Abstract
Aims
Myocardial injury (MINJ) in Coronavirus disease 2019 (COVID-19) identifies individuals at high mortality risk but its clinical relevance is less well established for Influenza and no comparative analyses evaluating frequency and clinical implications of MINJ among hospitalized patients with Influenza or COVID-19 are available.
Methods and results
Hospitalized adults with laboratory confirmed Influenza A or B or COVID-19 underwent highly sensitive cardiac T Troponin (hs-cTnT) measurement at admission in four regional hospitals in Canton Ticino, Switzerland. MINJ was defined as hs-cTnT >14 ng/L. Clinical, laboratory and outcome data were retrospectively collected. The primary outcome was mortality up to 28 days. Cox regression models were used to assess correlations between admission diagnosis, MINJ, and mortality. Clinical correlates of MINJ in both viral diseases were also identified. MINJ occurred in 94 (65.5%) out of 145 patients hospitalized for Influenza and 216 (47.8%) out of 452 patients hospitalized for COVID-19. Advanced age and renal impairment were factors associated with MINJ in both diseases. At 28 days, 7 (4.8%) deaths occurred among Influenza and 76 deaths (16.8%) among COVID-19 patients with a hazard ratio (HR) of 3.69 [95% confidence interval (CI) 1.70–8.00]. Adjusted Cox regression models showed admission diagnosis of COVID-19 [HR 6.41 (95% CI 4.05–10.14)] and MINJ [HR 8.01 (95% CI 4.64–13.82)] to be associated with mortality.
Conclusions
Myocardial injury is frequent among both viral diseases and increases the risk of death in both COVID-19 and Influenza. The absolute risk of death is considerably higher in patients admitted for COVID-19 when compared with Influenza.
Keywords: Influenza, COVID-19, Myocardial injury, Troponin, Mortality
Graphical Abstract
Graphical Abstract.
Introduction
Coronavirus disease 2019 (COVID-19) and Influenza are associated to various clinical manifestations ranging from mild flu-like symptoms to severe respiratory distress syndromes,1–3 whereas advanced age and pre-existing cardiovascular diseases have been identified as predisposing factors for mortality in both viral diseases.4–8
The occurrence of myocardial injury (MINJ) has been previously recognized as prognostic marker in hospitalized patients affected by different respiratory illnesses such as pneumonia, chronic obstructive pulmonary disease (COPD), sepsis, and acute respiratory distress syndrome (ARDS).9–11 MINJ similarly occurs in COVID-19 patients, and while its mechanism is poorly understood,12,13 studies have confirmed its negative prognostic role.12,14–17
Several similarities and differences have been drawn between Influenza and COVID-19 both from clinical and prognostic perspectives.18
Influenza and COVID-19 are both contagious respiratory viral illnesses with a relevant virulence able to cause pandemic events. Both clinical entities have been often compared on a clinical ground,19 and initial autoptic comparative studies have highlighted how specific pathophysiological mechanisms are shared between the two viral infections.20 One of the multiple aspects linking the severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and Influenza viruses is represented by the peculiar endothelial involvement leading to microvascular thrombosis and neo-angiogenesis,20 which might represent the mechanisms behind MINJ.
Thus, comparisons between COVID-19 and other viral disease might provide clinically relevant information confirming the hypothesis that presence of MINJ has a prognostic relevance in different viral diseases. It is in fact unknown whether MINJ occurs in COVID-19 with similar rates also in other viral respiratory diseases such as Influenza21–24 and no study has assessed the comparative prognostic role of MINJ for mortality in COVID-19 and Influenza patients.
Thus, the aim of the following analysis is to provide comparative frequency rates and prognostic impact of MINJ in hospitalized patients with Influenza and COVID-19 diseases.
Methods
Study design and objectives
During the 2018–2019 seasonal epidemic, a multicentre prospective cohort study was conducted to enroll consecutive adult patients (>18 years) hospitalized for Influenza between December 2018 and 31 March 2019 in the four regional hospitals (Lugano, Bellinzona e Valli, Locarno, and Mendrisio) in Canton Ticino, Switzerland with the main objective to assess the frequency and prognosis of patients with Influenza-associated MINJ defined as an increased high-sensitivity cardiac Troponin T (hs-cTnT) as measured within 48 h from admission. At the outbreak of the SARS-COV-2 pandemic in Ticino, we set up a retrospective cohort study, which closely mirrored the protocol of the prospective cohort study in Influenza patients in the same four hospitals, including patients hospitalized for COVID-19 between 24 February 2020 and 11 May 2020. Shortly after the initial pandemic wave, the four hospitals mandated the assessment of hs-cTnT within 48 h from admission as part of their standard procedure. The objectives of the retrospective cohort study were to assess the frequency and prognosis of patients with COVID-19 associated MINJ, and to compare frequency and prognosis between Influenza and COVID-19.
Patients
Consecutive patients hospitalized for laboratory-confirmed Influenza infection, based on reverse transcription polymerase chain positive reaction for Influenza A/B detection on nasopharyngeal swab, were prospectively included during the 2018–2019 Influenza epidemic. Consecutive patients hospitalized for laboratory-confirmed COVID-19, based on positive reverse transcription polymerase chain reaction on nasopharyngeal swab for SARS-COV-2 virus detection, were retrospectively included during the 2020 COVID-19 pandemic. Exclusion criteria for both cohorts were the absence of hs-cTnT measurement within 48 h from admission, presence of an overt acute coronary syndrome; Tako-Tsubo cardiomyopathy; severe renal disease (defined as glomerular filtration rate < 30 mL/min/1.73 m2); systolic aortic pressure >200 mmHg; sustained tachyarrhythmias (heart rate > 150 b.p.m.); haemoglobin <90 g/L; known congestive heart failure or left ventricular ejection fraction <50%; stroke; pulmonary embolism; suspect of confirmed rhabdomyolysis (e.g. burns); known neuromuscular disorders; HIV infection and current treatment with immune suppressive agents.
This study complies with the Declaration of Helsinki. The independent Cantonal Research Ethics Committee of Ticino, Switzerland, approved the prospective cohort study in patients with Influenza and all patients gave written informed consent. The protocol for SARS-COV-2 data collection was submitted on 9 May 2020 and approved by the independent Research Ethics Committee on 20 May 2020. The requirement for informed consent was waived in view of the emerging pandemic situation and the retrospective nature of data collection.
Data collection and outcomes
De-identified clinical data were extracted from the cantonal electronic medical record system. Data collection included the following parameters in both cohorts: demographic characteristics (age, sex), cardiovascular risk factors (hypertension, diabetes, body mass index >30), presence of chronic conditions (cardiovascular or COPD), laboratory values (haemoglobin, leucocytes, lymphocytes, C-reactive protein, creatinine, lactate dehydrogenase, arterial lactate, arterial partial oxygen pressure and saturation, hs-cTnT), and presence of radiologically confirmed pneumonia defined as evidence of lung infiltrates at chest X-ray or computed tomography scan. The first available laboratory test results obtained within 48 h from admission were considered as baseline values in both groups. Repeated hs-cTnT measurements were left at discretion of the treating physician and collected when available. Further details regarding data collection in each cohort are provided in the Supplementary material online, Appendix.
Troponin measurements were obtained in all patients with Elecsys Troponin T high-sensitivity assay (Roche, Basel, Switzerland) which employs an immunoassay sandwich technique through two monoclonal antibodies specifically directed against human cardiac T Troponin. Values were obtained by means of a chemiluminescent emission which is measured by a photomultiplier. Myocardial injury was defined as hs-cTnT level above the upper reference limit (99th percentile) for the normal population. The cut-off of hs-cTnT ≤14 ng/L and >14 ng/L was used to discriminate between absence and presence of MINJ. Normal ranges of laboratory values are reported in Supplementary material online, Table S1.
The primary outcome was all-cause mortality up to 28 days, secondary outcomes were admission to an intensive care unit (ICU), mechanical ventilation or mechanical haemodynamic support and length of in-hospital stay in both cohorts.
Statistical analysis
We report medians and interquartile ranges (IQRs) for continuous variables and counts and percent for categorical variables. The Mann–Whitney U test and the Fisher’s exact test were used for between-group comparisons. Missing values were imputed using chained equations multiple imputations. Five datasets were generated, using all common variables to both cohorts, including clinical outcomes. Supplementary material online, Table S2 presents the frequency of missing covariate data.
We estimated the propensity of being hospitalized with COVID-19 based on a logistic regression model that included all baseline characteristics, to enable analyses weighted by the inverse probability of the admission diagnosis and adjust analyses for differences in clinical and laboratory characteristics at admission between COVID-19 and Influenza patients. The statistical efficacy of the procedure was assessed by comparing standardized differences in arithmetic means or proportions between patients admitted for influenza and COVID-19 before and after inverse-probability weighing.25
We calculated mortality rates per 100 person-weeks with 95% confidence intervals (95% CIs) and plotted time-to-event curves for mortality up to 28 days using Kaplan–Meier estimates. Time-to-event curves were plotted separately for patients with Influenza and COVID-19 by presence of MINJ (hs-cTnT≤ vs. >14 ng/L) and compared using a log-rank test.
Primary analyses were based on Cox regression models weighted for the inverse probability of the admission diagnosis. Model 1 separately included type of admission diagnosis or MINJ as independent variables in two univariate analyses. Model 2 simultaneously included type of admission diagnosis and MINJ as independent variables in a single multivariable analysis.
Sensitivity analyses included unweighted univariate and multivariable analyses of the full cohorts with data available on MINJ (n = 597), unweighted and weighted analyses for the inverse probability of the admission diagnosis after trimming of propensity scores to account for residual confounding introduced by patients in the tails of distributions of propensity scores in patients with Influenza and patients with COVID-19.25 The larger value of the 2.5th percentile of the propensity score was found in patients admitted for Influenza and was used as lower cut-off, the smaller value of the 97.5th percentile was also found in patients admitted for Influenza and was used as upper cut-offs. Patients in both groups who had propensity scores outside of these two cut-offs were dropped, whereas patients with propensity scores between these cut-offs were retained in the sensitivity analyses (n = 524).25
Finally, we used multivariable logistic regression models separately in patients admitted for Influenza and COVID-19 to identify the independent associations of MINJ with baseline characteristics. Non-collinear, clinically meaningful covariates with P < 0.20 in univariate analyses were included in final models. Two-sided P-values <0.05 were considered statistically significant, all uncertainty intervals are two-sided 95% CIs. Statistical analyses were done in Stata (release 16, StataCorp, College Station, TX, USA). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines checklist for data reporting.26 The data underlying this article will be shared on reasonable request to the corresponding author.
Results
Influenza patients
A total of 145 patients were hospitalized for Influenza during the 2018–2019 epidemic and were prospectively enrolled. Of them, 94 (65.5%) had hs-cTnT values >14 ng/L at admission (Supplementary material online, Figure S1). Table 1 reports baseline clinical characteristics and in hospital outcomes in Influenza patients.
Table 1.
Baseline clinical characteristics, laboratory values, and outcome data in consecutive hospitalized patients with Influenza by presence of myocardial injury
| No. of obs. | All patients (n = 145) | No. of obs. | hs-cTnT ≤14 ng/L (n = 51) | No. of obs. | hs-cTnT >14 ng/L (n = 94) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 145 | 76 (67–85) | 51 | 69 (54–79) | 94 | 81 (72–88) | <0.001 |
| Male sex, n (%) | 145 | 64 (44.1) | 51 | 22 (43.1) | 94 | 42 (44.7) | 1.000 |
| Hypertension, n (%) | 145 | 93 (64.1) | 51 | 25 (49.0) | 94 | 68 (72.3) | 0.007 |
| Diabetes, n (%) | 145 | 32 (22.0) | 51 | 8 (15.7) | 94 | 24 (25.5) | 0.211 |
| COPD, n (%) | 145 | 28 (19.3) | 51 | 5 (9.8) | 94 | 23 (24) | 0.046 |
| CVD, n (%) | 145 | 39 (26.9) | 51 | 14 (27.4) | 94 | 25 (26.6) | 1.000 |
| BMI >30, n (%) | 145 | 13 (8.9) | 51 | 5 (9.8) | 94 | 8 (8.5) | 0.770 |
| Smoking, n (%) | 145 | 28 (19.3) | 51 | 10 (19.6) | 94 | 18 (19.1) | 1.000 |
| OSAS, n (%) | 145 | 8 (5.5) | 51 | 1 (1.9) | 94 | 7 (7.4) | 0.261 |
| Dyslipidaemia, n (%) | 145 | 59 (40.6) | 51 | 15 (29.4) | 94 | 44 (46.8) | 0.052 |
| Family history of CVD, n (%) | 145 | 6 (4.1) | 51 | 3 (5.8) | 94 | 3 (3.1) | 0.425 |
| LVEF < 50%, n (%) | 145 | 2 (1.3) | 51 | 0 (0) | 94 | 2 (2.1) | 0.541 |
| Previous cerebrovascular diseases, n (%) | 145 | 16 (11.0) | 51 | 1 (1.9) | 94 | 15 (16) | 0.011 |
| hs-cTnT, ng/L | 145 | 19 (9–43) | 51 | 7 (5–10) | 94 | 34 (20–57) | |
| Haemoglobin, g/L | 145 | 131(120–142) | 51 | 135 (125–145) | 94 | 128 (118–142) | 0.027 |
| Leucocytes, n*109/L | 131 | 6.7 (5.0–9.2) | 44 | 5.8 (4.3–7.6) | 87 | 7.1 (5.1–9.8) | 0.025 |
| Lymphocytes, n*109/L | 119 | 0.76 (0.48–1.14) | 41 | 0.76 (0.55–1.09) | 78 | 0.74 (0.46–1.14) | 0.319 |
| C reactive protein, mg/L | 145 | 34 (14–60) | 51 | 25 (10–50) | 94 | 40 (18–67) | 0.019 |
| Creatinine, µmol/L | 145 | 85 (68–110) | 51 | 77 (64–94) | 94 | 93 (74–123) | <0.001 |
| LDH, U/L | 89 | 419 (353–487) | 27 | 396 (344–443) | 62 | 443 (356–507) | 0.097 |
| Lactate, mmol/L, | 88 | 1.3 (0.9–1.8) | 27 | 1.3 (0.9–1.8) | 61 | 1.3 (0.9–1.6) | 0.615 |
| Pa O2, mmHg | 85 | 58 (49–76) | 26 | 55 (50–64) | 59 | 61 (48–84) | 0.248 |
| Sat O2, % | 88 | 91 (85–95) | 27 | 91 (87–94) | 61 | 91 (85–96) | 0.778 |
| Radiologically confirmed pneumonia, n (%) | 145 | 19 (13.1) | 51 | 2 (3.9) | 94 | 17 (18.0) | 0.018 |
| ICU admission, n (%) | 145 | 18 (12.4) | 51 | 2 (3.9) | 94 | 16 (17.0) | 0.032 |
| Mechanical ventilation, n (%) | 145 | 4 (2.7) | 51 | 0 (0) | 94 | 4 (4.2) | 0.298 |
| Mechanical haemodynamic support, n (%) | 145 | 1 (0.7) | 51 | 0 (0) | 94 | 1 (1.1) | 1.000 |
| Length of hospital stay, days | 145 | 7 (5–9) | 51 | 7 (5–7) | 94 | 8 (6–10) | 0.001 |
| Mortality at 28 days, n (%) | 145 | 7 (4.8) | 51 | 0 (0) | 94 | 7 (7.4) | 0.052 |
| Mortality at 1 year, n (%) | 145 | 3 (2.0) | 51 | 0 (0) | 87 | 3 (3) |
Data are shown as median (IQR) or absolute numbers and percentages.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular diseases; hs-cTnT, high-sensitivity cardiac Troponin T; ICU, intensive care unit; LDH, lactate dehydrogenase; LVEF < 50%, left ventricular ejection fraction below 50%; OSAS, obstructive sleep apnoea syndrome; Pa O2, arterial partial oxygen pressure; Sat O2, arterial oxygen saturation.
Median age in patients with baseline hs-cTnT values >14 ng/L was 81 years (IQR: 72–88), significantly higher than patients with hs-cTnT values ≤14 ng/L [69 years (IQR: 54–79), P < 0.001] as were associated comorbidities. Influenza patients with baseline hs-cTnT values >14 ng/L had lower haemoglobin values and higher leucocytes count, C-reactive protein and creatinine levels as compared to those with hs-cTnT values ≤14 ng/L.
Among the 18 Influenza patients requiring ICU admission, 16 (88.8%) had admission hs-cTnT values >14 ng/L (P = 0.032) and all 4 (2.7%) patients requiring mechanical ventilation had baseline hs-cTnT values >14 ng/L, 1 patient required mechanical circulatory support. Length of in-hospital stay was longer in patients with increased hs-cTnT values at admission.
A second hs-TnT assay was available in 74 Influenza patients. Repeated measurements confirmed the presence of MINJ in 63 (88.7%) out of 71 patients with baseline hs-TnT values >14 ng/l. Values ≤14 ng/L were also confirmed in 3 (100%) out 3 patients without MINJ at baseline. At repeated testing, modest fluctuations of hs-TnT values were observed with median relative variation of ±16% (IQR 6–31%) as compared to the basal value.
COVID-19 patients
Among 576 COVID-19 positive patients admitted during the defined time frame, 452 had at least a single hs-cTnT measurement obtained within 48 h from admission (Supplementary material online, Figure S1). Supplementary material online, Table S3 reports baseline clinical characteristics and laboratory values in hospitalized patients with COVID-19 according to the availability of hs-cTnT at admission. Baseline hs-cTnT values >14 ng/L was detected in 216 (47.8%) COVID-19 patients.
Patients with hs-cTnT values >14 ng/L were older with a median age of 78 years (IQR: 72–84) than those with hs-cTnT values ≤14 ng/L [61 years (IQR: 52–70), P < 0.001] and had a higher incidence of comorbidities. Table 2 reports baseline clinical characteristics and in hospital outcome data in COVID-19 patients.
Table 2.
Baseline clinical characteristics and in hospital outcome data in hospitalized patients with COVID-19 by presence of myocardial injury
| No. of obs | All patients (n = 452) | No. of obs | hs-cTnT ≤14 ng/L (n = 236) | No. of obs | hs-cTnT >14 ng/L (n = 216) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 452 | 71 (59–79) | 236 | 61 (52–70) | 216 | 78 (72–84) | <0.001 |
| Male sex, n (%) | 452 | 287 (63.5) | 236 | 140 (59.3) | 216 | 147 (68.0) | 0.063 |
| Hypertension, n (%) | 452 | 220 (48.7) | 236 | 93 (39.4) | 216 | 127 (58.8) | <0.001 |
| Diabetes, n (%) | 452 | 108 (23.9) | 236 | 48 (20.3) | 216 | 60 (27.8) | 0.077 |
| COPD, n (%) | 452 | 72 (15.9) | 236 | 31 (13.1) | 216 | 41 (19.0) | 0.096 |
| CVD, n (%) | 452 | 157 (34.7) | 236 | 66 (28.0) | 216 | 91 (42.1) | 0.002 |
| BMI >30, n (%) | 407 | 118 (28.9) | 204 | 67 (32.8) | 203 | 51 (25.1) | 0.101 |
| Cancer, n (%) | 452 | 53 (11.7) | 236 | 21 (8.9) | 216 | 32 (14.8) | 0.057 |
| Immunosuppression, n (%) | 452 | 23 (5.1) | 236 | 12 (5.1) | 216 | 11 (5.1) | 1.000 |
| Other, n (%) | 452 | 118 (26.1) | 236 | 62 (26.3) | 216 | 56 (25.9) | 1.000 |
| No comorbidities, n (%) | 452 | 75 (16.6) | 236 | 56 (23.7) | 216 | 19 (8.8) | <0.001 |
| ACEI/ARB, n (%)a | 452 | 83 (18.3) | 236 | 35 (14.8) | 215 | 48 (22.2) | 0.051 |
| Oral anticoagulant, n (%)a | 452 | 18 (4.0) | 236 | 6 (2.5) | 216 | 12 (5.5) | 0.147 |
| Statins, n (%)a | 452 | 72 (15.9) | 236 | 31 (13.1) | 216 | 41 (18.9) | 0.096 |
| Delay between symptoms and hospitalization, days | 438 | 6.4 (3.0–9.4) | 232 | 7.3 (4.8–9.7) | 206 | 4.7 (1.7–7.6) | <0.001 |
| Fever with BT > 37.5°, n (%) | 452 | 362 (80.1) | 236 | 197 (83.5) | 216 | 165 (76.4) | 0.077 |
| Dyspnoea, n (%) | 452 | 241 (53.3) | 236 | 135 (57.2) | 216 | 106 (49.0) | 0.090 |
| Cough, n (%) | 452 | 296 (65.5) | 236 | 170 (72.0) | 216 | 126 (58.3) | 0.003 |
| ARDS, n (%) | 452 | 70 (15.5) | 236 | 35 (14.8) | 216 | 35 (16.2) | 0.698 |
| Diarrhoea, n (%) | 452 | 89 (19.7) | 236 | 51 (21.6) | 216 | 38 (17.6) | 0.290 |
| Other associated symptoms, n (%) | 452 | 202 (44.7) | 236 | 107 (45.3) | 216 | 95 (44.0) | 0.777 |
| Radiologically confirmed pneumonia, n (%) | 452 | 240 (53.1) | 236 | 124 (52.5) | 216 | 116 (53.7) | 0.850 |
| Anticoagulants, n (%)b | 452 | 397 (87.8) | 236 | 215 (91.1) | 216 | 182 (84.3) | 0.031 |
| Hydroxychloroquine, n (%)b | 452 | 181 (40.0) | 236 | 107 (45.3) | 216 | 74 (34.2) | 0.021 |
| Lopinavir/Ritonavir, n (%)b | 452 | 139 (30.7) | 236 | 90 (38.1) | 216 | 49 (22.7) | <0.001 |
| Inotropes, n (%)b | 452 | 73 (16.1) | 236 | 39 (16.5) | 216 | 34 (15.7) | 0.898 |
| ICU admission, n (%) | 452 | 101 (22.5) | 236 | 50 (21.2) | 216 | 51 (23.6) | 0.307 |
| Mechanical ventilation, n (%) | 452 | 78 (18.3) | 236 | 41 (16.7) | 216 | 37 (17.1) | 1.000 |
| Length of ICU stay, days | 452 | 18.8 (4.5–31.5) | 236 | 21.3 (8.1–32.9) | 216 | 11.8 (1.9–31.5) | 0.163 |
| Mechanical haemodynamic support, n (%) | 452 | 0 (0) | 236 | 0 (0) | 216 | 0 (0) | |
| Length of hospital stay, days | 452 | 12.5 (7.3–23.8) | 236 | 11.8 (6.9–20.5) | 216 | 14.1 (7.9–27.5) | 0.024 |
| Mortality at 28 days, n (%) | 452 | 76 (16.8) | 236 | 12 (5.1) | 216 | 64 (29.6) | <0.001 |
Data are shown as median (IQR) or absolute numbers and percentages.
ACEI/ARB, angiotensin-converting enzyme or receptor inhibitors; ARDS, acute respiratory distress syndrome, defined accordingly to the Berlin Criteria; BMI, body mass index; BT, body temperature; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular diseases; ICU, intensive care unit.
Therapy at admission.
In hospital treatment.
COVID-19 patients with hs-cTnT values >14 ng/L at admission had lower haemoglobin and lymphocyte count and higher leucocytes, procalcitonin, d-dimer, creatinine and N-terminal prohormone of brain natriuretic peptide when compared with patients with hs-cTnT values ≤14 ng/L. Table 3 reports laboratory values in hospitalized patients with COVID-19 by admission hs-cTnT levels ≤14 ng/L or >14 ng/L.
Table 3.
Baseline laboratory values in hospitalized patients with COVID-19 by presence of myocardial injury
| No. of obs. | All patients (n = 452) | No. of obs. | hs-cTnT ≤14 ng/L (n = 236) | No. of obs. | hs-cTnT >14 ng/L (n = 216) | P-value | |
|---|---|---|---|---|---|---|---|
| Haemoglobin | 452 | 139 (127–150) | 236 | 142 (132–153) | 216 | 136 (119–147) | <0.001 |
| Leucocytes | 452 | 5.7 (4.4–7.8) | 236 | 5.4 (4.2–7.4) | 216 | 5.9 (4.7–8.1) | 0.030 |
| Lymphocytes | 447 | 0.83 (0.61–1.08) | 232 | 0.87 (0.66–1.12) | 215 | 0.77 (0.55–1.05) | 0.002 |
| Platelets | 452 | 176 (144–221) | 236 | 180 (149–230) | 216 | 171 (143–215) | 0.118 |
| C reactive protein | 452 | 66 (26–118) | 236 | 61 (21–104) | 216 | 72 (32–121) | 0.129 |
| Procalcitonin | 316 | 0.13 (0.06–0.37) | 176 | 0.10 (0.05–0.27) | 140 | 0.17 (0.07–0.78) | <0.001 |
| D-Dimer | 406 | 0.84 (0.5–1.4) | 217 | 0.69 (0.46–1.06) | 189 | 1.09 (0.66–1.77) | <0.001 |
| Ferritin | 274 | 797 (396–1324) | 147 | 747 (356–1341) | 127 | 803 (426–1291) | 0.516 |
| Creatinine | 452 | 89 (74–109) | 236 | 79 (67–95) | 216 | 101 (85–129) | <0.001 |
| eGFR (CKD-EPI) | 452 | 69 (52–88) | 236 | 82 (67–98) | 216 | 56 (41–72) | <0.001 |
| LDH | 439 | 504 (411–659) | 234 | 535 (420–662) | 205 | 490 (401–655) | 0.104 |
| Bilirubin | 416 | 8.2 (5.1–12.2) | 221 | 8.7 (5.4–12.0) | 195 | 8.2 (5.0–13.0) | 0.838 |
| NT-pro BNP | 401 | 240 (79–605) | 212 | 100 (46–285) | 189 | 557 (267–1669) | <0.001 |
| Lactates | 184 | 1.2 (0.9–1.7) | 97 | 1.1 (0.9–1.5) | 87 | 1.2 (0.9–1.8) | 0.167 |
| Pa O2 | 387 | 69 (61–81) | 210 | 70 (63–80) | 177 | 69 (60–81) | 0.646 |
| Pa CO2 | 386 | 34 (31–37) | 210 | 34 (31–37) | 176 | 34 (31–37) | 0.788 |
| P/F ratio | 279 | 295 (216–335) | 142 | 300 (232–342) | 137 | 289 (204–327) | 0.320 |
| Sat O2 | 440 | 95 (93–96) | 234 | 95 (93–96) | 206 | 95 (92–96) | 0.142 |
Data are shown as median (IQR).
eGFR (CKD-EPI), estimated glomerular filtration rate estimated according to the Chronic Kidney Disease Epidemiology Collaboration formula; LDH, lactate dehydrogenase; NT-pro BNP, N-terminal prohormone of brain natriuretic peptide; P/F, ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2); Pa CO2, arterial carbon dioxide partial pressure; Pa O2, arterial partial oxygen pressure; Sat O2, arterial oxygen saturation.
Out of 101 (22.5%) COVID-19 patients requiring ICU admission, 51 (50.4%) had baseline hs-cTnT values >14 ng/L. Overall, 78 (18.3%) patients required mechanical ventilation, 37 (47.4%) of them with associated hs-cTnT values >14 ng/L. None required mechanical circulatory support. Length of in-hospital stay was significantly longer in COVID-19 patients with increased hs-cTnT values.
A second hs-TnT assay was available in 316 COVID-19 patients. Repeated measurements confirmed the presence of MINJ in 141 (89.8%) out of 157 patients with baseline hs-TnT values >14 ng/L. Values ≤14 ng/L were also confirmed 148 (93.0%) out of 159 patients with basal values ≤14 ng/L. Modest fluctuations of hs-TnT at repeated testing were observed with a median relative variation of ±14% (IQR 4–27%) as compared to the basal value.
Predictors of myocardial injury in COVID-19 and Influenza
At logistic regression multivariable analyses, age [odds ratio (OR) 2.15 per 10 years increase; 95% CI 1.48–2.84; P < 0.001], creatinine (OR 3.14 per log unit; 95% CI 1.17–8.40; P = 0.009), COPD (OR 5.15; 95% CI 1.37–19.31; P = 0.015), and C-reactive protein (OR 1.82 per log unit; 1.16–2.87; P = 0.023) were recognized as independent predictors of baseline hs-cTnT values >14 ng/L in Influenza (Supplementary material online, Table S4).
Age (OR 4.41 per 10 years increase; 95% CI: 3.10–5.69; P < 0.001), creatinine (OR 2.62 per log unit; 1.51–4.54; P = 0.001), male sex (OR 3.33; 95% CI 1.72–6.25; P < 0.001), and haemoglobin (OR 0.98; 0.97–1.00; P = 0.023) emerged as independent correlates of baseline hs-cTnT values >14 ng/L in COVID-19 (Supplementary material online, Table S5).
Comparative analysis of patients hospitalized for Influenza or COVID-19
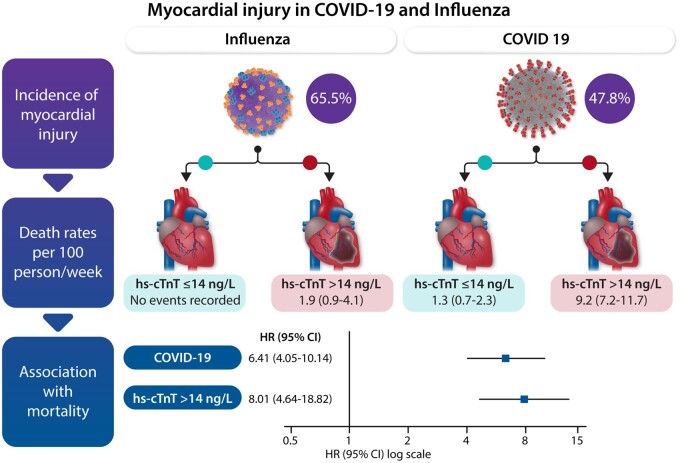
The likelihood of baseline hs-cTnT values >14 ng/L at admission was higher among Influenza than COVID-19 patients (risk ratio 1.36, 95% CI 1.16–1.58).
At a 28-days follow-up, 7 deaths (4.8%) occurred among patients with Influenza (rate 1.2 per 100 patients per week, 95% CI 0.6–2.6) when compared with 76 deaths (16.8%) among patients with COVID-19 (rate 4.7 per 100 patients per week, 95% CI 3.7–5.9), for an unadjusted hazard ratio (HR) of 3.69 (95% CI 1.70–8.00).
In patients with Influenza, all deaths were recorded among patients with baseline hs-cTnT >14 ng/L (1.9 per 100 patients per week, 95% CI 0.9–4.1).
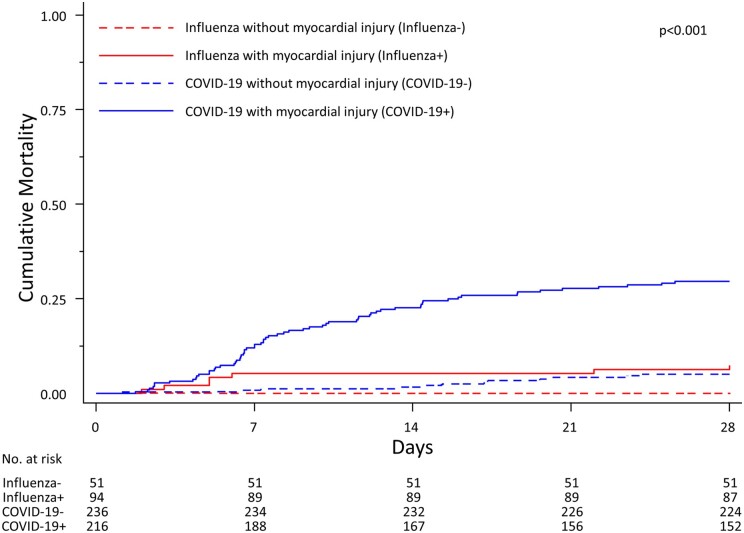
In patients with COVID-19, 64 deaths occurred among those with baseline hs-cTnT >14 ng/L (9.2 per 100 patients per week, 95% CI 7.2–11.7), whereas 12 deaths occurred in those without hs-cTnT elevations (1.3 per 100 patients per week, 95% CI 0.7–2.3). Mortality at 28 days was significantly different among the four groups (log-rank test P < 0.001), corresponding time-to-event curves are presented in Figure 1.
Figure 1.
Time-to event curves in hospitalized patients admitted for COVID-19 or Influenza. The P-value is from a log-rank test.
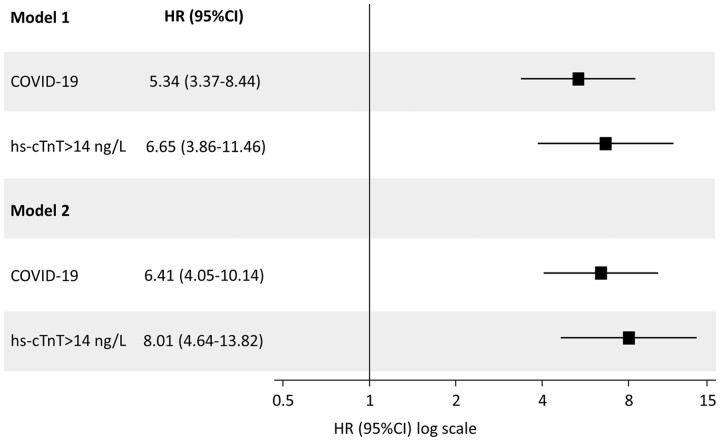
In adjusted Cox regression model, we found more than a five-fold increase in the hazard of death (HR 5.34, 95% CI 3.37–8.44) associated with COVID-19 at admission, and more than a six-fold increase associated with hs-cTnT elevations at admission (HR 6.65, 95% CI 3.86–11.46). These associations remained after mutual adjustment for admission diagnosis and hs-cTnT elevation, with an HR of 6.41 (95% CI 4.05–10.14) for COVID-19 and 8.01 (95% CI 4.64–13.82) for hs-cTnT elevation, respectively (Table 4). Figure 2 shows the associations of admission diagnosis and MINJ with mortality at 28 days.
Table 4.
Primary analyses: weighted univariate (Model 1) and multivariable (Model 2) Cox regression models
| HR (95% CI) | P-value | |
|---|---|---|
| Model 1 | ||
| COHORT | ||
| Influenza (n = 145) | 1 | |
| COVID-19 (n = 452) | 5.34 (3.37–8.44) | <0.001 |
| Myocardial injury | ||
| hs-cTnT ≤ 14 ng/L (n = 287) | 1 | |
| hs-cTnT >14 ng/L (n = 310) | 6.65 (3.86–11.46) | <0.001 |
| Model 2 | <0.001 | |
| COHORT | ||
| Influenza | 1 | |
| COVID-19 | 6.41 (4.05–10.14) | <0.001 |
| Myocardial injury | ||
| hs-cTnT ≤14 ng/L | 1 | |
| hs-cTnT >14 ng/L | 8.01 (4.64–13.82) | <0.001 |
Weighted analyses done after multiple imputation, with weights defined as the inverse probability of the admission diagnosis to adjust analyses for differences in clinical and laboratory characteristics at admission between COVID-19 and Influenza patients.
Figure 2:
Associations of admission diagnosis and myocardial injury with mortality at 28 days in hospitalized patients admitted for COVID-19 or Influenza after primary analysis. Model 1 separately included type of admission diagnosis or myocardial injury as independent variables. Model 2 simultaneously included type of admission diagnosis and myocardial injury as independent variables. Hazard ratios are for the association of admission diagnosis or myocardial injury with mortality at 28 days.
Sensitivity analysis
Sensitivity analysis included an unweighted univariate and multivariable Cox regression model on the full cohort (Supplementary material online, Table S6) and unweighted and weighted analyses for the inverse probability of the admission diagnosis after trimming of propensity scores.
After weighing for the inverse probability of admission diagnosis and trimming of propensity scores to account for residual confounding introduced by patients in the tails of distributions of propensity scores in the two groups, 524 (Influenza n = 118, COVID-19 n = 406) patients were retained. Standardized differences decreased considerably after inverse-probability weighing and were less than 0.20 throughout (Supplementary material online, Table S7).
Four deaths (3.4%) occurred in Influenza patients (rate 0.9 per 100 patients per week, 95% CI 0.3–2.2) when compared with 67 deaths (16.5%) among COVID-19 patients (rate 4.6 per 100 patients per week, 95% CI 3.6–5.8), for an unadjusted HR of 5.23 (95% CI 1.91–14.35).
In patients with Influenza, all deaths were recorded among patients with baseline hs-cTnT >14 ng/L (rate 1.40 per 100 patients per week, 95% CI 0.53–3.75).
In patients with COVID-19, 56 deaths occurred among those with hs-cTnT elevations (rate 8.59 per 100 patients per week, 95% CI 6.61–11.16), whereas 11 deaths occurred in those without hs-cTnT elevations (rate 1.36 per 100 patients per week, 95%-CI 0.75–2.46). Corresponding time-to-event curves are presented in Supplementary material online, Figure S2 while Supplementary material online, Tables S8 and S9 show results of the Cox models performed after trimming and inverse probability weighing confirming robustness of findings of the primary analysis.
In both viral diseases, higher hs-TnT baseline values were associated with an increased likelihood of all-cause mortality at 28 days. This association was confirmed when troponin was analysed as continuous variable [primary analysis population: HR 1.99 per log increase in troponin values (95% CI 1.73–2.21, P < 0,001); HR 1.92 per log increase in troponin values (95% CI 1.71–2.14, P < 0.001) in the population retained in the sensitivity analysis].
Discussion
The present study, which provides for the first-time comparative frequency distributions and prognostic impact of MINJ at admission among hospitalized patients with Influenza or COVID-19 can be summarized as follows:
Myocardial injury, while being common in both viral diseases, was significantly more frequent in hospitalized patients with Influenza as compared to COVID-19.
Age and renal impairment were recognized as factors associated with MINJ in both viral infections.
Admission diagnosis of COVID-19 and presence of MINJ remained independent predictors of in hospital mortality.
Among patients with MINJ, the absolute risk of death was six times higher in hospitalized patients with COVID-19 compared with Influenza.
All fatal events in Influenza patients occurred in patients with MINJ.
Following the first report by Huang et al.13 published soon after the outbreak of COVID-19 pandemic, early Chinese studies analysed the relationship between MINJ and in-hospital mortality.4,5,14 These studies suggested that the presence of increased troponin levels might identify a subgroup of patients at higher risk of in-hospital mortality. However, these studies used non-standardized definitions of MINJ, which was mainly based on non-high-sensitivity assays.
Subsequent analyses, based on a single highly sensitive troponin measurements with a single cut-off set above the 99th upper reference limit to discriminate between presence or absence of MINJ, confirmed the prognostic impact of MINJ in western populations.15,16 Type I and II myocardial infarction, the role of inflammation, endothelial damage and direct virally mediated myocardial damage were proposed to explain the occurrence of MINJ.12
Two comparative analyses on the incidence and prognostic relevance of MINJ in intubated patients with COVID-19 and non-COVID-19 related ARDS have been recently published.
Metkus et al.27 observed that half of patients with either COVID and non-COVID pneumonia-related ARDS showed MINJ with a higher mortality in the former group and a linear relationship between baseline troponin levels and likelihood of a fatal outcome. Nonetheless, association between MINJ and mortality mitigated and become no longer statistically significant after progressive covariate adjustment (including age, sex, renal function, bilirubin, Pa O2/FiO2 ratio, vasopressor, lactate levels) allowing authors to conclude that MINJ in COVID-19 was a function of baseline comorbidities in particular advanced age and multisystem organ dysfunction. Jirak et al.28 reported on a small cohort of patients with respiratory distress treated mainly with non-invasive ventilation with either COVID-19 or non-COVID related pneumonia. Aetiology of pneumonia in the non-COVID-19 was heterogeneous with a viral cause identified in less than 30% cases. MINJ and was higher in non-COVID-19 as compared to COVID-19 related pneumonias (78.1% vs. 96.4%, respectively).
Only a few retrospective studies evaluated the impact of MINJ in Influenza.21–24
Ludwig et al.21 retrospectively analysed 600 veterans with laboratory-confirmed Influenza infection and available cardiac biomarkers enrolled during 2010–2012 epidemics. Myocardial injury, defined as the presence of increased non-highly sensitive Troponin I or Creatine Kinase-MB values was observed in 24% of the patients and a cardiac cause was excluded only in 6.5%. Using a similar MINJ definition, Greaves et al.22 observed it in 12% of the 152 hospitalized patients with Influenza. More recently, MINJ was reported only in 2.9% of a large retrospective cohort of hospitalized patients with Influenza evaluated with low specificity assays during 2017–2018 seasonal epidemic.24 To date, only a single centre retrospective study used high-sensitivity troponin assays and observed MINJ in 31.8% of the 264 patients with laboratory-confirmed Influenza infection.23
Our data confirms that presence of MINJ is frequent among hospitalized COVID-19, shows that the occurrence of MINJ is at least as frequent, if not more frequent, among hospitalized Influenza patients. This observation carries relevant implications as it confirms that MINJ is a common finding in different acute respiratory diseases with a relative frequency higher in Influenza, as it was observed in patients with non-COVID-related pneumonia28 and that higher baseline troponin values were associated with an increased likelihood of all-cause mortality at 28 days. In addition, we originally observed that presence and extent of MINJ, with a linear relationship between baseline hs-TnT values and outcome, are able to stratify the likelihood of short term all-cause mortality in both viral diseases. This finding, while confirming previous observations on COVID-19 patients, extends this evidence to patients with Influenza.5,15,27
Our study suggests that the presence of MINJ, defined as a single hs-TnT value of above the 99th percentile upper reference limit obtained shortly after admission, identifies patients at greater risk of mortality in both respiratory viral diseases. While no fatal event was observed among Influenza patients without MINJ, the risk of mortality was similar among patients with influenza and MINJ and those with COVID-19 without MINJ. However, the presence of MINJ conferred a much greater risk of mortality among COVID-19 than Influenza patients so that the adjusted mortality risk was five-fold greater for the former compared with the latter group, with highly significant interaction testing. This observation was confirmed at multiple sensitivity and multivariable analyses. This finding carries relevant clinical values, providing a tool able to stratify prognosis at an early disease stage in both viral diseases. While the retrospective nature of our analysis does not allow to infer causative relationships, we concur with previous analyses of MINJ in viral diseases might be interpreted as a reliable marker, but not an ultimate determinant of fatal events.27
Both Influenza and SARS-COV2 have a peculiar endothelial tropism,29,30 which may explain our findings and provide connections between MINJ and outcomes.
Microvascular endothelial activation is known to be one of the pivotal pathogenic pathways in severe Influenza causing the expression of platelets-binding receptors triggering adhesion, activation, microvascular thrombi, vascular occlusion, and ischaemic damage.31
In COVID-19 patients, incidence of MINJ increases along with the severity of the clinical presentation30 nonetheless, as evidenced by magnetic resonance studies, cardiac involvement might occur independently from the severity of the clinical presentation and persists beyond the period of acute presentation.32
While the role of several proposed mechanism such as type I myocardial infarction, hypoxaemia, direct viral myocardial damage14,16,33,34 has not yet found definitive confirmation, the observed association of MINJ with several comorbidities, including advanced age, multisystem organ dysfunction, traditional cardiovascular risk factors, and renal impairment highlighted in previous reports5,14,16,23,27 for both viral respiratory diseases, supports the hypothesis of a key pathophysiological role for endothelium.29,30,35
All the above-mentioned mechanisms, while have been recently called upon in COVID-19, have been also widely reported previously for Influenza,18,20,36 adding similarities between the two viral entities.
There are several limitations to this study. The prospective inclusion of influenza patients was originally conceived as stand-alone cohort. Immediately after the COVID-19 outbreak, measurement of MINJ in the same hospitalized settings was implemented as part of the standard of care, but the data were only retrospectively collected after receiving institutional review board approval during the first wave pandemic. There were differences in baseline characteristics between hospitalized Influenza and COVID-19 patients. However, after accounting them in multiple multivariable analysis models, results remained consistent. However, we cannot exclude that covariates which were unmeasured might have biased our results. In particular, the decision to hospitalize a patient with COVID-19 might have reflected not only the severity of presentation but also the limited hospital capacity during the first COVID-19 pandemic wave. However, it is reassuring that the frequency of MINJ at presentation was in fact lower among COVID-19 when compared with influenza patients. Older age and renal insufficiency have been described as being associated with increased troponin levels even in the absence of concomitant viral infections, thus their relationship with virally mediated MINJ might not be mechanistic but potentially mediated by the role of several comorbidities.37 Nonetheless as no standard definition exists on alternative thresholds for myocardial biomarkers in specific subgroups of patients (e.g. elderly, chronic kidney disease, multiple comorbidities), the single hs-TnT cut-off has been used.
As no standard definition exists for how much rise and/or fall of hs-cTn concentration should be used to identify acute injury, our data cannot ultimately define the acute vs. chronic nature of observed cardiac injury. In patients with available repeated measurements, variations observed in both cohorts are close to a relative change ±20% to the baseline value, that has been proposed as a cut-off able to identify acute vs. chronic myocardial.
Nonetheless, from a clinical perspective, the independent association with short-term mortality observed in both cohorts strengthens the prognostic utility of increased troponin levels independently from an acute vs. chronic nature of MINJ. Cardiac imaging is not available in our data, as most patients were evaluated with informal point-of-care ultrasound. This did not allow us to collect and analyse data on ventricular function. Finally, further studies are needed to clarify whether triage and treatment of patients with COVID 19 or Influenza should be customized according to the presence of MINJ and to recognize the specific impact of different viral variants of concern.
In conclusion, MINJ is common in both influenza and COVID-19 viral diseases occurring in roughly one out of every two patients. Myocardial injury increases the risk of death in both COVID-19 and Influenza but its impact is six times greater on an absolute term among patients admitted for COVID-19 when compared with Influenza.
Lead author biography
Luigi Biasco, MD, PhD is an interventional cardiologist and researcher. After graduation and board certification in Cardiology and PhD degree at University of Turin he was awarded with 2014 EAPCI training fellowship completing his structural interventional training at Rigshospitalet, Copenhagen (DK) under the supervision of Prof. Lars Søndergaard. After a 3 years period in Cardiocentro Ticino he moved back to Italy, his home country. He is currently working as an interventional cardiologist in Turin. Since 2018 affiliated as a researcher at Università della Svizzera Italiana, Lugano (Switzerland). Authors of more than 50 publications in peer reviewed journals his main research interests are structural interventions (TAVI and mitral interventions), hemodynamic and myocardial injury in viral diseases. Active member of the EAPCI next generation group.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest: All authors have no conflict of interest to declare.
Supplementary Material
References
- 1. Burn E, You SC, Sena AG, Kostka K, Abedtash H, Abrahao MTF, Alberga A, Alghoul H, Alser O, Alshammari TM, Aragon M, Areia C, Banda JM, Cho J, Culhane AC, Davydov A, DeFalco FJ, Duarte-Salles T, DuVall S, Falconer T, Fernandez-Bertolin S, Gao W, Golozar A, Hardin J, Hripcsak G, Huser V, Jeon H, Jing Y, Jung CY, Kaas-Hansen BS, Kaduk D, Kent S, Kim Y, Kolovos S, Lane JCE, Lee H, Lynch KE, Makadia R, Matheny ME, Mehta PP, Morales DR, Natarajan K, Nyberg F, Ostropolets A, Park RW, Park J, Posada JD, Prats-Uribe A, Rao G, Reich C, Rho Y, Rijnbeek P, Schilling LM, Schuemie M, Shah NH, Shoaibi A, Song S, Spotnitz M, Suchard MA, Swerdel JN, Vizcaya D, Volpe S, Wen H, Williams AE, Yimer BB, Zhang L, Zhuk O, Prieto-Alhambra D, Ryan P.. Deep phenotyping of 34,128 adult patients hospitalised with COVID-19 in an international network study. Nat Commun 2020;11:5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paules C, Subbarao K.. Influenza. Lancet 2017;390:697–708. [DOI] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z.. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL.. Seasonal Influenza infections and cardiovascular disease mortality. JAMA Cardiol 2016;1:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brojakowska A, Eskandari A, Bisserier M, Bander J, Garikipati VNS, Hadri L, Goukassian DA, Fish KM.. Comorbidities, sequelae, blood biomarkers and their associated clinical outcomes in the Mount Sinai Health System COVID-19 patients. PLoS One 2021;16:e0253660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eskandari A, Brojakowska A, Bisserier M, Bander J, Garikipati VNS, Hadri L, Goukassian D, Fish K.. Retrospective analysis of demographic factors in COVID-19 patients entering the Mount Sinai Health System. PLoS One 2021;16:e0254707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vestjens SMT, Spoorenberg SMC, Rijkers GT, Grutters JC, Ten Berg JM, Noordzij PG, Van de Garde EMW, Bos WJW; Ovidius Study Group. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology 2017;22:1000–1006. [DOI] [PubMed] [Google Scholar]
- 10. Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega JC.. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med 2013;39:1181–1189. [DOI] [PubMed] [Google Scholar]
- 11. Hoiseth AD, Neukamm A, Karlsson BD, Omland T, Brekke PH, Soyseth V.. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011;66:775–781. [DOI] [PubMed] [Google Scholar]
- 12. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D.. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail 2020;26:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C.. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount Sinai CIC.. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Merlo M, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Specchia C, Metra M, Senni M.. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol 2020;5:1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B.. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latreille E, Lee WL.. Interactions of Influenza and SARS-CoV-2 with the lung endothelium: similarities, differences, and implications for therapy. Viruses 2021;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm (last accessed 23 August 2021).
- 20. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D.. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludwig A, Lucero-Obusan C, Schirmer P, Winston C, Holodniy M.. Acute cardiac injury events </=30 days after laboratory-confirmed influenza virus infection among U.S. veterans, 2010-2012. BMC Cardiovasc Disord 2015;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greaves K, Oxford JS, Price CP, Clarke GH, Crake T.. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch Intern Med 2003;163:165–168. [DOI] [PubMed] [Google Scholar]
- 23. Pizzini A, Burkert F, Theurl I, Weiss G, Bellmann-Weiler R.. Prognostic impact of high sensitive troponin T in patients with influenza virus infection: a retrospective analysis. Heart Lung 2020;49:105–109. [DOI] [PubMed] [Google Scholar]
- 24. Harris JE, Shah PJ, Korimilli V, Win H.. Frequency of troponin elevations in patients with influenza infection during the 2017-2018 influenza season. Int J Cardiol Heart Vasc 2019;22:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. da Costa BR, Gahl B, Jüni P.. Tools & techniques—statistics: propensity score techniques. EuroIntervention 2014;10:761–767. [DOI] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 27. Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, Thiemann DR, Trost JC, Hasan RK.. Myocardial injury in severe COVID-19 compared with non-COVID-19 acute respiratory distress syndrome. Circulation 2021;143:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jirak P, Larbig R, Shomanova Z, Frob EJ, Dankl D, Torgersen C, Frank N, Mahringer M, Butkiene D, Haake H, Salzer HJF, Tschoellitsch T, Lichtenauer M, Egle A, Lamprecht B, Reinecke H, Hoppe UC, Pistulli R, Motloch LJ.. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: results from a multicentre study. ESC Heart Fail 2021;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armstrong SM, Darwish I, Lee WL.. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence 2013;4:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Libby P, Luscher T.. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalil AC, Thomas PG.. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 2019;23:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E.. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guagliumi G, Sonzogni A, Pescetelli I, Pellegrini D, Finn AV.. Microthrombi and ST-segment-elevation myocardial infarction in COVID-19. Circulation 2020;142:804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E.. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H.. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engelmann B, Massberg S.. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 37. Sedighi SM, Fulop T, Mohammadpour A, Nguyen M, Prud’Homme P, Khalil A.. Elevated cardiac troponin levels in geriatric patients without ACS: role of comorbidities. CJC Open 2021;3:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest: All authors have no conflict of interest to declare.