Abstract
Background
The year 2020 was marked by the new coronavirus pandemic, resulting in millions of cases and deaths, placing healthcare workers at high risk of infection.
Aims
The aim of this study was to describe the role of an occupational health service during coronavirus disease 2019 pandemic in an oncologic hospital and characterize the most likely sources of viral infection.
Methods
The information of all healthcare workers with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from 11 March to 15 December 2020 was collected through an epidemiological survey conducted during contact tracing. The data extracted included gender, age, comorbidities, occupational group, source of infection, clinical presentation, duration of the disease, need for hospitalization and persistent or late symptoms after disease or upon returning to work.
Results
Out of a total of 2300 workers, 157 were infected, consisting of nurses (36%), nurse assistants (33%) and diagnostic and therapeutic professionals (10%). Physicians and administrative staff accounted for 8% each. The most frequently reported source of infection was occupational (43%), owing to worker-to-worker transmission (45%) and patient-to-worker transmission (36%). The most frequent moments of infection perceived corresponded to the removal of protective equipment during meals and moments of rest in the staff and changing rooms.
Conclusions
The study revealed that occupational transmission from patients and colleagues might be an important source of SARS-CoV-2 infection in healthcare workers. Spread between colleagues accounted for 45% of the occupational source infections reported. Implementing physical distancing measures and limiting the number of people in changing and rest rooms could significantly reduce infection and related absenteeism.
Keywords: Absenteeism, COVID-19 pandemic, healthcare workers, occupational health
Key learning points.
What is already known about this subject:
Healthcare workers are on the frontline of the fight against the new coronavirus pandemic. They are often at high risk of infection in the workplace and can transmit the disease to patients, colleagues and family.
When assessing the risk of infection with coronavirus disease 2019 in healthcare workers, it is vital to understand when and how they become infected in the hospital either by colleagues or patients.
What this study adds:
Healthcare workers may undervalue the risk of co-worker spread and overlook protective measures during breaks or mealtimes.
The moments of rest of the workers may have a substantial impact on infection transmission in the hospital settings.
What impact this may have on practice or policy:
Our findings may raise awareness among workers on the importance of maintaining all protective measures in all interactions with colleagues, including during breaks.
Strengthening administrative control measures, improving social distancing barriers and limiting the duration and number of people in the staff rooms could have a significant impact on reducing severe acute respiratory syndrome coronavirus 2 infection.
Introduction
Since December 2019, a highly infectious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), originating in Wuhan, China, has spread worldwide [1]. After a year since the start of the pandemic, the coronavirus disease 2019 (COVID-19) has infected more than 83 million people and caused more than 1.8 million deaths globally [2]. At the forefront of this crusade, healthcare workers (HCWs) have been and continue to be at excessive risk. In mid-August, it was estimated that about 300 000 HCWs had been infected with SARS-CoV-2, and more than 2500 perished worldwide [3]. These figures are underestimated, given that many countries do not accurately report their data [3]. HCWs are both victims and vectors of infection, so it is critical to guarantee their safety and mitigate transmission chains.
The main goal of occupational health services (OHS) is to protect HCWs from work-related risks. Consequently, during the COVID-19 pandemic, these services acquired a fundamental role in the care of the hospital personnel [4]. Notably, OHS had to adapt rapidly, implementing strategies that protect the health of HCWs and ensure the maintenance of healthcare. Additionally, OHS in many hospitals contribute to implementing infection control measures, the provision of personal protective equipment (PPE) and the systematic screening of HCWs, testing and contact tracing.
The Portuguese Oncology Institute of Porto (IPO-Porto) is a highly differentiated hospital that belongs to the National Health System (NHS). It has 2300 employees and provides specialized oncological diagnosis, treatment, research and education in Portugal’s northern region. When a SARS-CoV-2-infected patient is detected in IPO-Porto, he is referred to another institution of the NHS and only returns to IPO-Porto when considered cured.
The care of cancer patients during the pandemic can be compromised both by the delay in cancer diagnosis and treatment along with the susceptibility of immunosuppressed patients’ higher risk of infection [5]. Consequently, limiting nosocomial transmission of SARS-CoV-2 and preventing absenteeism is also one of OHS responsibilities.
The fundamental activity of OHS against COVID-19 is structured on a risk assessment process that mitigates viral transmission in the workplace and establishes preventive and protective measures to keep HCWs safe [6]. Due to the novelty of SARS-CoV-2, precise risk assessment frameworks are still under development, but risk control methods can be inferred from other biological risks [7]. One available approach is the hierarchy of controls, which consists of a system of interdependent strategies stratified from the most effective to the least effective protective strategies. The United States National Institute for Occupational Safety and Health (NIOSH) defines five steps of the hierarchy of controls: elimination, substitution, engineering controls, administrative controls and PPE [8].
Considering the hierarchy of control system, the most effective means to prevent transmission of SARS-CoV-2 is through the elimination of the hazard. This involves not only limiting visitors and working remotely (teleworking and teleconsultations), but also systematically conducting triage questionnaires on symptoms, both for patients and professionals whenever physical presence is necessary [9]. An extreme measure used at the onset of the pandemic was to temporarily suspend non-essential activity, which in an oncology hospital can have dramatic consequences. Additionally, identifying and screening suspected COVID-19 cases, isolation of index cases, and contact tracing and testing are basic occupational health procedures to eliminate the hazard inside healthcare settings. Mandatory screening of patients for SARS-CoV-2 infection before procedures and social isolation of positive cases are means to eliminate the risk and protect HCWs. The next applicable level of control is engineering controls consisting in optimizing ventilation and disinfection procedures for facilities and equipment. Next, the administrative controls are achieved with mandatory use of surgical masks within the institution, physical distancing attitudes, workers education, training and health surveillance. The last level of control strategy is the protection of workers with PPE in accordance with their risk. For example, during aerosol-generating procedures, the worker utilizes adequate full body protection, including isolation gowns with head covers, face shields and FFP2/N95 respirators, protective suit, gloves, boots and foot covers. This level includes not only the management of PPE provisions but also training, education and compliance with guidelines, especially during invasive airway procedures [7,10].
Overall, the nosocomial transmission of SARS-CoV-2 needs further understanding. It is important to mention that HCWs can be infected either in the community or within the hospital, being able to trigger or amplifying outbreaks [11,12]. The fear of spreading the infection to patients and colleagues in the hospital or relatives in the household raises mental health issues [13]. In addition to the risk of infection, the high workload leaves professionals at increased risk of anxiety, depression and burnout, undermining the health system’s capacity [13]. Characterizing the source of infection among workers can help discover additional intervention methods to reduce the transmission of SARS-CoV-2 in healthcare settings. Therefore, we analysed the reported sources of infection and epidemiological data of contact tracing survey of HCWs infected with SARS-CoV-2 within an oncology hospital.
Methods
IPO-Porto comprises medical and surgical oncology wards, intensive and intermediate care units, operating theatre, radiotherapy clinic, day-care clinic, pathology and clinic laboratories, research centre and multiple non-clinical services. The distribution of the 2300 employees consists of 736 nurses (32%), 552 nurse assistants (24%), 414 physicians (18%), 322 diagnostic and therapeutic professionals (14%), 253 administrative staff (11%) and 23 other (1%). Since 11 March 2020, when the first patient infected with SARS-CoV-2 was detected in our hospital, a systematic contact tracing of HCWs started under the coordination of OHS. Contact tracing of HCWs was carried out whenever they had direct contact with COVID-19 cases, either patients or co-workers. This intervention was performed in the first 24 h after the index-case lab result confirmation to minimize potential spread in hospital settings.
In order to achieve a low detection threshold, all professionals with any flu-like symptoms were tested. The epidemiological links within the healthcare settings were subsequently classified into high- and low-risk contacts, according to the level of exposure based on national and European guidelines [14,15]. According to the Portuguese General Directorate of Health (DGS) guidelines, high-risk contacts were self-isolated for 14 days after the last contact with the index-case, whereas low-risk contacts were allowed to work under active symptom monitorization. The list of contacts was provided by the hierarchical superior responsible for the case, either patient or worker. The occupational health physicians (OHPs) phoned the index-case to verify other possible contacts, called all the contacts and stratified the risk. Whenever indicated, SARS-CoV-2 detection test was arranged at an assembled test station. The swabs were performed by trained nurses according to standardized guidelines using nasopharyngeal specimens. In the virology laboratory, samples were assayed for SARS-CoV-2 real-time reverse transcriptase–polymerase chain reaction (RT-PCR). The results were sent to the OHPs defined as ‘detected’, ‘not detected’ or ‘inconclusive’. In case of an inconclusive result, the test was immediately repeated. While waiting for RT-PCR test result and whenever a SARS-CoV-2-infected worker was detected, they were restricted from work and isolated.
The return-to-work criteria were initially defined using a strategy based on two consecutive negative RT-PCR tests. On 14 October 2020, new national guidelines [16] changed the cure criteria. Thereafter, the isolation period was reduced to 10 days, for mild to moderate disease, or 20 days, for severe disease and immunosuppression, after the onset of symptoms. Cure criteria were defined as apyrexia and sustained symptomatic improvement for 3 consecutive days. For HCWs, besides the clinical criteria, an RT-PCR test was performed 10 days after the onset of symptoms. If the test was negative, the HCW returned to work. When the test result was positive, isolation was extended until 20 days after the onset of symptoms, at which point isolation was terminated without the need for an additional RT-PCR test. OHP reported cases infected with a clear occupational source as occupational disease to the respective government department for adequate compensation. Systematic contact tracing of each detected case was performed. During the pandemic, as state guidelines have changed, OHS has adapted its practice accordingly.
A database was created to keep records of test results, contact screenings, HCWs under active surveillance of symptoms and information on infected HCWs. All data were encrypted and stored on a dedicated database server to ensure protection and confidentiality. The information of all HCWs with SARS-CoV-2 infection was collected through an epidemiological survey conducted during contact screening, including gender, age, comorbidities, occupational group, source of infection, clinical presentation, duration of the disease, need for hospitalization and persistent symptoms after disease or upon returning to work. Persistent symptoms following infection were defined as symptoms that remained for more than 3 months after the cure date. The source of infection was categorized as unknown, social, occupational and familiar, depending on the contact tracing epidemiological survey outcome. An unknown source was defined when a worker with flu-like symptoms tested positive without any traced contacts. A social source was defined whenever the epidemiological link, whether colleague or not, was external to the hospital and not family. An occupational source was only considered when there was a clear exposure to a confirmed positive case in the workplace, and no interaction with other infected cases, apart from the contacts in hospital settings. Whenever the source of the infection was identified as occupational, OHP assessed the index-case, the risk of infection and the level of exposure, duration of contact, period of contagion, use of PPE during exposure, location and context of the contact, and other data relevant for the exposure risk [14]. Based on this investigation, the occupational infection was further classified as patient-to-HCW transmission, HCW-to-HCW transmission or undetermined. When an occupational source was assumed, but the worker had both colleague and patient exposure within the same possible contagious period, an undetermined occupational source was considered. This information allowed the OHS to identify places in the hospital setting with a higher propensity of exposure to COVID-19 cases: places where face-to-face contact (without proper PPE) occurred within 2 m for more than 15 min, or physical contact or having unprotected direct contact with infectious secretions. Ultimately, the ongoing investigation of sources and transmission sites was the mainstay of the COVID-19 risk assessment.
This study was approved by the Ethics Committee of the institution, and informed consent was obtained from all participants. We present an observational retrospective report of the data collected from infected HCWs from 11 March to 15 December 2020.
Results
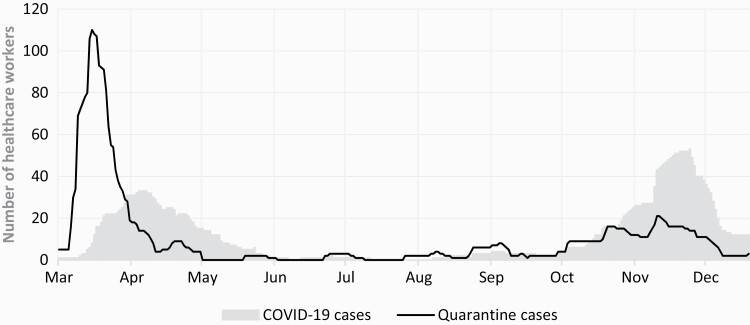
The first case of SARS-CoV-2 infection in HCWs at IPO-Porto was on 11 March 2020. From 11 March to 15 December 2020, 157 workers infected by SARS-CoV-2 were reported. Figure 1 shows the evolution of workforce loss turnover by highlighting quarantine and COVID-19 cases.
Figure 1.
Evolution of HCWs absenteeism during the first and second waves of the COVID-19 pandemic (11 March to 15 December 2020).
Table 1 summarizes epidemiological data on infected HCWs. Most of the participants were female (85%). Among the infected HCWs, the most frequent occupational groups were nurses (36%) and nurse assistants (33%), given they have the most direct contact with patients, followed by diagnostic and therapeutic professionals (radiographers, technical assistants and physiotherapists) with 10%. Physicians and administrative staff accounted for 8% each. Other occupational groups such as researchers, maintenance technicians and non-clinical staff were least represented (5%).
Table 1.
Characteristics of HCWs infected with SARS-CoV-2 from 11 March to 15 December 2020
| Variable | n (%) |
|---|---|
| Gender | |
| Female | 134 (85) |
| Male | 23 (15) |
| Age (years) | |
| Mean | 42 |
| Median | 41 |
| Range | 21–72 |
| Occupational groups | |
| Physician | 13 (8) |
| Nurse | 56 (36) |
| Nurse assistant | 52 (33) |
| Administrative staff | 13 (8) |
| Diagnostic and therapeutic professionals | 15 (10) |
| Other | 8 (5) |
| Source of infection | |
| Occupational | 67 (43) |
| Patient to worker | 24 (36) |
| Worker to worker | 30 (45) |
| Undetermined | 13 (19) |
| Familiar | 52 (33) |
| Social | 3 (2) |
| Unknown | 35 (22) |
| Chronic diseases | |
| Yes | 38 (24) |
| No | 119 (76) |
| Duration of the disease (days) | |
| Mean | 24 |
| Median | 20 |
| Range | 10–74 |
| Hospitalization | |
| Yes | 3 (2) |
| No | 154 (98) |
| Persistent symptoms | |
| Yes | 66 (42) |
| No | 91 (58) |
The most frequently reported source of infection was occupational (43%), which was attributed to HCW-to-HCW transmission (45%), patient-to-HCW transmission (36%) and undetermined (19%). All reported occupational infections occurred while using a surgical mask or without any mask. After investigating the places where most high-risk contacts occurred, it became apparent that the most frequent moments of transmission corresponded to meals and breaks in the staff and changing rooms without protective measures. The family source of infection occurred in 33% of the cases and the social source was reported in 2%. The source was unknown in 22% of the cases.
The mean duration of the disease was 24 days. National guidelines changed the cure criteria on October 14, consequently influencing the period of absenteeism [16]. Before this change, there were 49 HCWs infected, with a mean duration of the disease of 33 days, while of the 108 HCWs infected after October 14, the mean duration was reduced to 20 days. Of the latter, 15 HCWs returned to work early due to a negative RT-PCR detection test on the 10th day. Although 24% of the reported cases had comorbidities, only three cases (2%) were severe enough to require hospitalization, two requiring non-invasive ventilation. There were no HCW deaths related to COVID-19.
Discussion
This study analysed contact tracing surveys of HCWs in an oncology hospital and showed that the main source of SARS-CoV-2 infection was likely occupational. Interestingly, a significant portion of the cases reported an unknown source, which underlines the importance of transmission from asymptomatic patients [17,18]. On the other hand, our findings also show that contacts in the family household had a high prevalence (33%) in infected cases. This raises the question of whether symptom-based screening will effectively tackle the COVID-19 pandemic in healthcare settings [19].
This study has some limitations that need to be addressed, particularly the small sample size. Another concern is that the source of infection’s classification depended on the contact tracing survey, which may interfere with the validity of reported answers. In addition to recall bias, the asymptomatic transmission may be a confounding factor in some reported classifications of the source of infection. This would be better estimated by using whole-genome sequencing to establish clusters and transmission routes within healthcare settings.
In the 43% reported occupational infections investigation, there was an apparent epidemiological link when HCWs overlooked protective measures, particularly during meals and breaks in staff and dressing rooms. On the other hand, no occupational infections were reported while using FFP2/N95 respirators. These findings agree with previously published studies. Schneider et al. reported four healthcare-associated outbreaks of SARS-CoV-2 infections at a university hospital in Berlin, Germany, with 24 infected cases (23 HCWs and 1 patient) due to multiple unprotected contacts between HCWs [17]. In a study of 866 workers in a large university hospital in Helsinki, Finland, 54% of COVID-19 infections were confirmed or probable occupational, with 30% originating from co-workers [20]. Strengthening administrative control measures, improving social distancing barriers and limiting the number of people in staff and changing rooms could significantly reduce transmission in HCWs. Galanis et al. suggest that proper use of PPE and compliance with hygiene measures are associated with reduced seroprevalence of SARS-CoV-2 antibodies in HCWs [21]. Moreover, there is an urgent need to clarify the possibility of viable SARS-CoV-2 infection via airborne aerosols in asymptomatic patients [22]. Several studies support SARS-CoV-2 aerosol transmission [23,24], which has important implications in mitigation strategies, especially regarding indoor ventilation [25].
Most cases of SARS-CoV-2 infection reported were mild, which is in agreement with other studies [26]. There are still doubts about whether the severity of the disease may be related to the viral load of the initial inoculum [27], as is hypothesized in influenza [28,29]. If that is the case, HCWs may have a lower risk of a severe disease since they are better trained in the appropriate use of PPE and have access to FFP2/N95 respirators. On the other hand, IPO-Porto is a specialized oncology hospital and is not directly involved in treating COVID-19 patients. All cancer patients, even asymptomatic ones were tested regularly before hospital admission and before surgery, chemotherapy, radiotherapy or aerosol-generating procedures. If SARS-CoV-2 infection was detected, patients were referred for acute management in a general hospital. Thus, in our institution, the interaction between HCWs and COVID-19 patients is significantly lower than in other Portuguese hospitals, which may in part explain our analysis regarding occupational infections. To our knowledge, this is the first report of a Portuguese institution on this subject.
The loss of workforce in the first month of the pandemic (Figure 1) was exacerbated by both infected and quarantined HCWs. The peak of quarantine cases observed in the first wave may represent the first high-risk contacts with hospital inpatients, highlighting the initial shortcomings in infection control measures. Before October 14, when the national return-to-work criteria changed, HCWs were on sick leave for a longer period. This is evidenced by the reduction in the average duration of disease from 33 to 20 days. As the epidemiological understanding of COVID-19 evolved, we shifted from a definition of cure based exclusively on laboratory criteria (two negative tests) to a definition based on clinical criteria complemented with laboratory test results. The latter allows a quicker return to work, thus significantly shortening the period of absenteeism. In this regard, during the early stage of the pandemic, the testing capacity was not optimized. Therefore, quarantining personnel was a frequently used adaptive strategy of the OHS to ensure the isolation of high-risk cases. It is noteworthy that after the initial stage of the pandemic, quarantined cases decreased due to behavioural changes and progressive education regarding proper PPE usage, social distancing and handwashing measures.
A multidisciplinary occupational health team (physician, nurse, psychologist) was set up to monitor those absent from the workplace. Occupational nurses phoned quarantined or infected workers daily to carry out active disease surveillance. Once severe symptoms were identified, OHP would contact and refer the HCW to the emergency service. Whenever a mental health disturbance was identified, the occupational health psychologist would assist, and the OHP would subsequently follow up. Despite few serious cases, several workers (42%) have reported fatigue and intolerance to exercise upon returning to work. The persistent symptoms of the disease include respiratory, cardiovascular and neurological repercussions [30], which can impact HCWs’ working capacity. OHP proposed adjustments in the workload and tasks accordingly to their fitness to work.
Several questions remain regarding the spread of SARS-CoV-2 in HCWs and the best way to protect them. However, it is clear that the provision of safe and healthy workplaces for HCWs can prevent and mitigate the pandemic spread. When HCWs are on the verge of exhaustion and facing psychological distress, the central duty of occupational health is to support and protect workers’ health, providing them with the confidence to overcome this period as a strengthened society.
Competing interests
None declared.
References
- 1. Zhu N, Zhang D, Wang Wet al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus Disease (COVID-19): Weekly Epidemiological Update . 5 January 2021https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/2021 (8 January 2021, date last accessed).
- 3. Erdem H, Lucey DR. Healthcare worker infections and deaths due to COVID-19: a survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis 2021;102:239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chirico F, Magnavita N. The crucial role of occupational health surveillance for health-care workers during the COVID-19 pandemic. Workplace Health Saf 2021;69:5–6. [DOI] [PubMed] [Google Scholar]
- 5. Raymond E, Thieblemont C, Alran S, Faivre S. Impact of the COVID-19 outbreak on the management of patients with cancer. Target Oncol 2020;15:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chirico F, Magnavita N. COVID-19 infection in Italy: an occupational injury. S Afr Med J 2020;110:12944. [PubMed] [Google Scholar]
- 7. Zisook RE, Monnot A, Parker J, Gaffney S, Dotson S, Unice K. Assessing and managing the risks of COVID-19 in the workplace: applying industrial hygiene (IH)/occupational and environmental health and safety (OEHS) frameworks. Toxicol Ind Health 2020;36:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Occupational Safety and Health (NIOSH). Hierarchy of Controls . 2015. https://www.cdc.gov/niosh/topics/hierarchy/default.html (5 January 2021, date last accessed).
- 9. Rabeea K, Meyer J. How does the hierarchy of controls integrate with the epidemiologic triangle to help address and understand transmission of SARS-CoV-2? J Occup Environ Med 2020;62:e665–e668. [DOI] [PubMed] [Google Scholar]
- 10. de Perio MA, Dowell CH, Delaney LJet al. Strategies for optimizing the supply of N95 filtering facepiece respirators during the coronavirus disease 2019 (COVID-19) pandemic. Disaster Med Public Health Prep 2020;14:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asad H, Johnston C, Blyth Iet al. Health care workers and patients as Trojan horses: a COVID-19 ward outbreak. Infect Prevent Pract 2020;2:100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbas M, Robalo NT, Martischang Ret al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control 2021;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duarte I, Teixeira A, Castro Let al. Burnout among Portuguese healthcare workers during the COVID-19 pandemic. BMC Public Health 2020;20:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control. Contact Tracing: Public Health Management of Persons, Including Healthcare Workers, Who Have Had Contact With COVID-19 Cases in the European Union. Stockholm, Sweden: ECDC, 2020. https://www.ecdc.europa.eu/en/covid-19-contact-tracing-public-health-management#no-link (4 January 2021, date last accessed). [Google Scholar]
- 15. Direção Geral de Saúde (DGS). Norma nº 015/2020 de 24/07/2020. COVID-19: Rastreio de Contactos . 2020. https://covid19.min-saude.pt/normas/ (4 January 2021, date last accessed).
- 16. Direção Geral de Saúde (DGS). Norma nº 004/2020 de 14/10/2020. COVID-19: Abordagem do Doente com Suspeita ou Infeção por SARS-CoV-2. 2020. https://covid19.min-saude.pt/normas/ (4 January 2021, date last accessed).
- 17. Schneider S, Piening B, Nouri-Pasovsky PA, Krüger AC, Gastmeier P, Aghdassi SJS. SARS-coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control 2020;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao D, Wang M, Wang Met al. Asymptomatic infection by SARS-CoV-2 in healthcare workers: a study in a large teaching hospital in Wuhan, China. Int J Infect Dis 2020;99:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson MA, Quandelacy TM, Kada Set al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 2021;4:e2035057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oksanen LA, Sanmark E, Oksanen SAet al. Sources of healthcare workers’ COVID-19 infections and related safety guidelines. Int J Occup Med Environ Health 2021;34:239–249. [DOI] [PubMed] [Google Scholar]
- 21. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. J Hosp Infect 2021;108:120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Priyanka C, Choudhary OP, Singh I, Patra G. Aerosol transmission of SARS-CoV-2: the unresolved paradox. Travel Med Infect Dis 2020;37:101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacIntyre CR, Ananda-Rajah MR. Scientific evidence supports aerosol transmission of SARS-COV-2. Antimicrob Resist Infect Control 2020;9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang S, Mao Y, Jones RMet al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int 2020;144:106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jarvis MC. Aerosol transmission of SARS-CoV-2: physical principles and implications. Front Public Health 2020;8:590041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomez-Ochoa SA, Franco OH, Rojas LZet al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2021;190:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Damme W, Dahake R, van de Pas R, Vanham G, Assefa Y. COVID-19: does the infectious inoculum dose–response relationship contribute to understanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses 2021;146:110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paulo AC, Correia-Neves M, Domingos T, Murta AG, Pedrosa J. Influenza infectious dose may explain the high mortality of the second and third wave of 1918–1919 influenza pandemic. PLoS One 2010;5:e11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price I, Mochan-Keef ED, Swigon Det al. The inflammatory response to influenza A virus (H1N1): an experimental and mathematical study. J Theor Biol 2015;374:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang C, Huang L, Wang Yet al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]