Abstract
Background
The Adaptive Coronavirus Disease 2019 (COVID-19) Treatment Trial-1 (ACTT-1) found that remdesivir therapy hastened recovery in patients hospitalized with COVID-19, but the pathway for this improvement was not explored. We investigated how the dynamics of clinical progression changed along 4 pathways: recovery, improvement in respiratory therapy requirement, deterioration in respiratory therapy requirement, and death.
Methods
We analyzed trajectories of daily ordinal severity scores reflecting oxygen requirements of 1051 patients hospitalized with COVID-19 who participated in ACTT-1. We developed competing risks models that estimate the effect of remdesivir therapy on cumulative incidence of clinical improvement and deterioration, and multistate models that utilize the entirety of each patient’s clinical course to characterize the effect of remdesivir on progression along the 4 pathways above.
Results
Based on a competing risks analysis, remdesivir reduced clinical deterioration (hazard ratio [HR], 0.73; 95% confidence interval [CI]: .59–.91) and increased clinical improvement (HR, 1.22; 95% CI: 1.08, 1.39) relative to baseline. Our multistate models indicate that remdesivir inhibits worsening to ordinal scores of greater clinical severity among patients on room air or low-flow oxygen (HR, 0.74; 95% CI: .57–.94) and among patients receiving mechanical ventilation or high-flow oxygen/noninvasive positive-pressure ventilation (HR, 0.73; 95% CI: .53–1.00) at baseline. We also find that remdesivir reduces expected intensive care respiratory therapy utilization among patients not mechanically ventilated at baseline.
Conclusions
Remdesivir speeds time to recovery by preventing worsening to clinical states that would extend the course of hospitalization and increase intensive respiratory support, thereby reducing the overall demand for hospital care.
Keywords: clinical progression, critical care, multistate models, respiratory therapies
Remdesivir altered dynamics of clinical progression and sped recovery in hospitalized coronavirus disease 2019 (COVID-2019) patients in Adaptive COVID-19 Treatment Trial-1 (ACTT-1) by preventing clinical deterioration and reducing demand for supplemental oxygen and other respiratory therapies. Remdesivir may therefore improve outcomes and alleviate healthcare system capacity strain.
In response to the urgent need for therapeutic agents to treat coronavirus disease 2019 (COVID-19), the Adaptive COVID-19 Treatment Trial (ACTT-1) tested whether the antiviral agent remdesivir was effective in speeding recovery among hospitalized patients [1]. The primary analysis found that patients treated with remdesivir had faster time to recovery: a median of 10 days (95% confidence interval [CI]: 9–11) versus 15 days (95% CI, 13–18) for those given placebo (hazard ratio [HR] for recovery, 1.29; 95% CI: 1.12–1.49). Secondary analyses highlighted that new use of supportive respiratory therapies—low-flow oxygen, high-flow oxygen and noninvasive positive-pressure ventilation (NIPPV), and mechanical ventilation—was lower among patients treated with remdesivir. Understanding how remdesivir therapy changed the course of hospitalization and led patients to recover faster would provide important context for remdesivir treatment guidelines, which often stratify recommendations using the ACTT ordinal scale.
The secondary analysis presented in this manuscript leverages the temporally dense data from ACTT-1 to evaluate the effect of remdesivir on the clinical dynamics of hospitalized COVID-19 patients. We develop competing risks models to estimate the treatment effect on the cumulative incidence of patients who improved or worsened relative to baseline, and present descriptive analyses and multistate models (MSMs) that capture each patient’s clinical course, including intercurrent events, along 4 aspects of clinical progression: recovery, improvement leading to de-escalation of respiratory therapy, deterioration leading to escalation of respiratory therapy, and death. We find that remdesivir resulted in a more direct path to recovery and prevented clinical deterioration that would extend hospitalization and possibly require critical care respiratory therapy. Hospital capacity strain [2], particularly intensive care unit (ICU) capacity strain during the severe acute respiratory syndrome coronavirus 2 pandemic [3], has been shown to be associated with increased mortality and worse clinical outcomes. Therefore, our results have implications for healthcare leaders and policy makers tasked with resource allocation and hospital management.
METHODS
Definitions
Patients in ACTT-1 were assessed daily while hospitalized using an 8-category ordinal score (OS) scale indicating their worst clinical status during the prior 24 hours (Supplementary Figures 1 and 2). The categories are as follows: (1) not hospitalized and no limitations of activities; (2) not hospitalized, with limitation of activities, home oxygen requirement, or both; (3) hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalization was extended for infection-control or other nonmedical reasons); (4) hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related to COVID-19 or to other medical conditions); (5) hospitalized, requiring any supplemental oxygen; (6) hospitalized, requiring NIPPV or high-flow oxygen devices; (7,) hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; and (8) death. Patients in OS 1, 2, and 3 were considered “recovered.” Patients in OS 4 and 5 were generally treated outside ICU settings and, hence, categorized as receiving “non-ICU respiratory therapies” (although OS 4 patients did not receive respiratory therapy). Patients in OS 6 and 7 were generally treated within an ICU and were categorized as receiving “ICU respiratory therapies” (although treatment with high-flow oxygen was sometimes administered outside of an ICU).
Data Description
Supplementary Table 3 summarizes demographic characteristics of the 1062 patients in the ACTT-1 primary analysis [1]. We excluded 11 patients without a baseline ordinal score, leaving 1051 patients for this analysis. We limited data to each patient’s initial course of hospitalization, excluding post-discharge observations from normal study follow-up or hospital readmission, resulting in 155 patients with at least 1 timepoint removed.
Table 1 summarizes outcomes among ACTT-1 participants and characterizes clinical improvement and deterioration in the course of hospitalization, which are defined, respectively, as reaching a lower or higher severity ordinal score than baseline, irrespective of interim or subsequent clinical progression. Table 2 summarize the initial clinical progression among study participants, grouping their observed sequences of ordinal scores by their first observed transition—improvement or deterioration—and first 2 transitions. In summarizing the first 2 transitions, sustained improvement or recovery is improvement followed by no change or additional improvement (which for some patients is recovery); transient improvement is improvement followed by deterioration; transient deterioration is deterioration followed by improvement; and sustained deterioration or death is defined as deterioration followed by either no change or additional deterioration (which for some patients is death). Supplementary Figures 6 and 7 display initial progression and final outcomes as flows between ordinal scores.
Table 1.
Patient Outcomes and Dynamics of Clinical Progression
| Baseline Ordinal Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Room Air [4] | Supplemental Oxygen [5] | Noninvasive Ventilation/ High-flow Oxygen [6] | Invasive Ventilation [7] | ||||||
| Remdesivir | Placebo | Remdesivir | Placebo | Remdesivir | Placebo | Remdesivir | Placebo | Remdesivir | Placebo | |
| (N = 533) | (N = 518) | (N = 75) | (N = 63) | (N = 232) | (N = 203) | (N = 95) | (N = 98) | (N = 131) | (N = 154) | |
| Recovery | ||||||||||
| No. recovered (%) | 395 (74.1%) | 349 (67.4%) | 73 (97.3%) | 58 (92.1%) | 204 (87.9%) | 155 (76.4%) | 56 (58.9%) | 61 (62.2%) | 62 (47.3%) | 75 (48.7%) |
| HR (95% CI) | 1.26 (1.10, 1.45) | 1.23 (.87, 1.74) | 1.46 (1.20, 1.79) | 1.08 (.76, 1.52) | 1.00 (.73, 1.38) | |||||
| Any improvement relative to baseline | ||||||||||
| No. ever improved (%) | 444 (83.3%) | 404 (78.0%) | Equivalent to recovery | 207 (89.2%) | 165 (81.3%) | 73 (76.8%) | 78 (79.6%) | 91 (69.5%) | 103 (66.9%) | |
| HR (95% CI) | 1.22 (1.08, 1.39) | 1.38 (1.13, 1.68) | 1.10 (.83, 1.47) | 1.05 (.81, 1.35) | ||||||
| Any deterioration relative to baseline | ||||||||||
| No. ever worsened (%) | 143 (26.8%) | 176 (34.0%) | 23 (30.7%) | 25 (39.7%) | 58 (25.0%) | 76 (37.4%) | 35 (36.8%) | 46 (46.9%) | Equivalent to death | |
| HR (95% CI) | 0.73 (.59, .91) | 0.75 (.42, 1.31) | 0.61 (.44, .85) | 0.74 (.48, 1.14) | ||||||
| Death | ||||||||||
| No. died (%) | 56 (10.5%) | 71 (13.7%) | 2 (2.7%) | 2 (3.2%) | 9 (3.9%) | 21 (10.3%) | 18 (18.9%) | 19 (19.4%) | 27 (20.6%) | 29 (18.8%) |
| HR (95% CI) | 0.82 (.58, 1.16) | 0.84 (.12, 5.95) | 0.36 (.16, .77) | 1.02 (.54, 1.90) | 1.09 (.66, 1.83) |
Summary of overall outcomes among patients by treatment arm and baseline ordinal score. Any clinical improvement (deterioration) relative to baseline describes the clinical course of a patient and is counted as the patient ever reaching a less (more) severe clinical state than their status at baseline, irrespective of their interim or subsequent clinical progression. Subdistribution hazard ratios are estimated from competing risks regression models, as described in Fine and Gray [4], treating discharge and death as competing events. Overall estimates were obtained from competing risks models stratified by baseline severity score, whereas group-specific estimates were obtained from models fit separately to participants in each ordinal score group.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 2.
Initial Clinical Progression
| Baseline Ordinal Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Room Air [4] | Supplemental Oxygen [5] | Noninvasive Ventilation/ High-flow Oxygen [6] | Invasive Ventilation [7] | ||||||
| Remdesivir | Placebo | Remdesivir | Placebo | Remdesivir | Placebo | Remdesivir | Placebo | Remdesivir | Placebo | |
| (N = 533) | (N = 518) | (N = 75) | (N = 63) | (N = 232) | (N = 203) | (N = 95) | (N = 98) | (N = 131) | (N = 154) | |
| First transition | ||||||||||
| Improvement | 374 (70%) | 323 (62%) | 52 (69%) | 38 (60%) | 170 (73%) | 124 (61%) | 61 (64%) | 58 (59%) | 91 (69%) | 103 (67%) |
| Deterioration | 133 (25%) | 166 (32%) | 23 (31%) | 25 (40%) | 58 (25%) | 75 (37%) | 30 (32%) | 40 (41%) | 22 (17%) | 26 (17%) |
| No change | 26 (5%) | 29 (6%) | 0 (0%) | 0 (0%) | 4 (2%) | 4 (2%) | 4 (4%) | 0 (0%) | 18 (14%) | 25 (16%) |
| First 2 transitions | ||||||||||
| Sustained improvement or recovery | 325 (61%) | 264 (51%) | 52 (69%) | 38 (60%) | 157 (68%) | 107 (53%) | 44 (46%) | 46 (47%) | 72 (55%) | 73 (47%) |
| Transient improvement | 49 (9%) | 59 (11%) | Not applicable | 13 (6%) | 17 (8%) | 17 (18%) | 12 (12%) | 19 (15%) | 30 (19%) | |
| Transient deterioration | 80 (15%) | 81 (16%) | 20 (27%) | 18 (29%) | 46 (20%) | 40 (20%) | 14 (15%) | 23 (23%) | Not applicable | |
| Sustained deterioration or death | 53 (10%) | 85 (16%) | 3 (4%) | 7 (11%) | 12 (5%) | 35 (17%) | 16 (17%) | 17 (17%) | 22 (17%) | 26 (17%) |
Summary of patients’ first ordinal score transition and first two transitions. For the first transition, “improvement” (“deterioration”) is defined as being in a better (worse) ordinal score than baseline. For the first two transitions, “sustained improvement or recovery” is defined as having a first transition in the direction of improvement on the ordinal score followed by either no change or a second transition in the direction of improvement (which for some patients indicates recovery); “transient improvement” is defined as having a first transition in the direction of improvement followed by a second transition in the direction of deterioration; “transient deterioration” is defined as having a first transition in the direction of deterioration followed by a second transition in the direction of improvement; and “sustained deterioration or death” is defined as having a first transition in the direction of deterioration followed by either no change or a second transition in the direction of deterioration (which for some patients indicates death).
Competing Risks Models for Incidence of Key Events
We assess the effect of remdesivir treatment on cumulative incidence of recovery, clinical improvement relative to baseline, clinical deterioration relative to baseline, and death. The subdistribution hazard ratio for each of these events relates the relative hazard among participants who have not yet experienced an event to the change in cumulative incidence of that event and is estimated from 1 of 4 Fine-Gray competing risks models, treating death and recovery as competing events [4–6]. Models reporting overall estimates were stratified by baseline OS, although models for OS-specific effects were fit separately to data from each baseline OS group. Clinical improvement and deterioration relative to baseline characterize a patient’s clinical path and are not exclusive of each other or of death and recovery. The competing risks analyses consider only whether a patient experienced a particular event and each patient only contributes one observation per model.
Multistate Models for Clinical Progression
We analyze each patient’s clinical course using a time-inhomogeneous multistate Markov model that describes progression through the ACTT-1 ordinal scale. The MSM structure (Figure 1B) determines the states between which a patient may transition directly and reflects the dynamics of disease and clinical practice during ACTT-1. For instance, the model assumes that a patient on room air (OS 4) or receiving low-flow oxygen (OS 5) may be discharged, but a patient receiving NIPPV (OS 6) or invasive ventilation (OS 7) would receive nonintensive therapies prior to discharge. Note that OS transitions occur continuously throughout hospitalization, but the data are daily snapshots of clinical status. Hence, a patient may be observed transitioning from room air to ventilation if multiple transitions occur within a day. Discharge is treated as an absorbing state because we only analyze each patient’s initial hospitalization. The model is fit separately to patients who received non-ICU respiratory therapies (OS 4 and OS 5) or ICU respiratory therapies (OS 6 and OS 7) at baseline using the msm package in R version 4.0.3 [7, 8].
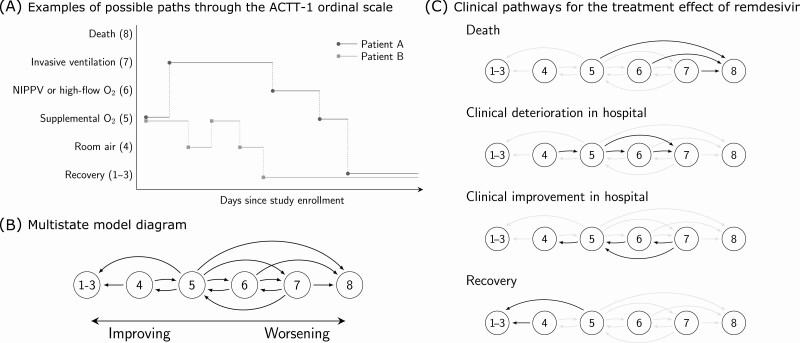
Figure 1.
Multistate model for clinical progression for patients enrolled in the Adaptive COVID-19 Treatment Trial-1 (ACTT-1). A, Examples of possible paths through the ACTT-1 ordinal score (OS) scale. Both patients A and B are on supplemental oxygen (OS 5) at baseline. A standard time-to-event analysis assesses whether treatment with remdesivir shortens the expected time until the patients enter the recovered state (OS 1–3). Multistate analysis assesses whether treatment with remdesivir alters the dynamics of how patients travel throughout the ordinal scale over the course of the study. B, Multistate model diagram. Patients transition between states continuously in time. Arrows indicate which direct transitions are possible. For example, a patient starting on room air may transition to discharge or supplemental oxygen. However, the model assumes that a patient on room air would not be intubated without first receiving supplemental oxygen, whether “observed” or not from the perspective of data capturing. Note that the data are daily snapshots of each patient’s status and that multiple transitions are possible within the same day. C, Clinical pathways for the treatment effect of remdesivir. Hazard ratio for remdesivir versus placebo is assumed to be common to all transitions within each transition group. For instance, we estimate that remdesivir slows down the rate of clinical deterioration within the hospital by a relative 26% (95% CI: 6%–43%) and that this effect applies to worsening from room air to supplemental oxygen (OS 4–5), supplemental oxygen to noninvasive positive-pressure ventilation (OS 5–6), or supplemental oxygen to invasive ventilation (OS 5–7). Sensitivity to groupings of transitions is explored in the supplement and the results are shown to be robust to how transitions are grouped. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; NIPPV, noninvasive positive-pressure ventilation.
We estimate the treatment effect of remdesivir along 4 clinical pathways defined by grouping related OS transitions (Figure 1C): recovery or cessation of hospital-based therapy, improvement leading to reduction in respiratory therapy requirements, deterioration leading to increase in respiratory therapy requirements, and death. In defining these pathways, we allow for the effect on transitions between levels of hospital-based respiratory therapy to differ from the effect on transitions leading to cessation of hospital-based therapy. The treatment effect is common to all transitions within each pathway and is interpreted as a common hazard ratio. We quantify uncertainty about common hazard ratios using bootstrap confidence intervals and obtain P-values via a rerandomization procedure that permutes treatment labels by baseline ordinal score. The Supplementary Appendix presents technical details about the model formulation and estimation procedures, along with sensitivity analyses establishing the robustness of our analysis to separately fitting the model in different baseline ordinal score groups, to alternative definitions of treatment effects, to model structures that aggregate various ordinal scores, and to changepoints in transition intensities.
RESULTS
Data Description and Competing Risks Analysis for the Incidence of Key Events
Our reanalysis of recovery and death (Table 1) confirms the benefit on recovery and inconclusive result on mortality reported in the primary ACTT-1 manuscript, with slight differences due to the use of OS, rather than baseline status, in stratification and minor changes in the data set. In addition, more patients treated with remdesivir reached a less severe OS than their baseline compared with placebo patients (83.3% vs 78.0%; HR, 1.22; 95% CI: 1.08–1.39), although fewer reached a more severe OS (36.8% vs 46.9%; HR 0.73; 95% CI: .59–.91). The reduction in patients who reached an OS worse than baseline was greatest among patients in OS 5 (25.0% vs 37.4%; HR 0.61; 95% CI: .44–.85).
Patients treated with remdesivir took a more direct path toward improvement compared with patients given placebo (Table 2 and Supplementary Figures 6 and 7). The first state transition among remdesivir patients more often resulted in clinical improvement (70% vs 62%) and less often in clinical deterioration (25% vs 32%). The decrease in initial deterioration on the remdesivir arm was particularly evident among patients who were not mechanically ventilated at baseline (OS 4, 31% versus 40%; OS 5, 25% vs 37%; OS 6, 32% vs 41%). The first two transitions were more often consistent with sustained improvement or recovery (61% vs 51%) and less often with sustained deterioration or death (10% vs 16%) among remdesivir patients. The contrast in transitions to more intensive respiratory therapies early in the clinical course is visually apparent in Supplementary Figures 6 and 7.
Remdesivir Treatment Compared to Placebo Reduces the Rate of Clinical Deterioration
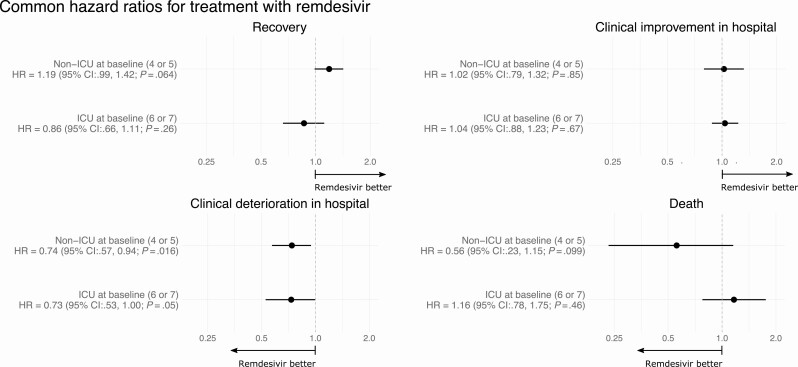
When examining the full trajectory of transitions between states using MSMs, we find that the rate of clinical deterioration within the hospital was lower among patients treated with remdesivir than those given placebo (Figure 2). Similar reductions in the rates of clinical deterioration within the hospital were estimated among patients receiving non-ICU respiratory therapies (HR, 0.74; 95% CI: .57–.94; P, .016) and ICU respiratory therapies (HR, 0.73; 95% CI: .53–1.00; P, .05) at baseline. We do not find evidence of a treatment effect on clinical improvement within the hospital. Although not statistically significant, the transition intensities leading directly to recovery were higher in the remdesivir arm than the placebo arm for patients receiving non-ICU respiratory therapies at baseline (HR, 1.19; 95% CI: .99–1.42; P = .064), and the intensities of transitions directly to death were lower (HR, 0.56; 95% CI: .23–1.15; P = .099). We do not find a similar pattern suggesting a multifaceted benefit among patients receiving ICU respiratory therapies at baseline.
Figure 2.
Effects of remdesivir on clinical progression. Common hazard ratios for transitions in each of the 4 clinical pathways diagrammed in Figure 1C. Separate models were fit to data from patients who received nonintensive therapies outside the ICU setting (“non-ICU”) at baseline and those receiving noninvasive positive-pressure ventilation or invasive ventilation (“ICU”) at baseline. For each hazard ratio (HR), bootstrap CIs, and P-values were obtained via a rerandomization procedure. Abbreviations: CI, confidence interval; ICU, intensive care unit.
Impact of Remdesivir Treatment in Patients Not Requiring ICU Respiratory Therapy at Baseline
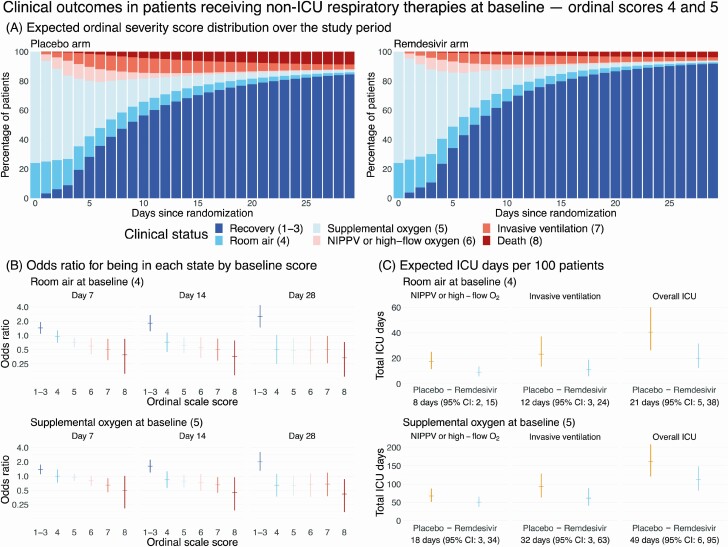
The consequence of a lower rate of clinical deterioration within the hospital is a shorter course of hospitalization and a lower probability of requiring ICU respiratory therapies. Figure 3A shows the MSM point estimates of the expected ordinal scale distribution by treatment arm for patients receiving non-ICU respiratory therapies at baseline (OS 4 and 5). The expected percentages of patients in OS 6 to 8 (ICU states; orange and red bars) are higher in the placebo arm throughout the study period, whereas recoveries (dark blue bars) accrue faster among patients treated with remdesivir. At 1-week post-randomization, baseline OS 4 and 5 patients on remdesivir have better odds of being in improved states (Figure 3B; detailed results in Supplementary Table 5). This improvement in the overall odds of recovery and death persists throughout the study period, suggesting that remdesivir does not merely delay the inevitable.
Figure 3.
Clinical impact of remdesivir in patients receiving non-ICU respiratory therapies at baseline ordinal scores 4 and 5. A, Stacked probability plots showing the expected distribution of ordinal severity scores by treatment arm at each day post-randomization among patients receiving nonintensive therapies outside the ICU setting at baseline, assuming the initial distribution of baseline ordinal score 4 and 5 patients is the same as was observed in the Adaptive Clinical Treatment Trial-1. B, Ratio of odds that a patient on room air (top row) or supplemental oxygen (bottom row) at baseline will be in each ordinal score on remdesivir versus placebo at days 7, 14, and 28 post-randomization. Each odds ratio is shown with a 95% bootstrap CI. C, Expected number of ICU days per 100 patients by baseline ordinal score. Each plot shows the total expected number of days per 100 patients on each arm along with 95% bootstrap CIs. Below each panel is the estimated difference in ICU days per 100 patients (placebo minus remdesivir). Abbreviations: CI, confidence interval; ICU, intensive care unit; NIPPV, noninvasive positive-pressure ventilation.
The area of each state in the stacked probability plot corresponds to the expected total resource utilization for the clinical course of COVID-19 at the population level, conditional on the initial distribution of OS 4 and 5 patients. Based on this model, we expect that remdesivir treatment would result in fewer patients worsening to ICU-level care, reducing expected ICU resource utilization (Figure 3C). Our model estimates that treatment with remdesivir results in an expected savings of 21 ICU therapy days (95% CI: 5–38 days) per 100 patients admitted on room air (OS 4) at baseline, and a savings of 49 ICU therapy days (95% CI: 6–95 days) per 100 patients initially on supplemental oxygen (OS 5).
Impact of Remdesivir Treatment in Patients Requiring ICU Respiratory Therapies at Baseline
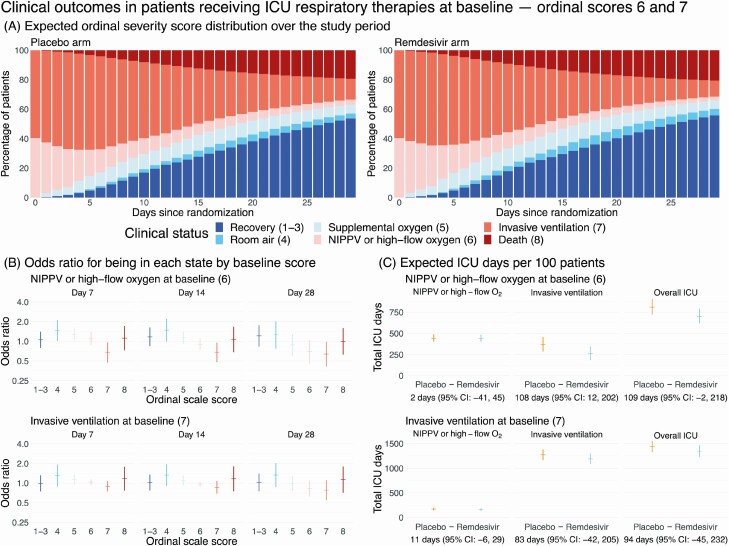
Figure 4A shows point estimates of the expected ordinal scale distribution by treatment arm for patients receiving ICU respiratory therapies at baseline (OS 6 and 7). The area of the invasive ventilation state (dark orange bars) is smaller in the remdesivir arm than in the placebo arm, although differences in the point estimates are difficult to discern visually. The odds of a patient treated with high-flow oxygen or NIPPV at baseline (OS 6) receiving mechanical ventilation at days 7, 14, and 28 are lower on remdesivir (Figure 4B; detailed results in Supplementary Table 5). We estimate an expected savings of 108 ventilation days (95% CI: 12–202 days) per 100 patients treated with remdesivir admitted on OS 6 at baseline (Figure 4C). However, treatment with remdesivir does not have a statistically significant effect on the expected utilization of NIPPV or high-flow oxygen among patients in OS 6 at baseline or on expected ICU resource utilization among patients on invasive ventilation at baseline.
Figure 4.
Clinical impact of remdesivir in patients receiving ICU respiratory therapies at baseline ordinal scores 6 and 7. A, Stacked probability plots showing the expected distribution of ordinal severity scores by treatment arm at each day post-randomization among patients receiving ICU respiratory therapies at baseline, assuming the initial distribution of baseline ordinal scores 6 and 7 patients is the same as was observed in the Adaptive Clinical Treatment Trial-1. B, Ratio of the odds that a patient on NIPPV (top row) or invasive ventilation (bottom row) at baseline will be in each ordinal score state on remdesivir versus placebo at days 7, 14, and 28 post-randomization. Each odds ratio is shown with a 95% bootstrap confidence interval. C, Expected number of ICU days per 100 patients by baseline ordinal score. Each plot shows the total expected number of days per 100 patients on each arm along with 95% bootstrap CIs. Below each panel is the estimated difference in ICU days per 100 patients (placebo minus remdesivir). Abbreviations: CI, confidence interval; ICU, intensive care unit; NIPPV, noninvasive positive-pressure ventilation.
DISCUSSION
The granularity of data collected in ACTT-1 enabled us to assess how remdesivir therapy affects distinct pathways for clinical progression. Multistate models have been leveraged in observational settings to gain critical insight into the clinical progression of hospitalized COVID-19 patients [9]. By using data from a randomized clinical trial, we were able to identify a distinct clinical pathway through which remdesivir led to faster recovery: patients treated with remdesivir were discharged sooner primarily because they tended not to worsen during hospitalization. This reduced treatment with supplemental oxygen and critical care therapies.
We hypothesize that recovery from COVID-19 depends more on the host’s immune system than on antiviral therapy, and that sustained viral suppression due to remdesivir is insufficient to reverse the inflammatory cascade associated with progressed disease. Recovery from COVID-19 may depend on both the prompt use of antivirals and modulation of the host immune system. Our analysis suggests that the primary pathway through which remdesivir alters the dynamics of clinical progression is by preventing respiratory deterioration. This may explain why patients who are later in their disease course, for example, on mechanical ventilation, experience less benefit from remdesivir therapy. Thus, an effective model for therapy may combine an intervention to prevent clinical decline with another to aid in recovery. For instance, the combination of baricitinib, a Janus kinase inhibitor, and remdesivir was superior to remdesivir monotherapy [10]: remdesivir may retard clinical decline, whereas baricitinib refines the immune response. To this point, a recent multistate analysis of a French database of patients with COVID-19 concluded that IL-6 antagonists, but not steroids, increased the probability of a patient being successfully extubated [11]. The model of one intervention preventing clinical decline and another aiding in recovery may also be the basis for combination therapy with steroids and sulfamethoxazole/trimethoprim to treat patients with AIDS and Pneumocystis jiroveci pneumonia [12].
Inpatient treatment for COVID-19 has presented unprecedented challenges to modern health care systems with many facilities episodically functioning at or above their nominal capacity. Historically, hospital capacity strain has been shown to be associated with worse clinical outcomes [2], and there is evidence that clinical outcomes for patients with COVID-19 have also been worse [3]. The major determinants of ICU capacity for a given hospital are staff, beds, ventilators, and—in many hospitals—high-flow oxygen and NIPPV ventilation devices. Multiple strategies to maintain or expand ICU bed availability have been employed during the pandemic with limited success, including postponing elective surgeries, building field hospitals, and deploying new and retired nursing staff [13]. The results of ACTT-1 showed that hospitalized COVID-19 patients who received remdesivir were ready for discharge sooner than patients receiving placebo [1]. Our models suggest that remdesivir treatment may provide additional benefits at the population level by reducing the need for oftentimes scarce ICU resources.
Clinical practice and COVID-19 treatment have evolved since the time of ACTT-1. Although we maintain that remdesivir therapy may benefit ICU resource utilization in the current clinical environment, changes in clinical practice and the prevalence of other therapies, such as dexamethasone and baricitinib, make it difficult to extrapolate the expected savings in resource utilization today. For example, our analysis regarded high-flow oxygenation therapy as an ICU-based treatment. Although high-flow nasal cannula oxygenation therapy has traditionally been limited to the ICU or intermediate care units [14], its use has expanded to some general wards, especially when ICU capacity was limited. Nonetheless, high-flow oxygenation must be administered by dedicated devices and managed by trained clinicians, representing potential resource limitations. We also acknowledge that intubation practices have changed, as early intubation was more prominent in the first few months of the pandemic when ACTT-1 was conducted [15]. It is uncertain whether or how more recent intubation practices affect the transportability of our estimates.
The results of this study have implications for COVID-19 clinical care and treatment guidelines that make recommendations about remdesivir therapy based on the ordinal scale used in ACTT. Currently, the World Health Organization does not recommend the use of remdesivir in any patients with COVID-19, and the National Institutes of Health does not advocate for or against the routine use of remdesivir in patients who are hospitalized but not requiring supplemental oxygen. Our analysis suggests that healthcare systems may benefit from reduced ICU strain if hospitalized patients not requiring supplemental oxygen are treated with remdesivir.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank Dean Follmann, PhD, Michael Proschan, PhD, Erica Brittain, PhD, and Gail Potter, PhD, for thoughtful suggestions that improved the analysis, Katelyn Le, MS, Sophia Charuhas, MA, and Stacy Kopka, MS, for help in preparing the manuscript for submission, and Alyssa La Regina for administrative support.
Financial support. This analysis used data from the Adaptive COVID-19 Treatment Trial (ACTT-1) trial (DOI:10.1056/NEJMoa2007764). The ACTT-1 trial was sponsored and primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Maryland. ACTT-1 was funded in part with federal funds from the NIAID and the National Cancer Institute, NIH, under contract HHSN261200800001E 75N910D00024, task order number 75N91019F00130/75N91020F00010, and by the Department of Defense, Defense Health Program. This trial has been supported in part by the NIAID of the NIH under award numbers UM1AI148684, UM1AI148576, UM1AI148573, UM1AI148575, UM1AI148452, UM1AI148685, UM1AI148450, and UM1AI148689. The trial has also been funded in part by the governments of Denmark, Japan, Mexico, and Singapore. The trial site in South Korea received funding from the Seoul National University Hospital. Support for the London International Coordinating Centre was also provided by the United Kingdom Medical Research Council (MRC_UU_12023/23). Collaborating investigators involved with collection of data during the ACTT-1 trial are noted in the Supplementary Appendix. The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institutes of Health or the Department of Health and Human Services, Walter Reed National Military Medical Center, the Department of Defense, or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. This work utilized the computational resources of the NIH HPC Biowulf computing cluster (http://hpc.nih.gov).
Potential conflicts of interest. N. H. reports owning less than $25 000 in Gilead stock from their wife’s prior job with company. P. T. reports being an investigator in the study and receiving funding from the NIH. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Jonathan Fintzi, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, Rockville, Maryland, USA.
Tyler Bonnett, Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research, Frederick, Maryland, USA.
Daniel A Sweeney, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of California, San Diego, La Jolla, California, USA.
Nikhil A Huprikar, Pulmonary and Critical Care Service, Department of Medicine, Walter Reed National Military Medical Center, Bethesda, Maryland, USA.
Anuradha Ganesan, Walter Reed National Military Medical Center, Infectious Disease Clinical Research Program, Department of Preventative Medicine and Biostatistics, Uniformed Services University of Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Maria G Frank, Department of Medicine, Denver Health Hospital Authority, Associate Professor of Medicine, University of Colorado School of Medicine, Aurora, Colorado, USA.
Susan L F McLellan, Division of Infectious Disease, University of Texas Medical Branch, Galveston, Texas, USA.
Lori E Dodd, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, Rockville, Maryland, USA.
Pablo Tebas, Division of Infectious Diseases/Clinical Trials Unit, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Aneesh K Mehta, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
References
- 1. Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. . Remdesivir for the treatment of Covid-19: final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guise JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med 2017; 32:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bravata DM, Perkins AJ, Myers LJ, et al. . Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs Hospitals during the COVID-19 pandemic. JAMA Netw Open 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 5. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36:4391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Therneau T. A package for survival analysis in R. R package version 3.2-7. 2020. [Google Scholar]
- 7. Jackson C. Multi-state models for panel data: the msm package for R. J Stat Softw 2011; 38:1–29. [Google Scholar]
- 8. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 9. Mody A, Lyons PG, Vazquez Guillamet C, et al. . The clinical course of coronavirus disease 2019 in a US hospital system: a multistate analysis. Am J Epidemiol 2021; 190:539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members. . Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021; 384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ursino M, Dupuis C, Buetti N, et al. . Multistate modeling of COVID-19 patients using a large multicentric prospective cohort of critically ill patients. J Clin Med 2021; 10:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medina I, Mills J, Leoung G, et al. . Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med 1990; 323:776–82. [DOI] [PubMed] [Google Scholar]
- 13. McCabe R, Schmit N, Christen P, et al. . Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med 2020; 18:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015; 148:253–61. [DOI] [PubMed] [Google Scholar]
- 15. Matta A, Chaudhary S, Bryan Lo K, et al. . Timing of intubation and its implications on outcomes in critically ill patients with coronavirus disease 2019 infection. Crit Care Explor 2020; 2:e0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.