ABSTRACT
A 67-year-old female with Type 2 diabetes mellitus developed nephrotic syndrome within 1 week of receiving the first dose of severe acute respiratory syndrome coronavirus 2 CoronaVac vaccine. A kidney biopsy was consistent with minimal change nephrotic syndrome and treatment was symptomatic with antiproteinuric therapy and improvement in proteinuria. Oedema returned within 1 week of the second dose of CoronaVac. On this occasion, acute kidney injury and massive proteinuria were noted. In kidney biopsy, glomeruli were normal, but tubulointerstitial inflammation consistent with acute tubulointerstitial nephritis was noted. Pulse followed by oral steroids was followed by recovery of kidney function. Proteinuria decreased after initiation of cyclosporine A.
Keywords: acute interstitial nephritis, acute kidney injury, COVID-19 vaccination, kidney biopsy, minimal change disease, nephrotic syndrome, proteinuria
INTRODUCTION
In this study we present a patient who applied with nephrotic syndrome findings ∼20 days after the first dose of CoronaVac vaccine and who had minimal change disease (MCD) on kidney biopsy, after which the proteinuria regressed to non-nephrotic levels. Due to the ongoing severe coronavirus disease 2019 (COVID-19)-related global pandemic conditions and the patient being in the high-risk group for severe infection, the patient received a second dose of CoronaVac vaccine, which is the only vaccine in use in Turkey with the indication for emergency use, after which the patient experienced excessively increased proteinuria and rapid deterioration in kidney functions.
CASE
A 67-year-old female with Type 2 diabetes mellitus received the first dose of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) CoronaVac on 13 February 2021. A second dose of vaccination was not allowed at this stage. On 3 March 2021, she complained of lower limb swelling that had started 2 weeks earlier and increased gradually, associated with 7 kg weight gain. Physical examination revealed weight 66 kg, blood pressure 150/95 mmHg and bilateral pretibial oedema. Biochemical examination showed fasting blood glucose 178 mg/dL, urea 21 mg/dL, creatinine 0.6 mg/dL, total cholesterol 524 mg/dL, total protein 4.1 g/dL, serum albumin 2.2 g/dL and 9.0 g protein in 24-h urine. A diagnosis of nephrotic syndrome was made. Serum complement C3 and C4 levels were within normal limits and anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, hepatitis B surface antigen, anti-hepatitis C antibody and anti-HIV antibody were negative. Urine sediment did not show haematuria. A SARS-CoV-2 reverse transcriptase polymerase chain reaction test was negative twice. There was no evidence of monoclonal gammopathy in serum and urine. Her only medication was once a day 60 mg gliclazide and once a day 100 mg sitagliptin, and proteinuria had not been assessed in the past. She was started on ramipril 10 mg once a day, valsartan 80 mg once a day, nebivolol 5 mg once a day, rosuvastatin 20 mg once a day and furosemide 40 mg orally once a day.
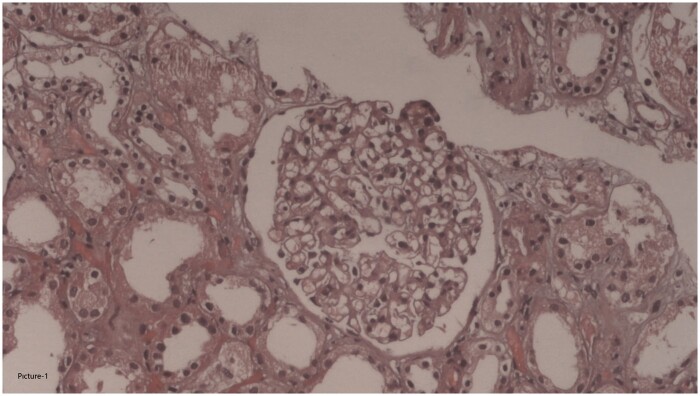
No retinopathy findings were detected in the fundus examination. A kidney biopsy performed on 17 March 2021 contained 45 glomeruli; one was globally sclerotic. The rest were of normal size and cellular structure. Interstitial inflammation, fibrosis or tubular atrophy were not observed. Vascular structures were normal. No amyloid deposition was detected in the Congo red staining. No evidence of immunoglobulin A (IgA), IgM, IgG, C3, C1q, fibrinogen, kappa light chain or lambda light chain deposition was found in eight glomeruli by immunofluorescence. Thus, the biopsy was consistent with minimal change glomerulopathy (Figure 1).
FIGURE 1:
Clinical laboratory findings of the patient after the first dose of the vaccine. MMF, mycophenolate mofetil.
During follow-up, proteinuria decreased to 2.5 g/day and weight to 62 kg with dual renin–angiotensin–aldosterone system (RAAS) blockade. It was decided to continue with dual RAAS blockade (Figure 2).
FIGURE 2:
Clinical laboratory findings of the patient after the second dose of the vaccine.
A second dose of CoronaVac vaccine, the only vaccine available at the time, was approved considering severe pandemic conditions and the patient’s high risk for severe infection. It was administered on 2 April 2021, while the patient was in partial remission.
Approximately 3 weeks after the second vaccine dose, the patient presented with headache and swelling all over the body. Symptoms had started 1 week after the second dose. Weight was 75 kg, arterial blood pressure 180/100 mmHg, urea 102 mg/dL, creatinine 4.2 mg/dL, total protein 4 g/dL, albumin 1.5 g/dL and proteinuria 18.6 g/day. The urinary sediment contained 6–7 white blood cell count and ++ haematuria. Ultrasound disclosed normal bilateral kidney size (right 127 mm, left 123 mm) and increased echogenicity suggesting parenchymal kidney disease.
A second kidney biopsy was performed on 30 April 2021 and then 500 mg pulse steroid therapy was started and repeated for 3 days. Anti-glomerular basement membrane antibody was negative, complement C3 and C4 levels were normal, and viral screening data were negative.
The second kidney biopsy contained 25 glomeruli with normal size and cellularity. Hydropic degeneration of proximal tubular cells and interstitial inflammation consisting of lymphocytes and eosinophils in the medullary area were observed. Proteinaceous material was detected in many tubule lumens. No pathological features or signs of vasculitis were detected in arteries and arterioles. A diagnosis of acute interstitial nephritis was made (Figure 3).
FIGURE 3:
Normal histological appearance of glomerulus seen in first renal biopsy. Normal interstitial area is also discernible (haematoxylen & eosin, ×200).
Treatment was continued with oral 1 mg/kg prednisolone. Serum creatinine peaked at 5.2 mg/dL, and had decreased to 1.12 mg/dL by 30 May 2021, but proteinuria continued at 16 g/day. Thus, the steroid dose was reduced, and 150 mg/day cyclosporine A was added in two divided doses on 28 May 2021. At last follow-up proteinuria was still 3 g/day (26 July 2021, Figure 4).
FIGURE 4:
Histological appearance of second renal biopsy. (A) Normal histological appearance of glomeruli (haematoxylen & eosin, ×200). (B) Interstitial inflammation composed predominantly of lymphocytes. Hyalin casts are also discernible inside the tubules (haematoxylen & eosin, ×400).
DISCUSSION
Severe COVID-19 is more frequent in patients over 65 years of age, and in high-risk patients such as those with cancer or other causes of immune deficiency, chronic kidney disease, diabetes, obesity and asthma [1, 2]. Vaccines are cost-effective in preventing COVID-19 disease and related deaths [3]. CoronaVac is a formalin-inactivated SARS-CoV-2 vaccine produced by Sinovac Life Science (Beijing, China) [4, 5], and it is the first vaccine approved for emergency use by the Ministry of Health in Turkey [6]. It started to be used as of January 2021, primarily for healthcare personnel and advanced age groups with a high risk of severe disease and mortality.
Post-vaccine nephrotic syndrome is not a rare condition. There are reports of MCD after influenza, tetanus and pneumococcal vaccination [7–9]. MCD and acute kidney injury were observed after SARS-CoV-2 mRNA Pfizer-BioNTech vaccination in a 50-year-old male. Complaints started a few days after vaccine administration, and responded to steroids [10]. In a second case reported after Pfizer-BioNTech vaccination, a 77-year-old male developed 23 g/day proteinuria and acute kidney injury, and biopsy suggested MCD and acute tubular injury [11]. A third case was a 63-year-old female who developed MCD with proteinuria of 13.4 g/day after the administration of SARS-CoV-2 mRNA Moderna vaccine [12]. Unlike prior cases, our case developed nephrotic syndrome after an inactivated vaccine, and it is the first case in which acute kidney injury due to acute interstitial nephritis was observed after the second dose of vaccine followed by the regression of proteinuria to non-nephrotic levels after dual RAAS blockade.
Vaccination may cause relapses in patients with nephrotic syndrome. Mild relapses were observed in 104 paediatric patients diagnosed with primary nephrotic syndrome after inactivated influenza vaccine was administered. Histopathological diagnoses, including MCD and focal segmental glomerulosclerosis, were made in 91% of patients, who continued immunosuppressive therapy. In this study, 104 paediatric patients received 208 flu vaccines. There were 261 nephrotic syndrome relapses between days −180 and +180. Compared with the relapse rate in the −180 to 0 interval (1.19 times/person-year), those in the 0 to +180 interval (1.2 to 1.3–1.5 times/person-year) days groups were slightly increased, but without significance. Despite this risk of relapse, the authors supported flu vaccination of paediatric nephrotic syndrome [13].
The present patient received a second dose of CoronaVac, considering that the high risk for severe COVID-19. However, more severe proteinuria and acute kidney injury due to acute interstitial nephritis developed. The patient, who was under follow-up after the first biopsy, did not have a history of drug use that could cause acute interstitial nephritis, except for the drugs that were started for the treatment of nephrotic syndrome.
We hypothesize that patient may have become sensitized to components of the first dose of vaccine.
PATIENT CONSENT
Written informed consent was obtained from the patient for the publication of the case report.
CONFLICT OF INTEREST STATEMENT
None declared.
Contributor Information
Suat Unver, Department of Nephrology, Liv Hospital Vadistanbul, İstanbul, Turkey.
Aptullah Haholu, Department of Pathology, Maltepe University Faculty of Medicine, Istanbul, Turkey.
Sukru Yildirim, Department of Pathology, Maltepe University Faculty of Medicine, Istanbul, Turkey.
REFERENCES
- 1. Mallah SI, Ghorab OK, Al-Salmi S et al. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob 2021; 20: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev 2021; 170: 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Zeng G, Pan H et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Z, Hu Y, Xu M et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; www.thelancet.com/infectionPublishedonline (27 March 2021, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavsan S. Sinovac’s COVID-19 shot is 83% effective, not 91%, Turkey says. Nikkei Asia. https://asia.nikkei.com/Spotlight/Coronavirus/COVID-vaccines/Sinovac-s-COVID-19-shot-is-83-effective-not-91-Turkey-says (9 April 2021, date last accessed)
- 7. Gutierrez S, Dotto B, Petiti JP et al. Minimal change disease following influenza vaccination and acute renal failure: just a coincidence? Nefrologia 2012; 32: 414–415 [DOI] [PubMed] [Google Scholar]
- 8. Kielstein JT, Termuhlen L, Sohn J et al. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol 2000; 54: 246–248 [PubMed] [Google Scholar]
- 9. Kikuchi Y, Imakiire T, Hyodo T et al. Minimal change nephrotic syndrome, lymphadenopathy and hyperimmunoglobulinemia after immunization with a pneumococcal vaccine. Clin Nephrol 2002; 58: 68–72 [DOI] [PubMed] [Google Scholar]
- 10. Lebedev L, Saxpojnikov M, Wechsler A et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021; 78: 142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Agati VD, Kudose S, Bomback AS et al. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int 2021; 100: 461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holzworth A, Couchot P, Cruz-Knight W et al. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 2021; 100: 463–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishimori S, Kamei K, Ando T et al. Influenza virus vaccination in children with nephrotic syndrome: insignificant risk of relapse. Clin Exp Nephrol 2020; 24: 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]