Abstract:
Cell saver blood reinfusion, a blood conservation technique recently available for pediatric use, is typically limited to 6 hours post processing to guard against bacterial contamination. We hypothesize that reinfusion of cell saver blood up to 24 hours post collection in children after cardiac surgery will not increase the incidence of hospital-acquired infections (HAI). The primary aim is to compare incidence of HAI between children receiving cell saver blood ≤6 hours vs. >6 to ≤24 hours from its collection. The secondary aim is to compare mortality and clinical outcomes. Retrospective chart review of children ≤18 years undergoing cardiac surgery with cardiopulmonary bypass (CPB) from 2013 to 2018 when cell saver collection and bedside temperature controlled storage became standard of care. Patients on extracorporeal membrane oxygenation (ECMO) within 48 hours postoperatively and those who did not receive cell saver were excluded. The primary outcome was HAI incidence postoperative days 0–6. Demographic data included diagnosis, surgical severity score, and clinical outcomes. 466 patients, 45% female. No significant between-group differences identified. There was no significant difference in HAI (control 8.5% vs. treatment 8.0%, p = .80) and death (control 7.9% vs. treatment 4.9%, p = .20). Noninferiority testing indicated the treatment group was not statistically inferior to the control group (p = .0028). Kaplan–Meier curve depicted similar status between-group rates of no infection or death; 92% treatment vs. 91% control. Total volume allogeneic red blood cell transfusion (allogeneic blood transfusion [ABT]) up to 24 hours postoperatively was significantly less in the treatment group, p < .0001. Incidence of HAI or mortality was not increased in patients receiving cell saver blood reinfusion >6 to ≤24 hours post collection. Treatment subjects received significantly less volume of ABT. Considering the risks of ABT, these findings support cell saver blood reinfusion up to 24 hours post collection.
Keywords: cell saver, blood conservation, transfusion, hospital-acquired infection
Despite significant advancements in the safety of screening, storage, and processing of blood products that have developed with advancements in technology and medicine, blood transfusion remains associated with significant risk (1–4). Additionally, the incidence of transfusion-transmitted infection (TTI) and transfusion-related immune modulation (TRIM) increases with increased donor exposure (5). Allogeneic blood transfusions (ABT) are associated with increased mortality and prolonged duration of mechanical ventilation, particularly in critically ill children and children undergoing cardiac surgery (6).
Although benefits of blood conservation strategies are increasingly recognized, blood transfusions remain common following cardiac surgical procedures, particularly in infants and children (2). Rates of hospital-acquired infections (HAI) post cardiac surgery ranges from 13% to 50% worldwide (7–11), with increasing number of transfusions associated with increased risk of infection (9,10,12).
Utilization of a cell saver device as a safe and effective blood conservation technique was, until recently, primarily used in adults (13). The device removes heparin, cell debris, and inflammatory byproducts, as well as plasma, clotting factors, and platelets that are normally present in whole blood from cardiopulmonary bypass (CPB) blood salvage. As seen specifically with the Fresenius Continuous Autotransfusion System™ (Terumo Medical Corporation, Somerset, NJ), the device concentrates the cell saver blood product to a hematocrit of 56–72% (14). This concentrated and washed blood can be reinfused to the patient potentially avoiding additional donor exposure. Limitations from small volume salvaged blood collection initially prevented its use, but newer devices allow for pediatric cell saver blood processing and reinfusion (15,16).
Administration of cell saver blood in infants limited to the first 6 hours post cardiac surgery has proven to be safe and effective in reducing postoperative ABT (17). Hishon et al. demonstrated that cell saver blood stored at room temperature for 18 hours showed minimal chemical deterioration and limited microbiologic contamination, leaving authors to conclude that advanced processing techniques allow cell saver blood to be safe for reinfusion up to 24 hours post collection when maintained in a bedside cooler and accessed using aseptic techniques (18).
Concern regarding findings of bacteremia in the red blood cells (RBCs) of cell saver blood in adults undergoing cardiac surgery has raised concerns regarding safety of its infusion (19). Bacterial culture growth demonstrated in 60–85% of salvaged blood, attributed to airborne bacteria in the environment, has not been directly correlated with postoperative infections (20). Despite evidence of bacterial contaminants and endotoxin presence documented in cell saver blood, no clinical impact has been found (including infection or postoperative bleeding), though determination of the timing of contamination was not elucidated (during surgery vs. upon blood draw) (21). Additionally, despite the unknown effects on antibiotic disposition with processing cell saver blood and the risk of contamination with gram-positive bacteria, the increased use of cell saver blood postoperatively has not demonstrated an increased infection rate (21,22).
In 2013, the first clinical trial reinfusing cell saver blood in pediatric cardiac surgery up to 24 hours post collection was performed at our institution by Cholette et al. Although not powered to evaluate clinical outcomes, it demonstrated a statistically significant reduction in ABT in the first 48 hours postoperatively in the cell saver blood group (23). These results led the University of Rochester Medical Center (URMC) Congenital Cardiac Surgical Program, in partnership with the URMC blood bank, to adopt cell saver blood collection and reinfusion up to 24 hours following its collection as standard of care.
In 2016, the American Association of Blood Banks (AABB) published updated standards regarding autologous blood collection and use, specifying that cell saver blood could be safely reinfused for up to 24 hours (24). Purpose of the current study was to confirm the safety of our cell saver protocol (reinfusing cell saver for up to 24 hours post its collection) specifically in pediatric cardiac surgery, focusing on the incidence of HAIs. Supported by the new AABB standards, we hypothesized that reinfusion of cell saver blood for up to 24 hours post its collection in children undergoing cardiac surgery would not increase the incidence of HAI. Primary aim of the current study is to examine clinical outcomes in children receiving cell saver blood for up to 24 hours from time of collection, compared to those receiving it in the first 6 hours following collection.
MATERIALS AND METHODS
This is a retrospective chart review of children ≤18 years who underwent cardiac surgery with CPB at the URMC from 2013 to 2018. Local URMC Research Subject Review Board approval for this study was obtained. Collection of cell saver blood maintained at the patient’s bedside in a temperature-controlled cooler for reinfusion up to 24 hours post collection was standard of care. Patients requiring extracorporeal membrane oxygenation (ECMO) within the first 48 hours postoperatively and those who did not receive any cell saver blood were excluded. Data from 466 patients were reviewed. The primary aim was to compare the incidence of HAI between children receiving cell saver blood ≤6 hours (control) vs. >6 to ≤24 hours (treatment) from time of collection. The secondary aim was to compare mortality and clinical outcomes between groups, including number and type of HAI, number and volume of blood product transfusions, volume of crystalloid infusion, duration of hospital stay, mechanical ventilation, central venous catheter access, indwelling urinary catheters, inotropic support, incidence of arterial or venous thrombosis, lowest hemoglobin levels, and incidence and duration of delayed sternal closure. Demographic data included age, gender, weight, race/ethnicity, chromosomal abnormalities, comorbidities, cardiac diagnosis, and surgical severity score (Society of Thoracic Surgeons--European Association for Cardio-Thoracic Surgery [STS-EACTS] score). Comorbidities included prematurity, nutritional deficiencies, arrhythmias, pulmonary hypertension, chronic lung disease, bronchotracheomalacia, airway obstruction (obstructive sleep apnea, supra or subglottic stenosis), kidney disease, gastrointestinal complications (necrotizing enterocolitis, tracheoesophageal fistula, Hirschsprung disease, biliary atresia), neurologic abnormalities (seizures, cerebral palsy, hypogenesis or dysplasia of the brain, hydrocephalus, Dandy–Walker malformation, spinal agenesis), cleft palate, limb abnormalities, vertebral anomalies, asplenia/polysplenia, and endocrine abnormalities (hypothyroidism, adrenal insufficiency).
Since 2003 our program’s goal was to collect CPB cell salvage and process into cell saver blood on all children presenting for cardiac surgery with CPB. The volume collected was dependent on patient weight/body surface area, initial hemoglobin, type of surgical procedure, CPB priming volume, and residual end-CPB volume. The Fresenius Continuous Autotransfusion System® (CATS) (Terumo Medical Corporation, Somerset, NJ) was used exclusively for all cases. The cell saver program used for all cases was the Quality Wash Setting. The same local URMC intraoperative cell saver protocol was used for all cases, which includes after completion of CPB, remaining pump salvage is infused into the Fresenius CATS cell saver device, with the resulting processed and washed volume collected. The moment the cell saver process is completed, the collection bag is disconnected, a green sticker labeling it as “autologous blood” is applied, and it is immediately placed in a temperature-controlled and temperature-monitored “CATS” cooler. This cooler is kept with the patient and maintained between 1°C and 6°C, in keeping with New York State blood bank regulation 58–2.25 and transferred to the intensive care unit with the patient, and maintained at the bedside according to URMC blood bank protocol with documented quality assurance checks every 4 hours (25). The cooler includes a continuous thermometer and alarm system that alarms if the internal temperature exceeds 6°C, indicating need to change the coolant.
Aliquots of cell saver blood are drawn off from the collection bag aseptically using a needleless adapter used for RBC transfusion. Cell saver blood was reinfused per local cardiac intensive care unit (CICU) blood transfusion guidelines to maintain hemodynamics and oxygenation if the patient’s hemoglobin was less than 13 g/dL in aliquot volumes at the discretion of the CICU attending, or in place of allogeneic red cell transfusion as clinically indicated in volumes of 10 mL/kg. The CPB antibiotic regimen was not changed throughout the duration of the study and included 25 mg/kg (up to 1 g) of cephazolin given in the bypass prime in all patients. The postoperative antibiotic regimen and ICU infection surveillance regimen were also not changed throughout the study.
Data collected from each subject’s medical record included cardiac diagnosis, type of surgery, STS-EACTS score, reoperation, duration of hospital stay, and total volume of cell saver blood collected. From the first postoperative 24 hours, total volumes of 1) cell saver blood, 2) crystalloid, 3) 5% albumin, 4) allogeneic RBC, 5) platelets, 6) cryoprecipitate, and 7) fresh frozen plasma administered were collected. The lowest hemoglobin levels on postoperative days (PODs) 0–2; baseline and peak creatinine levels in the first 48 hours postoperatively; durations of inhaled nitric oxide (iNO), delayed sternal closure, invasive mechanical ventilation, inotropic support, ECMO (if started after 48 hours postoperatively), central venous catheter access, and indwelling urinary catheter use were measured. The incidence of HAI as defined by Centers for Disease Control and Prevention (CDC) criteria (26), thrombosis, and death were collected.
Statistical Analysis
All statistical analyses were conducted using Version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC). Descriptive statistics such as mean, standard deviation, frequency, and percentage were used to summarize the patient characteristics. Using two sample t tests or chi square/Fisher’s exact test where appropriate, we determined whether there were important baseline differences between the study groups with respect to participant characteristics. The primary analyses were adjusted for these differences. If distributional assumptions associated with a particular statistical procedure were violated, appropriate transformations were made or nonparametric alternatives were used (e.g., Wilcoxon rank sum tests in place of two-sample t tests).
The infection rate was calculated and compared between study groups using the Pearson chi square test. A noninferiority test of two proportions was performed to evaluate whether the treatment group is inferior to the control group in rate of infection/death, with a noninferiority margin of 5%. Additionally, status as a composite of both infection and death was analyzed. Kaplan–Meier curves were used to display the incidence of infections by study group. Except for the noninferiority test, all tests were two-sided. The p values less than .05 were considered statistically significant.
RESULTS
About 504 children <18 years underwent cardiac surgery with CPB from 2013 to 2018. Of these, 20 children were excluded since they did not receive any cell saver blood. An additional 18 were excluded due to requiring ECMO within 48 hours of surgery. A total of 466 subjects were included in the analysis, 45% of whom were female (Table 1). There were 139 subjects in the control group and 327 subjects in the treatment group (Table 1). Treatment group subjects were significantly younger and smaller than control group subjects (Table 1); however, cardiac diagnosis and surgical severity scores were similar, and no significant between-group differences were found (Table 2).
Table 1.
Subject primary characteristics of both the control and treatment group.
| Characteristics | Subcategory | Control Group ≤6 Hours | Treatment Group >6 to ≤24 Hours | p Value |
|---|---|---|---|---|
| Total patients | – | 139 | 327 | – |
| Age (months) | – | 27.8 ± 55.8 | 13.6 ± 35.8 | .0014* |
| Gender (%) | Male | 63 | 51 | .0261* |
| Weight (kg) | – | 9.9 ± 12.1 | 7.8 ± 12.3 | <.0001* |
| Ethnicity (%) | Hispanic | 3 | 4 | .4719 |
| Non-Hispanic | 97 | 96 | – |
Mean weight, age, gender, and ethnicity noted with standard deviations, as well as p values.
p value indicates statistical significance.
Table 2.
Subject secondary characteristics of both the control and treatment group.
| Characteristics | Subcategory | Control Group ≤6 Hours | Treatment Group >6 to ≤24 Hours |
|---|---|---|---|
| Total patients | – | 139 | 327 |
| Chromosomal abnormality (%) | Trisomy 21 | 16 | 9 |
| DiGeorge syndrome | 1 | 5 | |
| Other | 5 | 4 | |
| Comorbidities (%) | Yes | 25 | 29 |
| Diagnosis (%) | TAPVR | 5 | 4 |
| Tricuspid atresia | 0 | <1 | |
| Pulmonary atresia/stenosis | 1 | 2 | |
| Truncus arteriosus | 1 | 2 | |
| ASD/VSD/PDA | 13 | 14 | |
| TOF | 20 | 18 | |
| DTGA ± VSD | 7 | 11 | |
| Coarctation ± VSD | 1 | 1 | |
| Hypoplastic/IAA | 1 | 5 | |
| HLHS | 9 | 6 | |
| AVSD | 12 | 7 | |
| DORV ± VSD | 2 | 2 | |
| LVOTO | 2 | 2 | |
| AVSD/TOF | 0 | <1 | |
| Other/mixed lesions | 26 | 25 | |
| STS-EACTS score (%) | 1–3 | 68 | 64 |
| 4–5 | 32 | 36 |
ASD, atrial septal defect; AVSD, atrioventricular septal defect; DORV, double outlet right ventricle; DTGA, D-transposition of the great arteries; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; LVOTO, left ventricular outflow tract obstruction; PDA, patent ductus arteriosus; TAPVR, total anomalous pulmonary venous return; TOF, Tetralogy of Fallot; VSD, ventricular septal defect.
In the first 24 hours postoperatively, most patients received a combination of cell saver blood, allogeneic blood products, crystalloid, and 5% albumin. Cell saver blood was the most prevalent form of volume replacement, with as expected greater mean volumes reinfused in the treatment group (101.7 mL ± 97.6 [control] and 115.2 mL ± 79.4 [treatment], p = .0003) (Table 3). However, with regard to infection there was no significant difference in the volume of cell saver blood reinfused (subjects with HAI 111.6 mL ± 87.5 and subjects without HAI 107.95 mL ± 54.9, p = .3575) (Table 4). Additionally, the volume of cell saver blood given in 24 hours was similar between subjects with HAI and death or status (110.1 mL ± 82.4) and those without (123.0 mL ± 112.1), p = .2428 (Table 4). Mean volume of crystalloid was similar between groups (59.8 mL ± 193.4 [control] and 49.8 ± 170.2 [treatment], p = .8828) (Table 3). Unsurprisingly allogeneic RBCs were transfused significantly less in treatment group compared to the control group subjects (p < .0001) (Table 3). Similarly, 5% albumin and cryoprecipitate were given in significantly smaller volumes in treatment group subjects (p = .0071 and .0477, respectively) (Table 3). Though these were significant findings in 24 hours, they did not prove to be statistically significant in a 7-day period (Table 5). Nadir hemoglobin levels were similar on POD 0 and 1, but were significantly higher on POD 2 in the treatment group (p < .0001) (Table 3).
Table 3.
Mean volume of each product received with standard deviations.
| Characteristics | Subcategory | Control Group ≤6 Hours | Treatment Group >6 to ≤24 Hours | p Value |
|---|---|---|---|---|
| Total volume in first 24 hours (mL) | Crystalloid | 59.8 ± 193.4 | 49.8 ± 170.2 | .8828 |
| Albumin | 35.5 + 111.2 | 10.4 ± 31.3 | .0071* | |
| Cell saver | 101.7 ± 97.6 | 115.2 ± 79.4 | .0003* | |
| PRBCs | 12.7 ± 60.3 | .4 ± 3.7 | <.0001* | |
| Platelets | 3.4 ± 21.5 | .6 ± 4.7 | .24 | |
| Cryoprecipitate | 1.1 ± 7.3 | .1 ± 1.9 | .0477* | |
| FFP | 2.71 ± 7.9 | 1.1 ± 8.3 | .6191 | |
| Hemoglobin (g/dL) | PODO | 11.7 ± 1.6 | 11.7 ± 1.4 | .7557 |
| POD 1 | 11.9 ± 1.9 | 11.6 ± 1.7 | .0766 | |
| POD 2 | 10.7 ± 1.8 | 11.8 ± 1.6 | <.0001* |
Nadir mean hemoglobin levels POD 0–2. FFP, fresh frozen plasma; POD, postoperative day; PRBCs, packed red blood cells.
p value indicates statistical significance.
Table 4.
Mean volume of cell saver blood received in a 24-hour period in subjects with HAI and subjects without HAI, and in subjects with HAI plus death (status) and those without.
| Category | Subcategory | Volume of Cell Saver in 24 Hours (mL) | p Value |
|---|---|---|---|
| Infection | HAI | 111.6 ± 87.5 | .3575 |
| No HAI | 107.95 ± 54.9 | ||
| Status | Status | 110.1 ± 82.4 | .2428 |
| No status | 123.0 ± 112.1 |
HAI, hospital-acquired infection.
Table 5.
Comparison of continuous variables and clinical outcomes between both the control and treatment group with means and standard deviations.
| Category | Control Group ≤6 Hours | Treatment Group >6 to ≤24 Hours | p Value |
|---|---|---|---|
| POD (days) | 3.2 ± 1.8 | 4.1 ± 2.2 | .1656 |
| Hospital LOS (days) | 18.5 ± 28.3 | 19.6 ± 25.8 | .0426* |
| OR PRBCs (mL) | 28.9 ± 72.5 | 38.2 ± 74.4 | .0708 |
| RBC transfusions in 7 days (#) | .4 ± 1.4 | .3 ± .8 | .7804 |
| PLT transfusions in 7 days (#) | .1 ± .7 | .1 ± .3 | .4815 |
| Cryo transfusions in 7 days (#) | 0 ± .3 | 0 ± .3 | .4818 |
| FFP transfusions in 7 days (#) | 0 ± .5 | 0 ± .2 | .7758 |
| Creatinine change (%) | .3 ± .5 | .8 ± 5.6 | .5709 |
| iNO duration (days) | 5.6 ± 5.9 | 6.7 ± 5.1 | .1295 |
| Mechanical ventilation duration (days) | 4.8 ± 9.7 | 5.1 ± 8.1 | .0654 |
| Delayed chest closure duration (days) | 7.2 ± 11.2 | 5 ± 9.1 | .3913 |
| Inotrope duration (days) | 4.3 ± 7.2 | 4.8 ± 7.4 | .1189 |
| ECMO duration (days) | 15 ± 16.8 | 7.2 ± 4.7 | .587 |
| CVC duration (days) | 9.2 ± 16.1 | 12.3 ± 20.2 | .0041* |
| Urinary catheter duration (days) | 1.7 ± 1.7 | 1.9 ± 2.1 | .456 |
CVC, central venous catheter; cryo, cryoprecipitate; ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; iNO, inhaled nitric oxide; LOS, length of stay; OR, operating room; PRBCs, packed red blood cells; PLT, platelet; POD, postoperative day; RBC, red blood cell.
p value indicates statistical significance.
The absolute number of infections was increased in the treatment group; however, the number of subjects was also more than double (n = 327 vs. n = 139) and the difference was not statistically significant (Table 6). Hospital length of stay was significantly longer in the treatment group (18.5 days ± 28.3 [control] and 19.9 days ± 25.8 [treatment], p = .0426), as was mean central venous catheter duration (9.2 days ± 16.1 [control] and 12.3 days ± 20.2 [treatment], p = .0041) (Table 5). Despite these findings, statistical analysis revealed no statistically significant difference in HAI (p = .80) and mortality (p = .20) between groups (Table 6).
Table 6.
Total number of patients with an infection in the first 7 days postoperatively, total number of deaths, and status (composite of both infection and death) in each group.
| Characteristics | Control Group ≤6 Hours | Treatment Group >6 to ≤24 Hours | p Value |
|---|---|---|---|
| Infection | 12 (8.63%) | 26 (7.95%) | .8056 |
| Death | 11 (7.91%) | 16 (4.89%) | .2016 |
| Status | 15 (10.79%) | 27 (8.26%) | .3821 |
Higher creatinine levels were seen in the treatment group, which also received larger volumes of RBCs intraoperatively, but neither difference was statistically significant (Table 5). Despite the larger volume of RBCs transfused intraoperatively, the initial hemoglobin levels immediately postoperation were not significantly different between groups (Table 3). The incidence of diagnosed chromosomal abnormalities, duration of iNO, ECMO, indwelling urinary catheters, delayed sternal closure, invasive mechanical ventilation, and the number of blood product transfusions given on POD 0–7 were not significantly different between groups.
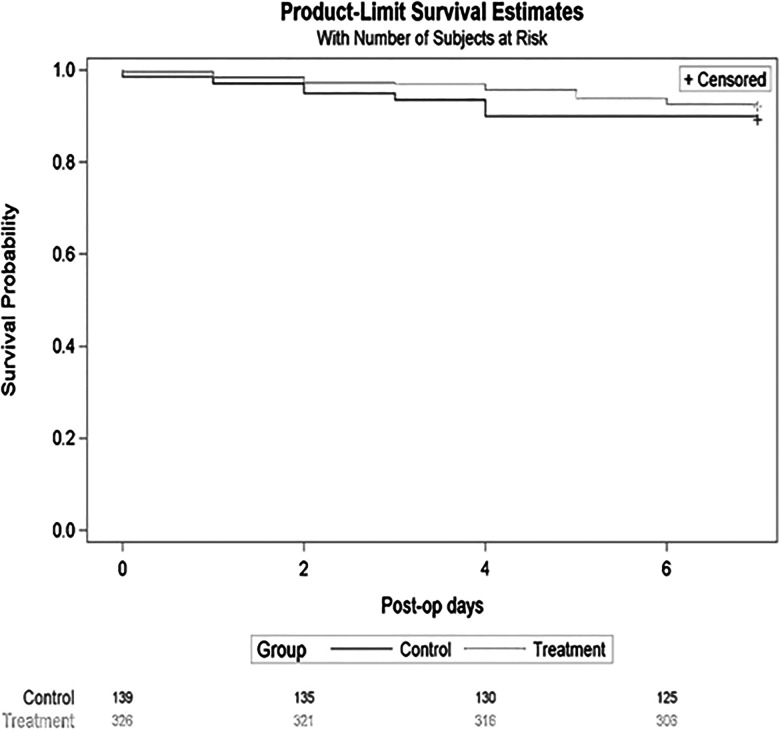
Status, a composite of both HAI and death, was similar between groups. The Kaplan–Meier curve depicted similar status rates of no infection or death between groups; 92% treatment vs. 91% control, p = .38 (Figure 1). Noninferiority testing indicated that the treatment group was not significantly inferior to the control group, p = .0028.
Figure 1.
Kaplan–Meier curves depict the time and number of infection/deaths for each group.
DISCUSSION
There have been numerous studies confirming increased morbidity and mortality associated with allogeneic RBC transfusions in infants and children undergoing cardiac surgery (3,4,6,9,10). Despite advances in surgical techniques, CPB strategies, and postoperative management, transfusions are often unavoidable with younger age, cyanotic heart disease, and CPB duration associated with increased blood product utilization (12).
Patient blood management programs have developed in attempt to limit overall blood product utilization, and include blood conservation techniques including cell saver collection and reinfusion (1,17). An initial study by Golab et al. first identified a reduction of allogeneic blood cell transfusions with no adverse effects in the pediatric cardiac population. This study, however, was limited to use for up to only 6 hours postoperatively. Limitations included protocol violations where ABTs were often given even when cell saver blood was available (17). Cholette et al. developed a protocol maintaining temperature-regulated cell saver blood at the patient’s bedside for reinfusion up to 24 hours following its processing. Results of this prospective randomized clinical trial, though not powered to assess for clinical outcomes, demonstrated a significant reduction in RBC and coagulant product transfusions and crystalloid/colloid infusion, decreased thrombosis, and shorter duration of inotropes and mechanical ventilation (23).
Nathan et al. (2018) described a significant reduction in postoperative transfusions when cell saver blood was used in their pediatric cardiac surgical program (1). This group limited their cell saver reinfusion to 4 hours from collection. Although they did not find any significant difference in hospital duration or postoperative ICU or hospital length of stay, they found a significant reduction in major adverse events when cell saver blood was used (22% vs. 16%).
We hypothesized that having cell saver blood available for reinfusion through the first 24 hours following its collection would reduce ABTs, and therefore reduce major adverse events associated with increased ABT number and volumes. Unique to our local practice is reinfusion of cell saver blood for up to 24 hours its processing, and we sought to provide data regarding safety of this practice, particularly related to HAI and mortality.
Nosocomial and HAI are important complications associated with congenital heart disease and post cardiac surgery (10,11,27,28). Ramifications of HAI include delayed chest closure, increased mechanical ventilation duration, increased inotrope duration, longer hospital stays, and increased mortality (8–10,12).
Our institutional practice is to maintain cell saver blood in a temperate-controlled cooler (equipped with temperature regulators and alarms) under blood bank protocol and quality assurance checks every 4 hours. The bedside cooler temperature is maintained between 1°C and 6°C as per New York State blood bank regulation 58–2.25, thus allowing blood to be stored up to 24 hours post collection (25). Strict blood bank temperature regulation, protocol adherence, and nursing protocols to ensure sterile reinfusion practice have been developed to prevent bacterial contamination and/or transfusion transmission of infection.
To evaluate the safety of this practice, we performed this retrospective study to evaluate the incidence of HAI between children receiving cell saver blood ≤6 hours vs. >6 to ≤24 hours from its collection. The secondary aim was to compare mortality and clinical outcomes. The composite outcome of HAI or death was evaluated as an additional measure given the low mortality rate within the studied population, allowing for better overall comparison between the two study groups. Given the known increase in infections and blood product transfusions, morbidity and mortality in patients requiring ECMO (28–31), we chose to exclude patients who required ECMO within the first 48 hours postoperatively. We collected data on other known risk factors for HAI including duration of mechanical ventilation, inotropes, nitric oxide, delayed chest closure, central venous catheters, indwelling urinary catheters, ECMO (post initial 48 hours), and hospital length of stay (4,6,7,28,31–35). These risk factors were accounted for to ultimately single out ABTs and cell saver blood use as an independent risk factor for HAI in this study, with direct comparison between the two groups. We chose to exclude patients who did not receive any cell saver blood in our analysis due to small number (n = 20) and due to older age and less complex surgical procedures.
We found no significant increased risk of HAI in patients who received cell saver blood for up to 24 hours postprocessing compared to those where cell saver blood was given only in the first 6 hours. The absolute number of infections was numerically higher in the treatment group, and larger subject numbers and equal-sized subject groups would help confirm our findings. Cell saver blood was not tested for bacterial growth throughout the 24-hour period. Future studies including microbiological testing of cell saver blood would be of interest, particularly when correlated with clinical data. Although there was a statistically significant reduction in the volume of albumin, cryoprecipitate, and allogeneic RBCs infused in the treatment group, there was no significant difference in total volume infused over the first 7 days postoperatively. This is likely due to inclusion of a very heterogeneous population with regard to cardiac diagnosis and surgery performed. Evaluation of a more homogeneous group, e.g., infants with a consistent heart defect and/or surgical repair might demonstrate a sustained difference in rate of ABT. Focus on neonates undergoing the highest risk surgical procedures that typically receive the largest amounts of volume and blood products would be of interest. Treatment group subjects received larger volume of RBCs in the operating room, though this did not reach statistical significance and initial intensive care unit (ICU) hemoglobin levels were similar. Larger studies of a more uniform population that include additional intraoperative variables that contribute to transfusion decisions (i.e., CPB flow rates, mixed venous saturations, mean blood pressures, lowest temperature) would help elucidate potential reasons for this difference. There was no significant difference in renal function, nitric oxide duration, mechanical ventilation duration, delayed chest closure duration, inotrope duration, ECMO duration, and indwelling urinary catheter duration between groups. There was no difference in bleeding, thrombosis, or number of reoperations between groups. Creatinine levels were higher in the treatment group though the difference was not statistically significant. Larger studies with a focused population in regard to age, cardiac diagnosis, and surgical procedure would be useful to explore for an impact of cell saver on renal function. Inclusion of data regarding fluid overload would also be of interest.
Mean hospital length of stay and central venous catheter duration were significantly longer in the treatment group though the overall ranges were very similar. Larger studies of a more uniform population may help discern the impact of cell saver blood on hospital length of stay. Used only for the first 24 hours following CPB, it would be interesting to discern whether this difference was maintained with larger subject numbers. The fact that the CVC duration was longer in the treatment group you would think would increase risk for central line–associated blood stream infection (CLABSI) and that was not found. Furthermore, the treatment group was younger and smaller than the control group, characteristics that are associated with greater incidence of HAI, but were not demonstrated in our results.
Limitations of this study include all those inherent to a retrospective study design. Though powered to evaluate clinical outcomes as demonstrated in the analysis, as a single-center study it may not be generalizable to other centers that use large numbers of coagulant products in the OR and postoperatively, or have increased incidence of bleeding complications and reoperations. The current study included a heterogeneous population in regard to cardiac morphology and surgical procedure; limitation to a younger cohort utilizing an infant CPB circuit (i.e., <8 kg) may reduce variations in CPB/surgical management with more uniform surgical complexity. A higher risk population (STAT categories >4) that typically receives larger volumes of ABT and have greater postoperative morbidity/mortality may more directly benefit from cell saver blood, and could be an avenue for further exploration in the future.
Utilization of cell saver blood as a blood conservation technique decreases the volume and number of ABTs in pediatric cardiac surgery (23). 2016 AABB standards support autologous blood reinfusion for up to 24 hours, and our data confirm the safety and feasibility of this practice in this especially vulnerable population of children undergoing cardiac surgery with CPB who are at high risk for HAI (24). This comparison of pediatric cardiac surgery patients receiving cell saver blood up to 6 hours vs. >6 to ≤24 hours post its collection demonstrates two key findings: 1) the incidence of HAI or adverse clinical outcomes was not increased in patients receiving cell saver blood reinfusion for more than 6 hours from collection and 2) treatment group subjects received significantly less volume of ABT when cell saver blood was available for 24 hours. Considering the risks associated with increased rates of ABT, in the absence of increased risk from later cell saver blood reinfusion, these findings support cell saver blood reinfusion up to 24 hours post collection.
Based upon this conclusion, the reinfusion of cell saver blood up to 24 hours proves to be a major advancement in pediatric cardiac surgical patient blood management programs. These findings support utilization of cell saver blood for up to 24 hours postprocessing when maintained under strict blood bank and nursing protocols. Our results affirm our local conservative transfusion practices to minimize ABT, and illustrate the value in interdepartmental partnerships (blood bank, cardiac surgery, perfusion, anesthesia, cardiac ICU) for patient blood management programs. We provide data to support adoption of similar strategies at other pediatric cardiac surgical centers. Studies at other large cardiac surgical centers are needed to confirm our results.
ACKNOWLEDGMENT
Dr. Laura Boulos earned Golisano Children’s Hospital Chair Fellow Award of $1,500.
REFERENCES
- 1.Nathan M, Tishler B, Gauvreau K, et al. A red cell preservation strategy reduces postoperative transfusions in pediatric heart surgery patients. Paediatr Anaesth. 2018;28:450–7. [DOI] [PubMed] [Google Scholar]
- 2.Salvin JW, Scheurer MA, Laussen PC, et al. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann Thorac Surg. 2011;91:204–11. [DOI] [PubMed] [Google Scholar]
- 3.Costello JM, Graham DA, Forbes Morrow D, et al. Risk factors for surgical site infection after cardiac surgery in children. Ann Thorac Surg. 2010;89:1833–42. [DOI] [PubMed] [Google Scholar]
- 4.Costello JM, Graham DA, Forbes Morrow D, et al. Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med. 2009;10:453–9. [DOI] [PubMed] [Google Scholar]
- 5.Kneyber M, Hersi M, Twisk JWR, et al. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33:1414–22. [DOI] [PubMed] [Google Scholar]
- 6.Turcotte RF, Brozovich A, Corda R, et al. Health care-associated infections in children after cardiac surgery. Pediatr Cardiol. 2014;35:1448–55. [DOI] [PubMed] [Google Scholar]
- 7.Levy I, Ovadia B, Erez E, et al. Nosocomial infections after cardiac surgery in infants and children: Incidence and risk factors. J Hosp Infect. 2003;53:111–6. [DOI] [PubMed] [Google Scholar]
- 8.Hatachi T, Tachibana K, Inata Y, et al. Risk factors for healthcare-associated infections after pediatric cardiac surgery. Pediatr Crit Care Med. 2018;19:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasija S, Makhija N, Kiran U, et al. Nosocomial infections in infants and children after cardiac surgery. Indian J Thorac Cardiovasc Surg. 2008;24:233–9. [Google Scholar]
- 10.Garcia H, Cervantes-Luna B, Gonzalez-Cabello H, et al. Risk factors for nosocomial infections after cardiac surgery in newborns with congenital heart disease. Pediatr Neonatol. 2018;59:404–9. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RS, Shekerdemian LS.. Avoidance of hospital-acquired infections in pediatric cardiac surgical patients. Pediatr Crit Care Med. 2016;17(Supp 1):S279–S86. [DOI] [PubMed] [Google Scholar]
- 12.Szekely A, Cserep Z, Sapi E, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. 2009;87:187–97. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Bainbridge D, Martin J, et al. The efficacy of an intraoperative cell saver during cardiac surgery: A meta-analysis of randomized trials. Anesth Analg. 2009;109:320–30. [DOI] [PubMed] [Google Scholar]
- 14.Fresenius Kabi LLC; Terumo Cardiovascular Group . Fresinius Continuous Autotransfusion System® (CATS). 2019. Available at: http://www.terumocv.com/doc/897262_CATSmart-Flex-Wash-and-Learning-Brochure_DEC2019_FINAL-LR.pdf. Accessed April 20, 2021.
- 15.Booke M, Hagemann O, Van Aken H, et al. Intraoperative autotransfusion in small children: An in vitro investigation to study its feasibility. Anesth Analg. 1999;88:763–5. [DOI] [PubMed] [Google Scholar]
- 16.Sistino JJ, Owitz D, Mongero LB.. Heparin washout in the pediatric cell saver bowl. J Extra Corpor Technol. 1992;24:94–6. [PubMed] [Google Scholar]
- 17.Golab HD, Scohy TV, de Jong PL, et al. Intraoperative cell salvage in infants undergoing elective cardiac surgery: A prospective trial. Eur J Cardiothorac Surg. 2008;34:354–9. [DOI] [PubMed] [Google Scholar]
- 18.Hishon ML, Ryan A, Lithgow P, et al. An evaluation of changes in composition and contamination of salvaged blood from the cardiopulmonary bypass circuit of pediatric patients. Heart Lung. 1995;24:307–11. [DOI] [PubMed] [Google Scholar]
- 19.Luque-Oliveros M. Bacteremia in the red blood cells obtained from the cell saver in patients submitted to heart surgery. Rev Lat Am Enfermagem. 2020;28:e3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida T, Nakano K, Nakatani H, et al. Bacteriological evaluation of the cardiac surgery environment accompanying hospital relocation. Surg Today. 2006;36:504–7. [DOI] [PubMed] [Google Scholar]
- 21.Bland LA, Villarino ME, Arduino MJ, et al. Bacteriologic and endotoxin analysis of salvaged blood used in autologous transfusions during cardiac operations. J Thorac Cardiovasc Surg. 1992;103:582–8. [PubMed] [Google Scholar]
- 22.Lasko MJ, Conelius AM, Serrano OK, et al. Impact of intraoperative cell salvage on concentrations of antibiotics used for surgical prophylaxis. Antimicrob Agents Chemother. 2020;64:e01725–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholette J, Powers K, Alfieris G, et al. Transfusion of cell saver salvaged blood in neonates and infants undergoing open heart surgery significantly reduces RBC and coagulant product transfusions and donor exposures: Results of a prospective, randomized, clinical trial. Pediatr Crit Care Med. 2013;14:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Association of Blood Banks (AABB) . Standards for Perioperative Autologous Blood Collection and Administration, 7th ed. Bethesda, MD: AABB; 2017.
- 25.American Association of Blood Banks (AABB) . Standard for Blood Banks and Transfusion Services, 26th ed. Bethesda, MD: AABB; 2009.
- 26.Center for Disease Control and Prevention . Identifying healthcare-associated infections (HAI) for NHSN surveillance. 2019. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/2PSC_IdentifyingHAIs_NHSNcurrent.pdf. Accessed January 12, 2020.
- 27.Sarvikivi E, Lyytikainen O, Nieminen H, et al. Nosocomial infections after pediatric cardiac surgery. Am J Infect Control. 2008;36:564–9. [DOI] [PubMed] [Google Scholar]
- 28.Alten JA, Fazlur Rahman AKM, Zaccagni HJ, et al. The epidemiology of health-care associated infections in pediatric cardiac intensive care units. Pediatr Infect Dis J. 2018;37:768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrup EA, Yuerek M, Griffis HM, et al. Hospital-acquired infection in pediatric subjects with congenital heart disease post-cardiotomy supported on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020;21:e1020–5. [DOI] [PubMed] [Google Scholar]
- 30.Cashen K, Reeder R, Dalton HJ, et al. Acquired infection during neonatal and pediatric extracorporeal membrane oxygenation. Perfusion. 2018;33:472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harder EE, Gaies MG, Yu S, et al. Risk factors for surgical site infection in pediatric cardiac surgery patients undergoing delayed sternal closure. J Thorac Cardiovasc Surg. 2013;146:326–33. [DOI] [PubMed] [Google Scholar]
- 32.Shaath GA, Jijeh A, Faruqui F, et al. Ventilator-associated pneumonia in children after cardiac surgery. Pediatr Cardiol. 2014;35:627–31. [DOI] [PubMed] [Google Scholar]
- 33.Abou Elella R, Najm HK, Balkhy H, et al. Impact of bloodstream infection on the outcome of children undergoing cardiac surgery. Pediatr Cardiol. 2010;31:483–9. [DOI] [PubMed] [Google Scholar]
- 34.Matlow AG, Wray RD, Cox PN.. Nosocomial urinary tract infections in children in a pediatric intensive care unit: A follow-up after 10 years. Pediatr Crit Care Med. 2003;4:74–7. [DOI] [PubMed] [Google Scholar]
- 35.Lee NG, Marchalik D, Lipsky A, et al. Risk factors for catheter associated urinary tract infections in a pediatric institution. J Urol. 2016;195:1306–11. [DOI] [PubMed] [Google Scholar]