
In 2015, we compared the Terumo CDI 500 and the Spectrum Medical M4 to each other and both devices to the ABL90 FLEX benchtop blood analyzer (1). At that time, the Spectrum technology was just emerging on the U.S. market providing an alternative to the longstanding CDI 500. The benchtop blood analyzer is the gold standard for blood oxygen saturation (SO2) and hemoglobin analysis. However, the benchtop analyzer only provides values at a given point in time. The 2019 American Society of Extracorporeal Technology’s Standards and Guidelines for Pediatric Perfusion recommend continuous monitoring of blood gases in standard 7.7 (2), and in the field of congenital cardiopulmonary bypass (CPB), the continuous measurement of SO2 and hemoglobin values has been commonplace for many years. The CDI 500 uses minimally invasive technology in the form of inline cuvettes to measure venous saturation (SvO2) and hemoglobin levels during CPB and has been found to be an effective monitoring tool (3–6). While this system is considered a trending device, it plays an important role in CPB management and improves the maintenance of appropriate blood gas parameters (7).
Recently, the Spectrum Medical M4 has been surpassed by the Quantum system providing measurement of some of the same values as the CDI 500 utilizing noninvasive, reusable technology. In the same manner as the M4 system, the Quantum device allows the clinician to monitor arterial and venous SO2, and hemoglobin with reusable sensors that clamp on to the outside of the CPB circuit tubing.
After our institution upgraded from the M4 to the Quantum platform, we repeated our previous study and conducted an analysis examining the accuracy of the Quantum technology and the CDI 500 as they compare to each other and the ABL90 FLEX benchtop blood gas analyzer.
METHODS
Patients were retrospectively identified by searching the Department of Cardiovascular Perfusion’s (DCP) VISION database for all patients undergoing cardiac surgery requiring CPB between August 1, 2018 and November 1, 2019, who were monitored using the Terumo CDI 500 (Terumo Cardiovascular, Ann Arbor, MI) and Spectrum Medical Quantum (Spectrum Medical, Fort Mill, NC) systems. Procedures from all Society of Thoracic Surgeons STAT categories were represented in the data set. Any patients missing any SvO2 or hemoglobin data necessary for analysis were excluded. The institutional review board approved this study.
Equipment
The ABL90 FLEX (Radiometer, Brea, CA) was used as the benchtop blood analyzer. Our standard of care utilizes the CDI 500 incorporated into all CPB circuits in the same location via the venous line. Also in the normal routine, the Spectrum Medical Quantum SvO2 and hemoglobin analyzers were incorporated into the CPB circuit and simultaneous data was collected from both the CDI 500 and Quantum devices. Manufacturer’s instructions for use were followed for both devices. Gas calibration was completed for the CDI 500 before use as well as in vivo calibration with each ABL90 result (8). The Quantum saturation and hemoglobin probes were factory reset to default values before each case and calibrated with each ABL90 sample at the same time as the CDI 500. Only values after the first in vivo calibration were included for comparison. The operational ranges of the Quantum hemoglobin and SvO2 are 5–16.6 g/dL and 25–100% respectively (9). Operational ranges of the CDI 500 for the hemoglobin and SvO2 are 5.6–12.6 g/dL and 60–100%, respectively (10). Only values resulted by the ABL90 that fell within the operational range of the devices were analyzed.
Data Collection
SvO2 and hemoglobin values generated by the Quantum, CDI 500, and ABL90 analyzers were collected for this study. Data generated by the Quantum and CDI 500 were measured via online and inline sensors and electronically captured as a matter of routine during all CPB cases. Data generated by the Quantum was collected every second. Data produced by the CDI 500 was collected every 6 seconds, the limit of the device. This data was electronically transmitted to the DCP database at the conclusion of each case. During data extraction, 60 measurements from the Quantum and 10 from the CDI 500 were averaged over 1 minute to provide a single value for the minute.
Blood analysis on the ABL90 benchtop device was performed at a minimum of every hour during CPB as a matter of routine. A blood sample size of approximately .5 mL was drawn from the CPB circuit every hour or more frequently if clinically required. This sample was immediately analyzed using the ABL90 blood analyzer and results were electronically transmitted to the DCP’s database at the conclusion of each case. Values provided by the ABL90, which may have been inaccurate because of analyzer error as determined by the analyzer or inaccurate because of sample acquisition error as determined by the clinician were discarded and not reported as a matter of routine. SvO2 and hemoglobin values from the ABL90 were electronically timestamped for comparison to the corresponding values from the Quantum and CDI 500 devices.
Statistical Analysis
Measurements are summarized as mean, standard deviation, median, minimum, and maximum by devices (Quantum, CDI 500, and ABL90). The specific parameters compared between the devices were SvO2 and hemoglobin. The parameters were compared with Bland–Altman analysis. Since each subject had repeated measures, the study had both between-subject and within-subject variations. Limit of agreements (95% LoA) for this type of clustered data was proposed for two conditions: one is when the quantity of the measure within a series of observations for each subject does not vary much (i.e., the true value is constant), and the other is when the quantity of the measure varies (i.e., the true value varies) (11). The later approach is chosen to calculate 95% LoA, since patients’ SvO2 and hemoglobin values vary during CPB. Values for the CDI 500 and Quantum SvO2 were categorized as a percentage of values that fall within an absolute value of ±3%, ±3.1–5%, and ±>5% from the ABL90. Hemoglobin values from the CDI 500 and Quantum were categorized as a percentage of values that fall within an absolute value of ±1 g/dL, ±1.1–2 g/dL, and >2 g/dL.
RESULTS
There were 519 patients included in the analysis. Data points numbering 69,404 and 70,598 were analyzed when comparing the CDI 500 to the Quantum for hemoglobin and SvO2, respectively. Comparison of hemoglobin for the CDI 500 and Quantum vs ABL90 used 2283 data points in each group. When analyzing the SvO2 data for the CDI 500 and Quantum versus ABL90, 1,414 data points in each group were compared.
Hemoglobin Measurement
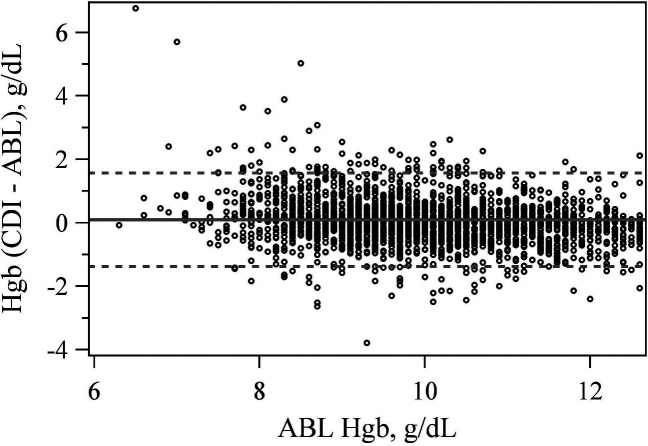
The CDI 500 reported hemoglobin within 1 g/dL of the ABL90 86.9% of the time (Table 1). The mean difference between the CDI 500 and ABL90 hemoglobin measurement was significantly different from zero at +.096 g/dL (p < .001) with LoA of −1.374 and 1.566 g/dL (Figure 1).
Table 1.
Comparison of CDI 500, quantum, and ABL 90 SvO2 and hemoglobin absolute values.
| CDI-ABL Hgb | N = 2,283 |
| <1 g/dL | 1,984 (86.9%) |
| 1.1–2 g/dL | 259 (11.3%) |
| >2 g/dL | 40 (1.8%) |
| Quantum-ABL Hgb | N = 2,283 |
| <1 g/dL | 1,998 (87.5%) |
| 1.1–2 g/dL | 232 (10.2%) |
| >2 g/dL | 53 (2.3%) |
| CDI-ABL SvO2 | N = 1,414 |
| ≤3% | 862 (61.0%) |
| 3.1–5% | 213 (15.1%) |
| 5.1–10% | 229 (16.2%) |
| >10.1 | 110 (7.8%) |
| Quantum-ABL SvO2 | N = 1,414 |
| ≤3% | 819 (57.9%) |
| 3.1–5% | 202 (14.3%) |
| 5.1–10% | 254 (18.0%) |
| >10.1 | 139 (9.8%) |
SvO2, venous saturation. N = number of data points in the group. % = percentage of total data points that fell within the group.
Figure 1.
Bland–Altman plot comparing hemoglobin values between the CDI 500 and ABL 90.
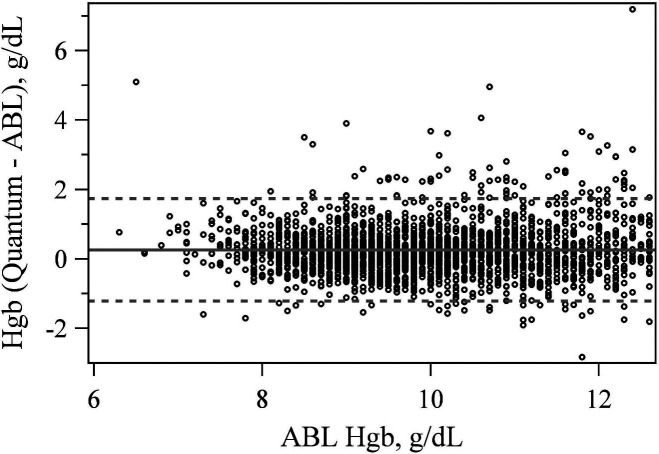
The Quantum reported hemoglobin within 1 g/dL of the ABL90 87.5% of the time (Table 1). The mean difference between the Quantum and ABL90 hemoglobin measurement was significantly different from zero at +.261 g/dL (p < .001) with LoA of −1.218 and 1.740 g/dL (Figure 2).
Figure 2.
Bland–Altman plot comparing hemoglobin values between the quantum and ABL 90.
SvO2 Measurement
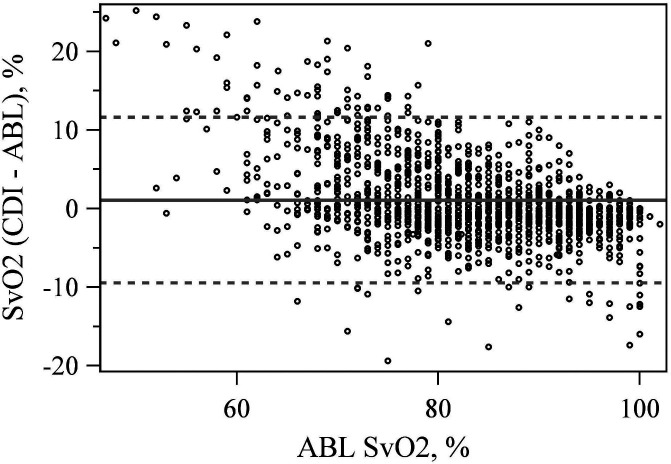
The CDI 500 reported SvO2 within 3% of the ABL90 61.0% of the time and within 5% of the ABL90 76.1% of the time (Table 1). The mean difference between the CDI 500 and ABL90 SvO2 measurement was significant 1.067% (p < .001) with LoA at −9.466% and 11.605% (Figure 3).
Figure 3.
Bland–Altman plot comparing venous saturation (SvO2) values between the CDI 500 and ABL 90.
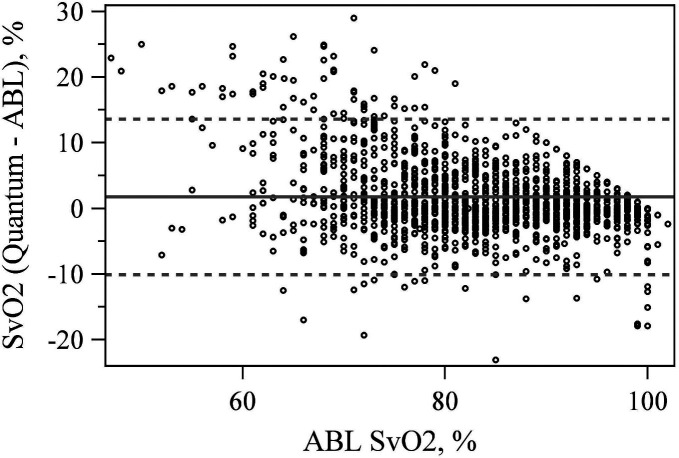
The Quantum reported SvO2 within 3% of the ABL90 57.9% of the time and within 5% of the ABL90 72.2% of the time (Table 1). The mean difference between the Quantum and ABL90 SvO2 measurement was significant at 1.761% (p < .001) with LoA equal to −10.086% and 13.609% (Figure 4).
Figure 4.
Bland–Altman plot comparing venous saturation (SvO2) values between the quantum and ABL 90.
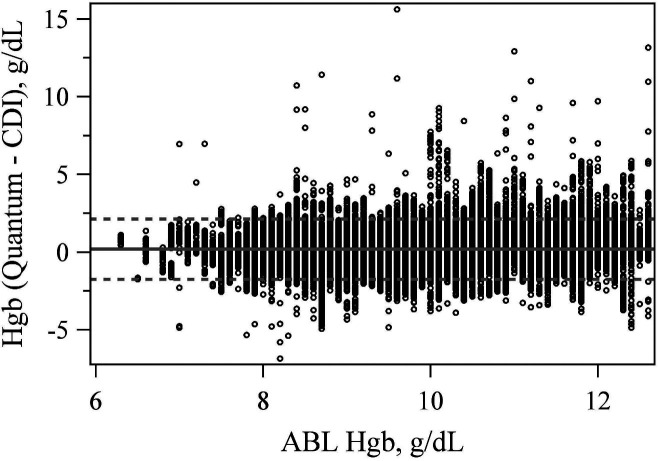
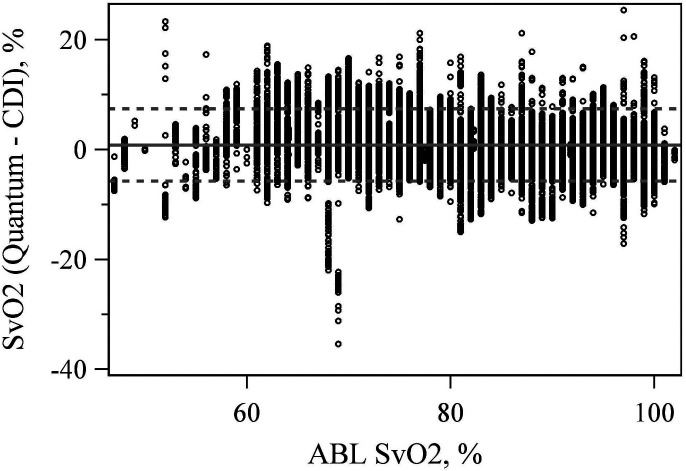
CDI 500 Compared to Quantum
The mean difference between the CDI 500 and Quantum hemoglobin measurement equaled .194 (p < .001). The LoA were −1.744 and 2.132 g/dL (Figure 5). The mean difference between the CDI 500 and Quantum SvO2 measurement was also significantly different from zero at 0.861 (p < .001) with LoA of −5.719% and 7.441% (Figure 6).
Figure 5.
Bland–Altman plot comparing hemoglobin values between the CDI 500 and quantum at the true ABL90 value.
Figure 6.
Bland–Altman plot comparing venous saturation (SvO2) values between the CDI 500 and quantum at the true ABL90 value.
DISCUSSION
All device comparisons were statistically significantly different when compared to zero difference, likely because of the large data set as the magnitudes of these differences are all quite small and may not be clinically significant. Although the reader should judge for themselves based upon their specific practice, in our opinion, the 95% LoA was too large for either the CDI 500 or Quantum hemoglobin or SvO2 values to be substituted for ABL90 values. As recommended by the manufacturers, the CDI 500 and Quantum should only be used as a trending device.
The CDI 500 utilizes single-use inline cuvettes and the Quantum incorporates clamp on or online technology reusable from patient to patient. One benefit of the Quantum technology is the elimination of the disposable cuvette resulting in a cost reduction. Additionally, the quantum device does not require gas calibration before use as is recommended for the CDI 500 representing both a preparation time and cost savings.
Following our original study, a few changes were made to our clinical practice in an attempt to improve the accuracy of these devices. Specifically, regarding the Spectrum Medical online technology measuring hemoglobin, we observed the rate of and location at which fluids are administered during CPB seemed to have a significant effect upon the M4 readings. We hypothesized this was due to the M4’s sensitivity, rate of sampling, and location of the sensor on the circuit in relation to fluid administration. The sensor is placed just distal to the oxygenator outlet on our circuit. When fluids administered to the manifold, which empties directly into the venous line, passed the sensor, the fluctuation in hemoglobin concentration caused a significant fluctuation in the sensors reading. The sensor’s response was likely appropriate at the time. However, the reading did not appear to settle out as quickly as it changed with volume administration. Adding volume directly to the venous reservoir cardiotomy seems to reduce this effect, theoretically, by allowing better mixing of the fluids before crossing the sensor. This change to our practice continued through the time period of this study and may have had an impact on the improved results related to the Quantum platform as related to the hemoglobin measurements.
At the time of our previous study, the manufacturer’s recommendation with regard to calibrating the M4’s hemoglobin sensor at the beginning of the case was that recalibration was not suggested as the case progresses. Since our previous study, Spectrum Medical has modified the Quantum’s instructions for use to allow for periodic calibration of the sensors. We have observed calibrating with each benchtop analyzer result appears to bring the Quantum’s hemoglobin reading more closely in line with the benchtop analyzer. Additionally, at times as a result of the software design in place at the time of our previous study, the M4 would not allow the capture of the current values and the clinician was required to wait for the device to allow the values to be captured. Since the completion of that study and also with the Quantum hardware, Spectrum Medical has updated the software and the value capture function has been significantly improved resulting in no capture delays. These improvements in the technology also likely impacted the improved performance.
Although this study did not directly examine the influence of individual parameters such as hemoglobin, SvO2, and temperature upon the device’s readings, each of these parameters were within the device’s operational limits. One limitation to this study was the comparison of venous hemoglobin measurements from the CDI 500 to arterial hemoglobin measurements from the Quantum. However, the difference in these measurements did not appear to significantly impact the findings as the magnitude of difference in these measurements between the two devices, while significantly different from zero (p < .001), was quite small at .194 g/dL.
The Quantum and CDI 500 are not expected to be as accurate as the benchtop analyzer, yet they provide an important role in patient care. In this patient population and the specific values examined, the CDI 500 fared slightly better in comparison to the ABL90 than the Quantum regarding the SvO2 measurement while the Quantum edged out the CDI 500 in relation to the hemoglobin. However, the differences between the two trending devices as they compared to the ABL90 were small and the devices could be used interchangeably to trend hemoglobin and SvO2. In addition to the measurements examined here, these devices each have benefits and capabilities outside the scope of this paper and each application should be considered when choosing a platform.
REFERENCES
- 1.Reagor JA, Gao Z, Lombardi JP, et al. . Accuracy of the spectrum medical M4 and terumo CDI 500 compared to the radiometer ABL90 FLEX benchtop blood analyzer. Perfusion. 2017;32:523–8. [DOI] [PubMed] [Google Scholar]
- 2.AmSECT . Standards and Guidelines for Pediatric and Congenital Perfusion Practice. 2019. Available at: https://www.amsect.org/p/cm/ld/fid=1697. [DOI] [PubMed] [Google Scholar]
- 3.Southworth R, Sutton R, Fau - Mize S, et al. . Clinical evaluation of a new in-line continuous blood gas monitor. J Extra Corpor Technol. 1998;32:166–70. [PubMed] [Google Scholar]
- 4.Schreur A, Niles S, Fau - Ploessl J, et al. . Use of the CDI blood parameter monitoring system 500 for continuous blood gas measurement during extracorporeal membrane oxygenation simulation. J Extra Corpor Technol. 2005;37:377–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Gruber M, Breu A, Frauendorf M, et al. . Washing of banked blood by three different blood salvage devices. Transfusion. 2013;53:1001–9. [DOI] [PubMed] [Google Scholar]
- 6.Bellaiche AL, Nielsen PF, Fau - Brantlov S, et al. . Clinical evaluation of the accuracy and precision of the CDI 500 in-line blood gas monitor with and without gas calibration. J Extra Corpor Technol. 2011;43:53–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Ottens J.Tuble SC, Fau - Sanderson AJ, et al. . Improving cardiopulmonary bypass: does continuous blood gas monitoring have a role to play? J Extra Corpor Technol. 2007;39:313. [PMC free article] [PubMed] [Google Scholar]
- 8.Manual O. CDI Blood Parameter Monitoring System 500, 236603J ed. Ann Arbor, MI: Terumo Cardiovascular System Corporation; 2015. [Google Scholar]
- 9.Quantum Workstation (QWS) User Manual, SUM-32000001_v10b ed. Fort Mill, SC: Spectrum Medical; 2019. [Google Scholar]
- 10.CDI 500 Operators Manual, Supplement, 855401 R/B ed. Ann Arbor, MI: Terumo Cardiovascular System Corporation; 2014. [Google Scholar]
- 11.Bland JM, Altman DG.. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82. [DOI] [PubMed] [Google Scholar]