Abstract
Background
In August 2020, in the context of COVID-19 pandemics, an autochthonous dengue outbreak was identified for the first time in Italy.
Methods
Following the reporting of the index case of autochthonous dengue, epidemiological investigation, vector control and substances of human origin safety measures were immediately activated, according to the national arbovirus surveillance plan. Dengue cases were followed-up with weekly visits and laboratory tests until recovery and clearance of viral RNA from blood.
Results
The primary dengue case was identified in a young woman, who developed fever after returning from Indonesia to northern Italy, on 27 July 2020. She spent the mandatory quarantine for COVID-19 at home with relatives, six of whom developed dengue within two weeks. Epidemiological investigation identified further five autochthonous dengue cases among people who lived or stayed near the residence of the primary case. The last case of the outbreak developed fever on 29 September 2020. Dengue cases had a mild febrile illness, except one with persistent asthenia and myalgia. DENV-1 RNA was detected in blood and/or urine in all autochthonous cases, up to 35 days after fever onset. All cases developed IgM and IgG antibodies which cross-reacted with West Nile virus (WNV) and other flaviviruses. Sequencing of the full viral genome from blood samples showed over 99% nucleotide identity with DENV-1 strains isolated in China in 2014–2015; phylogenetic analysis classified the virus within Genotype I. Entomological site inspection identified a high density of Aedes albopictus mosquitoes, which conceivably sustained local DENV-1 transmission. Aedes koreicus mosquitoes were also collected in the site.
Conclusions
Areas in Europe with high density of Aedes mosquitoes should be considered at risk for dengue transmission. The presence of endemic flaviviruses, such as WNV, might pose problems in the laboratory diagnosis.
Keywords: DENV, surveillance, follow-up, urine, saliva, Aedes albopictus, Aedes koreicus
Background
In the recent years, European countries reported an increased activity of vector-borne viruses, whose expansion is greatly affected by the major drivers of emerging infectious diseases, including warming climate, land use, global travel and trade.1 This is the case of the unprecedented number of West Nile virus (WNV) infections that have been reported by several European countries where the virus has become endemic,2 including Italy,3 Greece,4 Spain,5 Germany6 and the Netherlands,7 which recorded summer heat waves during the last years. In addition, autochthonous transmission of dengue virus (DENV), chikungunya virus (CHIKV) and Zika virus (ZIKV) has been identified in several countries, such as the large chikungunya outbreaks which occurred in Italy in 2007 and 2017, resulting in about 200 and 500 human infections, respectively;8,9 the dengue outbreaks which occurred in France since 2010,10 in Croatia in 201011 and in Spain in 2018;12 the autochthonous vector-borne transmission of ZIKV identified in France in 2019.13 The risk of introduction of these tropical viruses, resulting in local outbreaks, is expected to even worsen in the near future, since Aedes albopictus and other invasive Aedes mosquitoes, which are competent vectors for transmission, are endemic in southern European countries and are rapidly expanding their geographic range to northern Europe.14,15
Notwithstanding effective surveillance systems and vector control programs are in place in many countries, identification and prevention of vector-borne outbreaks remains challenging and the occurrence of outbreaks unpredictable. Actually, long delays in the diagnosis and reporting of imported cases of vector-borne viral infections have been identified as the main driver for autochthonous transmission of dengue and chikungunya in Europe.16 This is even more challenging in the context of the ongoing SARS-CoV-2 pandemics, which poses problems not only for the differential diagnosis among febrile conditions but also because of the under-testing and under-reporting of non-COVID-19 conditions. On the other hand, arbovirus transmission is likely to have increased during COVID-19 lockdown because of its effects on routine vector control programs and on the contact rate between human and mosquito populations.17
In this report, we describe the results of the clinical and laboratory investigation of 11 dengue cases involved in the autochthonous outbreak, which occurred in northern Italy, in August–September 2020, in the context of the COVID-19 pandemics, highlighting pitfalls in diagnosis and early management of the outbreak. The results of entomological investigation and vector control measures that were undertaken are also reported. Preliminary results of the first five cases of this outbreak and the implementation of surveillance and control measures have been described in a previous communication.18
Methods
Ethics statement
The cases reported in this study were investigated with routine procedures according to the national surveillance plan for arbovirus infections. Therefore, no approval was required from the ethics committee. Written informed consent was obtained from the study subjects regarding the publication of this paper.
Surveillance protocol for imported arbovirus infections
In 2020, in the Veneto Region, the surveillance program of autochthonous and imported arbovirus infections was integrated with COVID-19 surveillance, because of the overlaps of symptoms and clinical presentation of these febrile conditions. According to the program, clinical surveillance of imported arbovirus infections had to be carried out throughout the year, while the activation of entomological surveillance had to be carried out during the period of vector circulation, from June to November. Suspected imported cases of arbovirus (DENV, ZIKV and CHIKV) infection were defined as subjects with onset of fever (≥38°C) within a week, who returned from potentially endemic areas within two weeks, without other obvious causes of fever.19 Suspected cases were referred to an emergency department and/or infectious disease unit for clinical evaluation and laboratory testing for SARS-CoV-2, malaria (in case of travel to endemic areas) and arbovirus diagnosis and were quarantined at home with the recommendation to adopt measures to avoid mosquito bite. A case was defined as probable if anti-DENV, ZIKV or CHIKV IgM antibodies were detected in a single serum sample. A case was defined as confirmed if the virus was isolated in culture or its nucleic acids were detected in biological fluids (blood, urine or saliva), if DENV NS1 antigen was detected in blood, if seroconversion or 4-fold increase of antibodies titers were demonstrated in two serum samples collected at least two weeks apart, or if high titer IgM were detected and confirmed by neutralization assays. According to the program, all probable and confirmed cases had to be immediately reported to the local and regional hygiene and public health units, which had to collect relevant personal information (e.g. mosquito bite) to guide the activation of vector control measures within 24 h from the notification. Surveillance measures for imported arbovirus infections were applied also to suspected autochthonous outbreaks, i.e. two or more suspected cases occurring within 30 days in a small geographic area. With the identification of a confirmed or probable autochthonous dengue outbreak, the following activities had to be done: entomological investigation, enhancement of human surveillance with active case finding, implementation of vector control measures and of safety measures for substances of human origin.
Entomological investigation and interventions
Within 24 h from the notification of a suspected or confirmed case of imported arbovirus infection, site inspection had to be done by public health technicians, who verify the presence of Ae. albopictus adults and larvae and, if not already in place, set up BG-sentinel traps and ovitraps to collect Aedes spp. mosquitoes. Identification of Ae. albopictus was based on morphology, while Aedes koreicus was confirmed by molecular testing. If Ae. albopictus and/or Ae. koreicus were detected, mosquito disinfestation had to be conducted within 24 h from the notification of the case in the area comprised in a radius of 200 m around the house of the case and extended to sensible places in the nearby. Disinfestations included treatment with larvicides and adulticides and removal of breeding sites. The effectiveness of disinfestations was assessed by monitoring mosquito density before and after treatment.
Substances of human origin safety measures
According to the surveillance plan, when autochthonous arbovirus transmission was identified, screening by nucleic acid testing (NAT) had to be performed in all blood, organ, tissue and hematopoietic stem cell donors who were resident or had been for at least one night in the province of virus transmission during the 28 days before donation.
Laboratory methods
For molecular testing of arboviruses, total nucleic acids were purified from 200 μL of plasma, urine and saliva by using a MagNA Pure 96 System (Roche Applied Sciences, Basel, Switzerland). DENV RNA was amplified by in-house real-time RT-PCR methods which allowed discrimination among the different serotypes, according to the Center for Disease Control and Prevention protocol.20 WNV, USUV, ZIKV and CHIKV RNA testing were performed by in-house real-time RT-PCR methods, as previously described.21–23 Real-time RT-PCR assays were carried on using a one-step real-time PCR kit (Thermo Fisher Scientific, Waltham, MA, USA) and run on ABI 7900HT Sequence Detection Systems (Thermo Fisher Scientific). DENV NS1 antigen was detected in plasma by using a rapid immuno-chromatographic assay (Dengue NS1 Ag Strips, Bio-Rad, Hercules, CA, USA). Anti-DENV, WNV, ZIKV and CHIKV IgM and IgG antibodies were detected by a chemiluminescence immunoassay (CLIA) in a Thunderbolt instrument (VirClia, Vircell, Granada, Spain).
DENV NAT screening of donors of substances of human origin was done by using the cobas® CHIKV/DENV real-time PCR assay on a cobas® 6800 System (Roche) and the Procleix Dengue Virus Assay on a Procleix Panther System (Grifols Italia S.p.A., Milan, Italy).
Screening of viral infection in mosquitoes was done by a broad-range nested RT-PCR assay targeting viruses of the Flavivirus genus,24 followed by sequencing of positive results. All mosquito samples were also sent to the national reference laboratory (Department of Infectious Diseases, Istituto Superiore di Sanità, Rome, Italy) for confirmation.
DENV-1 genome sequencing and phylogenetic analysis
DENV-1 full genome sequencing was performed by PCR amplification using the primer sets designed by Stubbs et al.25 followed by Sanger sequencing. Briefly, total RNA was purified from plasma samples using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), reversed transcribed using Superscript IV (Thermo Fisher Scientific) and amplified in 40 overlapping amplicons covering the full genome of DENV-1. Amplicons were sequenced by using a Brilliant Dye terminator 1.1. kit (NimaGen, Nijmegen, The Netherlands) on a 3730xl Genetic Analyser (Thermo Fisher Scientific). Molecular phylogenetic analysis of DENV-1 was performed by using the Maximum Likelihood method based on the Tamura-Nei model26,27 with the genome sequence obtained in this study and with related DENV-1 genome sequences available in GenBank, as previously described.28,29
Results
Epidemiological and clinical investigation
A cluster of 11 cases of autochthonous dengue was identified in Vicenza Province, Veneto region, northern Italy, August–September 2020, secondary to a primary case imported from Indonesia. Preliminary data on this outbreak have been previous reported.18 The time line of events in this outbreak is represented in Figure 1. Demographic, clinical and baseline laboratory findings in the primary imported dengue case and in the 11 secondary autochthonous dengue cases are reported in Table 1.
Figure 1.
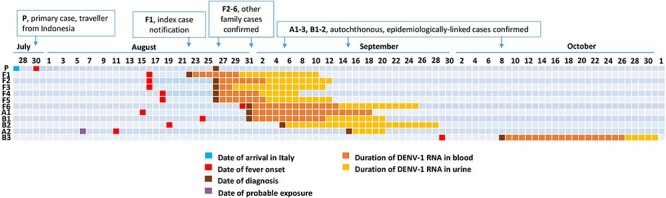
Timeline of the dengue outbreak, northern Italy, 2020. Each case is indicated in rows. P represents the primary case; F1–F6 represent household contacts of P; A1 and A2 and B1–B3 represent two clusters epidemiologically-linked to P and F1–6. Each line represents a day, from July 27th to November 1st, 2020. Date of relevant events, such as the arrival in Italy of the primary case, symptom onset, laboratory diagnosis, probable exposure of a dengue case who was not resident in the affected area, duration of DENV-1 RNA detection in blood and urine, and vector control interventions and indicated in the figure.
Table 1.
Demographic, clinical and baseline laboratory findings in the primary dengue case and 11 secondary autochthonous dengue cases, northern Italy, 2020
| Cases, ID* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F1 | F2 | F3 | F4 | F5 | F6 | A1 | B1 | B2 | A2 | B3 | |
| Demographic data | ||||||||||||
| Sex | F | M | F | M | M | M | M | F | F | M | F | F |
| Age, years | 30s | 50s | 50s | 20s | 20s | <10 | 10s | 50s | 60s | 70s | 50s | 70s |
| Days from symptom onset to diagnosis | 27 | 10 | 6 | 10 | 8 | 8 | 1 | 16 | 7 | 16 | 35 | 7 |
| Symptoms | ||||||||||||
| Duration of symptoms (days) | 4 | 7 | 5 | 4 | 4 | 5 | 4 | 65 | 5 | 5 | 7 | 7 |
| Fever (temperature, C) | 38 | 39 | 38 | 38 | 38.5 | 39 | 39 | 39.5 | 39.3 | 39.3 | 38.5 | 38 |
| Malaise | + | − | − | − | − | − | − | + | − | + | − | + |
| Arthralgia | + | + | + | + | − | − | + | + | + | − | − | + |
| Myalgia | + | + | + | + | − | − | − | + | + | + | − | + |
| Headache | + | + | + | + | − | − | + | + | + | − | + | + |
| Rash | + | − | − | − | − | − | + | + | + | + | − | + |
| Pruritus | + | + | + | + | + | + | + | + | + | + | − | + |
| Gastrointestinal | − | − | − | − | − | − | − | − | + | + | − | − |
| Virological tests | ||||||||||||
| DENV RNA in blood (CT) | − | 32.7 | 29.0 | 39.1 | 29.4 | 29.9 | 16.8 | 31.2 | 34.4 | − | − | 28.9 |
| DENV RNA in urine (CT) | − | 28.3 | 28.3 | 36.7 | 31.3 | 28.3 | 37.4 | 31.7 | 33.6 | 29.3 | 35.4 | 30.5 |
| DENV RNA in saliva (CT) | − | 35.4 | 35.2 | − | 35.8 | 29.9 | 36.2 | − | 33.6 | ND | ND | 30.4 |
| DENV NS1 Antigen (LFA) | − | − | + | − | + | + | + | − | − | − | − | + |
| DENV IgM (AU/mL) | 2.60 | 10.37 | − | 6.95 | 10.37 | 14.27 | − | 10.72 | − | 13.77 | 6.99 | 13.45 |
| DENV IgG (AU/mL) | 3.10 | − | − | − | − | − | − | 1.01 | 2.73 | 1.12 | 1.63 | − |
| ZIKV IgM (AU/mL) | − | − | − | 1.15 | − | 1.56 | − | − | − | − | − | − |
| ZIKV IgG (AU/mL) | 4.05 | − | − | − | − | − | − | − | 2.81 | − | − | − |
| WNV IgM (AU/mL) | − | 4.04 | 2.85 | 4.41 | 1.83 | 6.37 | − | 3.22 | 1.45 | 3.52 | 3.16 | 1.63 |
| WNV IgG (AU/mL) | 1.87 | − | − | − | − | − | − | 1.61 | 1.69 | 1.57 | 1.69 | − |
| CHIKV IgM (AU/mL) | − | 2.63 | − | − | − | − | − | − | − | − | − | − |
| CHIKV IgG (AU/mL) | − | − | − | − | − | − | − | − | − | − | − | − |
| Blood tests** | ||||||||||||
| WBC count (×109/L) | 6.4 | 6.3 | 5.7 | 4.5 | 5.7 | 5.3 | 3.1 | 5.2 | 11.8 | 5.4 | 4.9 | 2.6 |
| RBC count (×1012/L) | 4.08 | 4.94 | 4.45 | 4.82 | 4.71 | 4.33 | 4.97 | 4.73 | 4.97 | 4.93 | 4.68 | 4.81 |
| Hematocrit (L/L) | 0.38 | 0.45 | 0.40 | 0.43 | 0.42 | 0.34 | 0.44 | 0.42 | 0.43 | 0.43 | 0.41 | 0.45 |
| Platelet count (×109/L) | 196 | 329 | 299 | 294 | 234 | 475 | 156 | 350 | 146 | 320 | 172 | 60 |
Present or positive result; −: absent, undetectable or negative result; CT: Real-time RT-PCR threshold cycle; ND: non done; LFA: lateral flow assay; AU/mL: arbitrary unit/mL measured by chemiluminescent immunoassays (CLIA); WBC: white blood cell; RBC: red blood cell.
* *Normal range: WBC count: 3.5–12.5 × 109/L; Red blood cell count: 4.10–5.65 × 1012/L; Hematocrit: 0.39–0.49 L/L; Platelet count: 110–330 × 109/L.
The index case (case F1) was a woman in her fifties from Vicenza province with no recent travel history, who developed fever and arthralgia on 16 August 2020. She presented to the infectious diseases unit of Vicenza City Hospital on August 21, when symptoms had already disappeared. She referred that other household contacts had similar symptoms, including a relative who developed fever on July 30 (case P), three days after returning from a 16-month stay in West Sumatra, Indonesia, and who spent the mandatory 14-days quarantine for COVID-19 at her home. Molecular testing on a nasopharyngeal swab excluded SARS-CoV-2 infection in the index case, while investigation of vector-borne viruses demonstrated DENV-1 RNA in blood, urine and saliva. Following notification of this case of autochthonous dengue to the public health authority of the Veneto Region on 26 August, vector control measures were immediately implemented and general practitioners of the zone were alerted for referring cases of unexplained fever, according to regional and national arbovirus surveillance plans.
Epidemiological investigation and active search of other cases of DENV infection was done among household contacts and people living close to the imported case, including both asymptomatic and symptomatic individuals, by serological testing and by molecular testing of blood, urine and saliva samples. In addition, all individuals with symptoms consistent with arbovirus infection and living in Vicenza province or who travelled in Vicenza province during the 28 days before symptom onset were tested for DENV, besides autochthonous arboviruses, during the period from August to November 2020. This investigation allowed detecting DENV-1 infection in further 11 subjects with symptoms: i.e. the above described traveller returning from Indonesia (primary case, P) and 10 subjects with no travel history outside Veneto region in the 28 days before symptom onset (autochthonous secondary cases), including other five family contacts of the primary case (cases F2–F6) who lived at a distance <50 m from the primary case, two subjects (cases A1 and A2) who lived between 50- and 100-m distance from the primary case, and three cases (cases B1–B3) who lived at about 300 m from the primary case (Figure 2). The area where dengue cases were identified was characterized by a rural setting with few residential houses with gardens and low population density.
Figure 2.
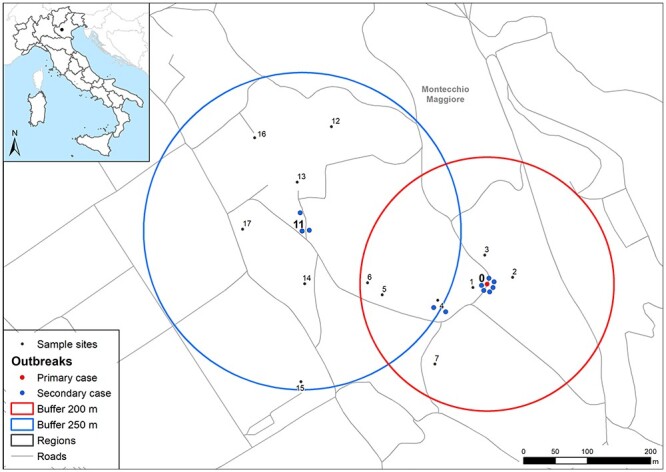
Map of dengue cases, entomological surveillance and control areas. Numbers correspond to sites monitored with mosquito traps as reported in Supplementary Table 1. The red circle represents the area of 200 m of radius around primary case (site number 0), while the blue circle the area of 250 m used after the finding of autochthonous cases (site 11) out of the first surveillance area. Sites 9 (hospital) and 10 (Sovizzo municipality) are not shown on the map.
Autochthonous dengue cases developed symptoms between 11 August and 29 September 2020 (Figure 1). The most common signs and symptoms were fever, pruritus, headache, arthralgia and myalgia (Table 1). Symptoms completely resolved in 4–8 days in all cases, except a patient (case A1) who developed severe asthenia and myalgia, which persisted for about two months and required steroid treatment. This patient had viral RNA still detectable in blood one month after the onset of symptoms.
Laboratory diagnosis and follow-up
Initially, after the onset of fever, the primary dengue case was only investigated for SARS-CoV-2 infection, while diagnosis of DENV infection was retrospective and based on serology and neutralization assays. In fact, the patient was first tested for dengue diagnosis 27 days after fever onset, when molecular tests for DENV and other arboviruses (WNV, USUV, ZIKV and CHIKV) on blood, urine and saliva gave negative results, while serology tests showed positive results for DENV IgM and IgG antibodies (IgG/IgM ratio > 1) and for ZIKV IgG and WNV IgG antibodies. Neutralization assays showed a higher increase of neutralizing antibody titres against DENV-1 than other DENV serotypes (32- vs. 8-fold increase, respectively) in serum samples collected in a 2-week interval. At variance, neutralization assays against ZIKV and WNV were negative. These results were consistent with a recent DENV-1 infection in a subject with previous DENV immunity.
In all autochthonous cases, DENV-1 infection was confirmed by detection of viral RNA in either blood, urine or saliva (Table 1). In addition, follow-up laboratory testing (Figure 3) showed that all subjects seroconverted and developed DENV IgM and IgG antibodies, which were confirmed by neutralization assays as DENV-1-specific (i.e. in convalescent sera, the neutralization titres against DENV-1 were 4- to 8-fold higher than the neutralization titres against DENV-2). Detection of antibodies against other arboviruses (WNV, ZIKV and CHIKV), performed by CLIA at the time of first evaluation, demonstrated the presence of cross-reactive anti-WNV IgM/IgG antibodies in all patients with detectable anti-DENV IgM/IgG antibodies, while false-positive CHIKV and ZIKV serology results were less common (Table 1). In one of these autochthonous dengue cases (case B1), in whom high DENV IgG antibody levels were detected at the time of diagnosis, prior WNV immunity was demonstrated by neutralization assay.
Figure 3.
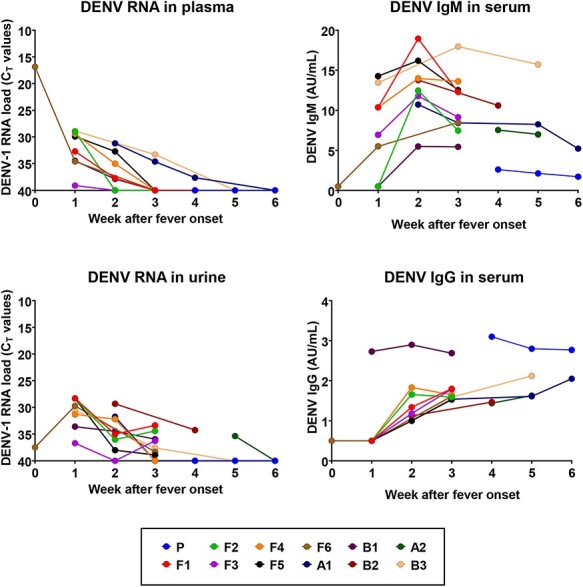
DENV-1 RNA load in blood and urine, reported as threshold cycles (CT) values, and serum anti-DENV IgM and IgG antibody levels, reported as AU/mL, determined in the primary dengue case (P) and the autochthonous dengue cases (F1–6, A1, A2, B1–3) during follow-up, up to 6 weeks after fever onset. Case identification is the same of Table 1 and Figure 1.
All dengue cases were followed-up with weekly visits and laboratory tests until recovery from symptoms and until DENV RNA was undetectable in plasma. In most patients, viral RNA was detectable within the first two weeks after the onset of symptoms, with the exception of two cases (cases A1 and B3), in whom DENV-1 RNA was detected in blood up to 24 and 35 days after symptom onset (Figures 1 and 3). Shedding of DENV-1 RNA in urine lasted for over 3 weeks in most patients. In one patient (case B2), who was evaluated for the first time on day 35 after symptom onset, DENV-1 RNA was still detectable in urine, but not in blood (Figures 1 and 3).
In most patients with autochthonous dengue, anti-DENV IgM antibodies appeared one week after the onset of symptoms and peaked two weeks after the onset of symptoms, while anti-DENV IgG antibodies appeared during the second week after symptom onset (Figure 3).
DENV-1 genome sequencing and phylogenetic analysis
DENV-1 full genome sequence (GenBank accession no. MZ291446) was obtained from an autochthonous case with high viral load in plasma, who was identified in the initial phase of the outbreak in mid-August 2020 (case F6). Partial genome sequencing of a region of about 2000 bp in the 5′ end of the genome, including the E gene, was performed in other three autochthonous cases, including the last case of the outbreak, who was identified in the end of September 2020. In addition, full viral genome sequencing was performed in a case of imported DENV-1 infection, who developed symptoms the day after returning from the French Antilles, in mid-August 2021. Alignment of the DENV-1 full genome sequence associated with the autochthonous outbreak showed over 99% nucleotide identity with DENV-1 genomes isolated in China, in 2014–15.30–32
Phylogenetic analysis classified the virus within DENV-1 Genotype I (Supplementary Figure 1). Partial genome sequencing showed that the autochthonous cases were infected with the same DENV-1 strain (100% identity). At variance, the genome from the DENV-1 case imported from the French Antilles showed the highest nucleotide identity (about 98%) with DENV-1 strains isolated in South and Central America classified as Genotype III.
DENV NAT screening of donors of substances of human origin
In the period from August 2020 to the end of November 2020, DENV NAT screening was performed in all blood, organ, stem cell and tissue donors who were resident in Vicenza province or stayed in the Vicenza province during the four weeks before donation. No viraemic donors were identified. Notably, one of the autochthonous dengue cases donated blood a few hours before travelling to the area of exposure. Retrospective DENV NAT analysis of this blood donation excluded DENV infection.
Entomological investigation and vector control measures
Entomological survey and vector control activities were implemented the same day of index case confirmation (Figure 1). Vector control measures, such as removal of standing waters, larvicidal and adulticidal treatments, were conducted for three consecutive days within 200-m radius area around the residence of index case (Figure 2). The entomological survey was also extended to other areas where the cases stayed before the period of isolation. After 5 days, a second survey was carried out to assess the presence of Aedes mosquitoes and the effectiveness of the disinfestation measures. Standard ovitraps and BG Sentinel® traps (Biogents, Germany) baited with their lure and CO2 were used to collect Aedes eggs and adult mosquitoes, respectively; ovitraps run for one week and BG-Sentinel for 24 h. When looking for the presence of adult mosquitoes by direct observation, and by larval search, Ae. albopictus and Ae. koreicus were identified (Supplementary Table 1). Since one of the secondary cases confirmed in September was located outside the 200 m area, a new surveillance area of radius 250 m was considered around this case (Figure 2), where entomological survey and vector control activities were also implemented (Supplementary Table 1). All mosquitoes tested with pan-Flavivirus RT-PCR assay were DENV-negative.
Discussion
In this study, we present the results of the integrated epidemiological, clinical, virological and entomological investigations on the first dengue outbreak reported in Italy, which occurred in August–September 2020. The outbreak involved 11 autochthonous cases secondary to an imported case from Indonesia, who was identified retrospectively. Transmission to six household contacts occurred during the 2-week period of COVID-19 quarantine of the primary case and further five autochthonous cases were identified within a distance of 300 m from the primary case. Quarantine and social distancing measures for COVID-19 have been associated with a rise of dengue cases in endemic countries, presumably because of the increased peridomestic contact between humans and mosquitoes.33 On the other hand, these containment measures might have reduced the risk of spillover dengue transmission in surrounding areas.34
In our study, active case finding was focused on all household contacts of the primary case and only on subjects with febrile illness who lived or stayed in the Vicenza province. Thus, we cannot exclude that further asymptomatic cases or cases with mild symptoms, who represent about 80% of DENV infections,35 were missed by the epidemiological investigation. However, in the autochthonous dengue outbreak here reported, the rapid implementation of vector control measures, the ecological setting with low population density and the end of the summer season probably mitigated the risk of onward virus transmission.
All dengue cases had a mild febrile illness with unspecific symptoms that could be misdiagnosed as West Nile fever and had antibodies that cross-reacted with WNV IgM and IgG serology tests. Thus, even in the context of a highly endemic setting for WNV and/or Usutu virus, such as northern Italy,22,36 diagnosis should not rely solely on a positive IgM and/or IgG result but should require confirmation by neutralization assay or, if available, a positive molecular test.37,38 Notably, molecular testing allowed the confirmation of DENV-1 infection in all autochthonous cases, even when samples were collected after the acute phase. In most cases, the duration of DENV RNA in blood and urine was 2 and 3 weeks, respectively, and in two cases, DENV RNA could be detected in blood and urine up to 4 and 5 weeks after symptom onset.
Entomological investigation indicated that the outbreak was conceivably sustained by Ae. albopictus, which was found to be abundant in the area. Aedes albopictus is a competent vector for DENV-1, as shown during other autochthonous dengue outbreaks in Europe, which were maintained by this mosquito species.10,39,40 Another invasive Aedes mosquito species, Ae. koreicus, is spreading in northern Italy, including Vicenza province,41 and was detected in the sites involved in the dengue outbreak. Vector competence of Ae. koreicus has been demonstrated for CHIKV,42 while it is still unknown whether it is able to transmit DENV and other arboviruses.43 The geographic expansion of invasive mosquitoes represents a serious threat for European countries, making them more vulnerable to arbovirus incursions.44,45 Actually, the risk of autochthonous transmission of dengue and chikungunya is not negligible in European regions where Ae. albopictus mosquitoes are established, and these infections should be considered in the differential diagnosis of febrile illness in travellers returning from these areas during the summer season.46 Notably, in Italy and in other Southern Mediterranean countries, most dengue cases are imported from endemic areas during the period from July to September, which corresponds to the peak of vector activity, and, with the increasing global mobility, the number of imported cases has raised during the last two decades.47–49 The risk of population exposure to dengue and other vector-borne diseases is predicted to further increase in the future because of the global warming and, as consequences, the geographic expansion of vectors towards higher altitudes and temperate regions and the longer duration of the transmission season in temperate countries.50–52
Often, the primary cases of autochthonous outbreaks of DENV, CHIKV and ZIKV in European countries are not identified, notwithstanding the implementation of effective surveillance programs for vector-borne infections. This is the case, e.g. of the large chikungunya outbreak in Italy, 2017, which was unrecognized for about one month;53 the cases of vector-borne transmission of ZIKV in France, 201913; the dengue outbreaks in Croatia in 201011 and in southern France in 2020,54 which were only identified through travellers to Germany and to the Netherlands, respectively. A study estimating the number of imported dengue cases in Italy suggested that only about 3% of the total infections are actually reported by the surveillance system, probably because most infections are unapparent or mild and do not require access to the health care system.48 Nonetheless, only the dengue outbreak here described has occurred in Italy so far. This suggests that the ecological conditions in Italy, like in other areas in Southern Europe, are not optimal for dengue transmission.55 One of the reasons is the absence of Aedes aegypti, the primary vector for dengue, which is more efficient than Aedes albobictus in DENV transmission. However, further research is needed to determine the competence of European Ae. albopictus strains and the threshold levels of mosquito and human population density to allow autochthonous transmission of dengue in Southern Europe.
All these data emphasize the importance of strengthening surveillance programs and public health interventions, in particular, vector control measures and campaigns to raise awareness among physicians and the general population on the risk of vector borne infections.
Actually, the reduction of vector control activities, the decrease of diagnostic testing and the increased outdoors hiking and sport activities during the COVID-19 emergency in 2020 have been considered as relevant co-factors for the resurgence of West Nile neuroinvasive disease in Spain5 and tick-borne encephalitis in Switzerland.56 These events probably contributed to the expansion of Aedes mosquito population, to the delay in the diagnosis of the imported dengue case, and hence, the occurrence of the autochthonous dengue outbreak here described.
As public health response, in 2021, the Veneto Region enhanced vector control measures and information activities and revised the surveillance plan for arbovirus infections to highlight the importance of including DENV, ZIKV and CHIKV infections in the differential diagnosis of febrile illness in subjects without travel history to endemic countries.
Supplementary Material
Contributor Information
Luisa Barzon, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Department of Molecular Medicine, University of Padova, via A. Gabelli 63, 35121 Padova, Italy; Microbiology and Virology Unit, Padova University Hospital, Via Giustiniani 2, 35128 Padova, Italy.
Federico Gobbi, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Department of Infectious/Tropical Diseases and Microbiology, IRCCS Sacro Cuore Don Calabria Hospital, Via Luigi Rizzardi 4, 37024, Negrar di Valpolicella, Verona, Italy.
Gioia Capelli, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Istituto Zooprofilattico Sperimentale delle Venezie, Viale dell'Università 10, 35020 Legnaro, Padova, Italy.
Fabrizio Montarsi, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Istituto Zooprofilattico Sperimentale delle Venezie, Viale dell'Università 10, 35020 Legnaro, Padova, Italy.
Simone Martini, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Entostudio s.r.l., Viale del Lavoro, 66, 35020 Ponte San Nicolò, Padova, Italy.
Silvia Riccetti, Department of Molecular Medicine, University of Padova, via A. Gabelli 63, 35121 Padova, Italy.
Alessandro Sinigaglia, Department of Molecular Medicine, University of Padova, via A. Gabelli 63, 35121 Padova, Italy.
Monia Pacenti, Microbiology and Virology Unit, Padova University Hospital, Via Giustiniani 2, 35128 Padova, Italy.
Giacomina Pavan, Department of Microbiology, St. Bortolo Hospital, Viale Ferdinando Rodolfi 37, 36100 Vicenza, Italy.
Mario Rassu, Department of Microbiology, St. Bortolo Hospital, Viale Ferdinando Rodolfi 37, 36100 Vicenza, Italy.
Maria Teresa Padovan, Department of Public Health, Azienda AULSS8 Berica, Viale Ferdinando Rodolfi 37, 36100 Vicenza, Italy.
Vinicio Manfrin, Department of Infectious Diseases, St. Bortolo Hospital, Viale Ferdinando Rodolfi 37, 36100 Vicenza, Italy.
Francesca Zanella, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Direzione Prevenzione, Sicurezza Alimentare Veterinaria, Dorsoduro, 3493 - Rio Nuovo - 30123 Venice, Italy.
Francesca Russo, Veneto Region Arbovirosis Task Force, Dorsoduro, 3493 - Rio Nuovo - 30123 Venezia, Italy; Direzione Prevenzione, Sicurezza Alimentare Veterinaria, Dorsoduro, 3493 - Rio Nuovo - 30123 Venice, Italy.
Felice Foglia, Department of Public Health, Azienda AULSS8 Berica, Viale Ferdinando Rodolfi 37, 36100 Vicenza, Italy.
Luca Lazzarini, Department of Infectious Diseases, St. Bortolo Hospital, Viale Ferdinando Rodolfi 37, 36100 Vicenza, Italy.
Authors’ contributions
Conceptualization, L.B.; clinical investigation, F.G., V.M., L.L.; virological investigation, L.B., S.R., A.S., M.P, G.P., M.R.; entomological investigation, G.C., F.M., S.M.; epidemiological investigation, M.T.P., F.Z., F.R., F.F.; data curation, L.B., G.C., L.L.; writing—original draft preparation, L.B.; figures, L.B., F.M., G.C., writing—review and editing, all authors; supervision, L.B., F.Z., F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Veneto Region and by the European Union's Horizon 2020 research and innovation programme [VEO, grant number 874735].
Conflict of interest: The authors have declared no conflicts of interest.
References
- 1. Barzon L. Ongoing and emerging arbovirus threats in Europe. J Clin Virol 2018; 107:38–47. [DOI] [PubMed] [Google Scholar]
- 2. Young JJ, Haussig JM, Aberle SW et al. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill 2021; 26:2001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pacenti M, Sinigaglia A, Franchin E et al. Human West Nile virus lineage 2 infection: Epidemiological, clinical, and virological findings. Viruses 2020; 12:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pervanidou D, Vakali A, Georgakopoulou T et al. West Nile virus in humans, Greece, 2018: the largest seasonal number of cases, 9 years after its emergence in the country. Euro Surveill 2020; 25:1900543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. García San Miguel Rodríguez-Alarcón L, Fernández-Martínez B, Sierra Moros MJ et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill 2021; 26:2002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pietsch C, Michalski D, Münch J et al. Autochthonous West Nile virus infection outbreak in humans, Leipzig, Germany, August to September 2020. Euro Surveill 2020; 25:2001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vlaskamp DR, Thijsen SF, Reimerink J et al. First autochthonous human West Nile virus infections in the Netherlands, July to August 2020. Euro Surveill 2020; 25:2001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rezza G, Nicoletti L, Angelini R et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 2007; 370:1840–6. [DOI] [PubMed] [Google Scholar]
- 9. Vairo F, Mammone A, Lanini S et al. Local transmission of chikungunya in Rome and the Lazio region. Italy. PLoS One 2018; 13:e0208896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Succo T, Leparc-Goffart I, Ferré J et al. Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Euro Surveill 2016; 21: pii=30240. [DOI] [PubMed] [Google Scholar]
- 11. Gjenero-Margan I, Aleraj B, Krajcar D et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill 2011; 16:19805. [PubMed] [Google Scholar]
- 12. Monge S, García-Ortúzar V, López Hernández B et al. Characterization of the first autochthonous dengue outbreak in Spain (August-September 2018). Acta Trop 2020; 205:105402. [DOI] [PubMed] [Google Scholar]
- 13. Giron S, Franke F, Decoppet A et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill 2019; 24:1900655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gossner C, Ducheyne E, Schaffner F. Increased risk for autochthonous vector-borne infections transmitted by Aedes albopictus in continental Europe. Euro Surveill 2018; 23: pii=1800268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franklinos LHV, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. Lancet Infect Dis 2019; 19:e302–12. [DOI] [PubMed] [Google Scholar]
- 16. Jourdain F, Roiz D, de Valk H et al. From importation to autochthonous transmission: drivers of chikungunya and dengue emergence in a temperate area. PLoS Negl Trop Dis 2020; 14:e0008320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brady O, Wilder-Smith A. What is the impact of lockdowns on dengue? Curr Infect Dis Rep 2021; 23:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazzarini L, Barzon L, Foglia F et al. First autochthonous dengue outbreak in Italy, August 2020. Euro Surveill 2020; 25:2001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gobbi F, Barzon L, Capelli G et al. Surveillance for West Nile, dengue, and chikungunya virus infections, Veneto Region, Italy, 2010. Emerg Infect Dis 2012; 18:671–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santago GA, Vergne E, Quiles Y et al. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis 2013; 7:e2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barzon L, Pacenti M, Franchin E et al. Excretion of West Nile virus in urine during acute infection. J Infect Dis 2013; 208:1086–92. [DOI] [PubMed] [Google Scholar]
- 22. Pacenti M, Sinigaglia A, Martello T et al. Clinical and virological findings in patients with Usutu virus infection, northern Italy, 2018. Euro Surveill 2019; 24:1900180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barzon L, Percivalle E, Pacenti M et al. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin Infect Dis 2018; 66:1173–80. [DOI] [PubMed] [Google Scholar]
- 24. Ravagnan S, Montarsi F, Cazzin S et al. First report outside Eastern Europe of West Nile virus lineage 2 related to the Volgograd 2007 strain, northeastern Italy, 2014. Parasit Vectors 2015; 8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stubbs SCB, Blacklaws BA, Yohan B et al. Assessment of a multiplex PCR and Nanopore-based method for dengue virus sequencing in Indonesia. Virol J 2020; 17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993; 10:512–26. [DOI] [PubMed] [Google Scholar]
- 27. Kumar S, Stecher G, Li M et al. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 2018; 35:1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barzon L, Pacenti M, Franchin E et al. Whole genome sequencing and phylogenetic analysis of West Nile virus lineage 1 and lineage 2 from human cases of infection, Italy, August 2013. Euro Surveill 2013; 18:20591. [DOI] [PubMed] [Google Scholar]
- 29. Barzon L, Papa A, Lavezzo E et al. Phylogenetic characterization of Central/Southern European lineage 2 West Nile virus: analysis of human outbreaks in Italy and Greece, 2013-2014. Clin Microbiol Infect 2015; 21:1122.e1–10. [DOI] [PubMed] [Google Scholar]
- 30. Zhao H, Zhang FC, Zhu Q et al. Epidemiological and virological characterizations of the 2014 dengue outbreak in Guangzhou. China PLoS One 2016; 11:e0156548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma M, Wu S, He Z et al. New genotype invasion of dengue virus serotype 1 drove massive outbreak in Guangzhou. China Parasit Vectors 2021; 14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koo C, Tien WP, Xu H et al. Highly selective transmission success of dengue virus type 1 lineages in a dynamic virus population: an evolutionary and fitness perspective. iScience 2018; 6:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim JT, Chew LZX, Choo ELW et al. Increased dengue transmissions in Singapore attributable to SARS-CoV-2 social distancing measures. J Infect Dis 2021; 223:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lim JT, Dickens BL, Ong J et al. Decreased dengue transmission in migrant worker populations in Singapore attributable to SARS-CoV-2 quarantine measures. J Travel Med 2021; 28:taaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez-Vegas C, Hamer DH, Chen LH et al. Prevalence of dengue virus infection in US travelers who have lived in or traveled to dengue-endemic countries. J Travel Med 2013; 20:352–60. [DOI] [PubMed] [Google Scholar]
- 36. Barzon L, Squarzon L, Cattai M et al. West Nile virus infection in Veneto region, Italy, 2008-2009. Euro Surveill 2009; 14:19289. [DOI] [PubMed] [Google Scholar]
- 37. Barzon L, Pacenti M, Ulbert S, Palù G. Latest developments and challenges in the diagnosis of human West Nile virus infection. Expert Rev Anti Infect Ther 2015; 13:327–42. [DOI] [PubMed] [Google Scholar]
- 38. Sinigaglia A, Pacenti M, Martello T et al. West Nile virus infection in individuals with pre-existing Usutu virus immunity, northern Italy, 2018. Euro Surveill 2019; 24:1900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. La Ruche G, Souares Y, Armengaud A et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill 2010; 15:19676. [PubMed] [Google Scholar]
- 40. Marchand E, Prat C, Jeannin C et al. Autochthonous case of dengue in France, October 2013. Euro Surveill 2013; 18:20661. [DOI] [PubMed] [Google Scholar]
- 41. Gradoni F, Bertola M, Carlin S et al. Geographical data on the occurrence and spreading of invasive Aedes mosquito species in Northeast Italy. Data Brief 2021; 36:107047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ciocchetta S, Prow NA, Darbro JM et al. The new European invader Aedes (Finlaya) koreicus: a potential vector of chikungunya virus. Pathog Glob Health 2018; 112:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect 2013; 19:685–92. [DOI] [PubMed] [Google Scholar]
- 44. Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis 2014; 14:1271–80. [DOI] [PubMed] [Google Scholar]
- 45. Medlock JM, Vaux AG, Cull B et al. Detection of the invasive mosquito species Aedes albopictus in southern England. Lancet Infect Dis 2017; 17:140. [DOI] [PubMed] [Google Scholar]
- 46. Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med 2019; 26:taz062. [DOI] [PubMed] [Google Scholar]
- 47. Neumayr A, Muñoz J, Schunk M et al. Sentinel surveillance of imported dengue via travellers to Europe 2012 to 2014: TropNet data from the DengueTools Research Initiative. Euro Surveill 2017; 22:30433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quam MB, Khan K, Sears J et al. Estimating air travel-associated importations of dengue virus into Italy. J Travel Med 2015; 22:186–93. [DOI] [PubMed] [Google Scholar]
- 49. Redondo-Bravo L, Ruiz-Huerta C, Gomez-Barroso D et al. Imported dengue in Spain: a nationwide analysis with predictive time series analyses. J Travel Med 2019; 26:taz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Colón-González FJ, Sewe MO, Tompkins AM et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health 2021; 5:e404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lillepold K, Rocklöv J, Liu-Helmersson J et al. More arboviral disease outbreaks in continental Europe due to the warming climate? J Travel Med 2019; 26:taz017. [DOI] [PubMed] [Google Scholar]
- 52. Liu-Helmersson J, Brännström Å, Sewe MO et al. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front Public Health 2019; 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Venturi G, Di Luca M, Fortuna C et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill 2017; 22:17–00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Massad E, Amaku M, Coutinho FAB et al. Estimating the probability of dengue virus introduction and secondary autochthonous cases in Europe. Sci Rep 2018; 8:4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vermeulen TD, Reimerink J, Reusken C et al. Autochthonous dengue in two Dutch tourists visiting Département Var, southern France, July 2020. Euro Surveill 2020; 25:2001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med 2020; 27:taaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


