Abstract
Background
The use of remdesivir has demonstrated a significant reduction in the time to recovery in patients with COVID-19. However, the impact on mortality is still controversial. Therefore, it is necessary to evaluate whether there is a specific subgroup of patients in whom an active antiviral therapy also reduces the mortality.
Methods
Patients admitted for >48 h in our hospital for a SARS-CoV-2 confirmed or suspected infection from February 2020 to February 2021 were retrospectively analysed. The primary outcome of the study was mortality at 30 days. Univariate and multivariate analyses were performed to identify predictors of mortality.
Results
In total, 2607 patients (438 receiving remdesivir and 2169 not) were included with a median (IQR) age of 65 (54–77) years and 58% were male. Four hundred and seventy-six were admitted to the ICU (18.3%) and 264 required invasive mechanical ventilation (10.1%). The global 30 day mortality rate was 10.7%. Pre-admission symptom duration of 4–6 days and ≤3 days was associated with a 1.5- and 2.5-fold increase in the mortality rate, respectively, in comparison with >6 days and treatment with remdesivir was independently associated with a lower mortality rate (OR = 0.382, 95% CI = 0.218–0.671). The analysis showed that the major difference was among patients with shorter pre-admission symptom duration (<6 days).
Conclusions
Patients with ≤3 days and 4–6 days from symptom onset to admission are associated with a 2.5- and 1.5-fold higher risk of death, respectively. Remdesivir was associated with 62% reduced odds of death versus standard-of-care and its survival benefit increased with shorter duration of symptoms.
Introduction
Up to April 2021, more than 140 million cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection had been detected around the world with more than 3 million related deaths. A recent analysis of 44 415 confirmed cases in China described that 81% were asymptomatic or mild, 14% were severe and 5% were critical with an overall mortality of 2.3%.1 Among patients that require hospitalization, both the rate of ICU admission and mortality are around 20%.2–4 On 1 May 2020, remdesivir received FDA emergency use authorization for hospitalized patients with COVID-19 and was officially approved on 22 October 2020 and received a conditional marketing authorization valid throughout the EU on 3 July 2020. Initial clinical trials using a control arm demonstrated the superiority of remdesivir in terms of clinical status improvement at day 285 or at day 11;6 however, initial clinical trials performed in China7 and the Solidarity trial8 did not find benefit in either clinical improvement or mortality. Different outcomes and the potential influence of when remdesivir was administered after symptom onset could explain the apparently controversial results in the aforementioned trials.
Recent data showed that, in patients admitted for COVID-19, both a shorter time since the onset of symptoms to testing and a lower cycle threshold (Ct) value of real-time RT–PCR (rRT–PCR) at admission (meaning high viral load) are independent predictors of mortality.9 The highest mortality rate was observed in those patients with ≤3 days from symptom onset to test. The ACTT-1 trial randomized patients to remdesivir versus placebo and the subgroup analysis demonstrated that the major benefit, in terms of time to recovery, was observed in patients with <10 days from symptom onset to remdesivir.5 In addition, in Supplementary Material, the authors showed that the major difference in time to recovery was observed in those with <6 days from symptom onset (10 days versus 24 days, HR = 1.92). However, the potential impact of remdesivir on the mortality rate according to the length of pre-admission symptom duration is not known.
The aim of our study was to retrospectively evaluate the outcome of COVID-19 patients admitted to our hospital from February 2020 to January 2021 and to define the impact of remdesivir administration on the mortality rate according to pre-admission symptom duration.
Methods
Study design and patients
This observational cohort study was performed at Hospital Clinic of Barcelona (Spain), a 700 bed university centre that provides care for an urban population of 500 000 adults. All patients admitted for ≥48 h with COVID-19 confirmed by rRT–PCR performed on nasopharyngeal throat swabs or a clinical picture highly suggestive of COVID-19 between 18 February 2020 and 24 February 2021 were included. Deaths that occurred within the first 48 h were included in the analysis. The Institutional Ethics Committee of Hospital Clinic of Barcelona approved the study and, due to the nature of the retrospective data review, waived the need for informed consent from individual patients (HCB/2020/0273).
From February 2020 to June 2020, patients receiving remdesivir were those included in two clinical trials. From July 2020, remdesivir treatment had to be approved by the Spanish Agency of Drugs and Health Products. Criteria to prescribe remdesivir included hospitalized patients with severe pneumonia due to SARS-CoV-2 documented by rRT–PCR, serology or antigen test and all of the following characteristics: (i) aged >12 years and >40 kg; (ii) need of supplemental low-flow oxygen; (iii) ≤7 days from symptom onset to remdesivir prescription; and (iv) met at least two of these three criteria: respiratory rate ≥24 breaths per min (bpm), oxygen saturation at ambient air ≤94% or PaO2/FiO2 <300 mmHg. Exclusion criteria included requirement of supplemental high-flow oxygen, mechanical ventilation, vasoactive drugs, extracorporeal membrane oxygenation or fulfilling the criteria for multiorgan failure at the moment of prescription. Contraindications included AST and ALT ≥5 times the normal range values, glomerular filtration <30 mL/min, haemodialysis or peritoneal dialysis. Exceptionally, some patients not fulfilling these criteria received remdesivir because the physician in charge considered it necessary due to comorbidity, severity or other criteria. The duration of remdesivir treatment in our institutional protocol was 5 days.
Data collection
Data were retrospectively collected for all patients included in the study from the electronic health records (EHRs). An intelligent system was used to retrieve the high-quality data from EHRs (SILDv1.0 system, S34M@) as previously described.10 Variables included were age, sex, pre-admission duration of symptoms in days, comorbidities (hypertension, chronic heart disease, diabetes mellitus, chronic liver disease, chronic kidney disease, chronic obstructive pulmonary disease, haematological neoplasia and solid neoplasia), respiratory rate and ambient air arterial oxygen saturation (SaO2) measured with a pulse oximeter at admission, creatinine, lymphocyte count, C-reactive protein and lactate dehydrogenase within the first 24 h from hospital admission. Information on the need of ICU admission and invasive mechanical ventilation was gathered and information on treatment with remdesivir and the use of steroids or tocilizumab within the first 3 days from admission was also gathered. The primary endpoint was mortality at 30 days.
Statistical analysis
Categorical variables were described using the absolute number and percentage and continuous variables were dichotomized according to the median. Categorical variables were compared using a χ2 test or Fisher’s exact test when necessary. The cumulative probability of dying within 30 days after hospital admission by pre-admission duration of symptoms is shown using a Kaplan–Meier plot and the differences were evaluated using the Log Rank test. For multivariable analysis, variables with a P value ≤0.05 in the univariable analysis were subjected to further selection by using a forward logistic regression method. Interactions between remdesivir and other variables were explored. The calibration of the model was assessed by means of the Hosmer–Lemeshow goodness-of-fit test and the area under the receiver operating characteristic (ROC) curve was used to measure the predictive ability of the model. Statistical significance was defined as a two-tailed P value <0.05. The propensity score (PS) to define the characteristics of patients that received remdesivir was calculated using a multivariate analysis with remdesivir as the dependent variable (results provided in the Supplementary data available at JAC Online). The analysis was performed in SPSS version 26 (SPSS Inc., Chicago, IL, USA).
Results
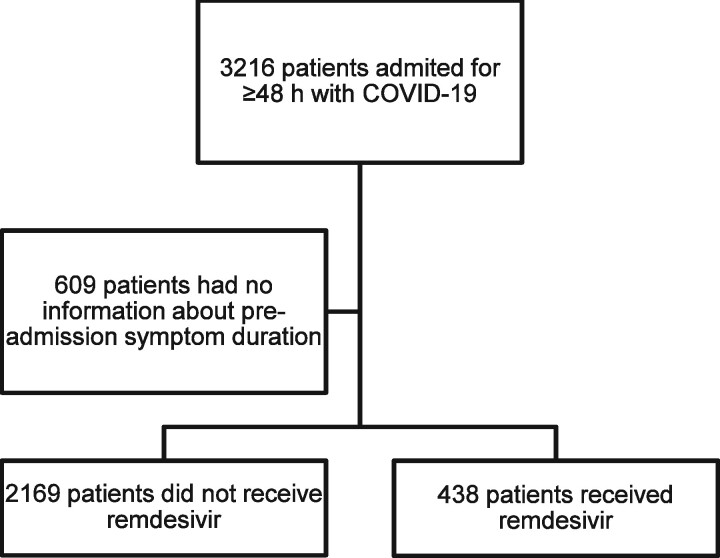
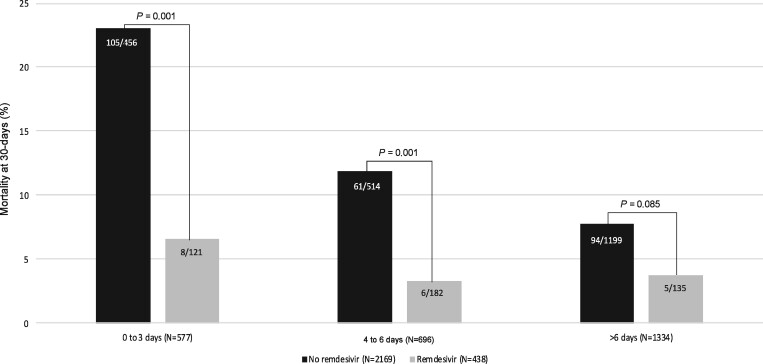
The population evaluated (n = 2607) is shown in Figure 1. The median (IQR) age was 65 (54–77) years and 58% were male. The median (IQR) pre-admission duration of symptoms was 7 (4–9) days. The most common comorbidities were hypertension (45.8%), chronic heart disease (24.4%), chronic pulmonary disease (24.2%), diabetes mellitus (11.4%), solid neoplasia (14.2%) and chronic renal failure (12.3%). A total of 476 patients were admitted to the ICU (18.3%) and 264 required invasive mechanical ventilation (10.1%). The global 30 day mortality rate was 10.7%; 4.3% among those that received remdesivir (19 out of 438) and 12% among those who did not receive remdesivir (260 out of 2169, P < 0.001). The corresponding rates for patients receiving or not receiving remdesivir stratified by pre-admission symptom duration are depicted in Figure 2. The major difference was observed in patients with a short duration of symptoms, 6.6% versus 23%, respectively, for ≤3 days and 3.3% versus 11.9%, respectively, for 4–6 days (P = 0.001 for both comparisons). There was a trend among patients with pre-admission duration of symptoms >6 days, but the difference was not statistically significant (3.7% versus 7.8%, respectively, P = 0.085).
Figure 1.
Flowchart of the population selected for the analysis.
Figure 2.
Mortality rate at 30 days by remdesivir treatment and the pre-test duration of symptoms (proportion comparisons using χ2 test).
Variables associated with 30 day mortality are shown in Table 1. Age, comorbidities (chronic heart disease, hypertension, diabetes mellitus, chronic renal failure, chronic pulmonary disease, haematological malignancies and solid neoplasm), pre-admission symptom duration (Figure 3), respiratory failure at admission (respiratory rate >21 bpm and SaO2 ≤94%), absence of fever and the need of invasive mechanical ventilation were significantly associated with mortality. Among biochemical parameters, high serum concentrations of creatinine, lactate dehydrogenase and C-reactive protein, as well as lymphopenia, were also associated with a higher mortality rate. Treatment with remdesivir appeared as the only protective factor. Independent factors associated with mortality are depicted in Table 2. Our analysis showed the importance of pre-admission duration of symptoms, showing a 1.5- and 2.5-fold increase in the mortality rate in those with 4–6 days and ≤3 days, respectively, compared with those with >6 days. As expected, age, renal failure, lymphopenia and C-reactive protein were also independent predictors of mortality. After adjusting for all these variables, remdesivir remained as an independent protective factor (OR = 0.382, 95% CI = 0.218–0.671). Similar results were obtained using the variable duration of symptoms as a continuous one in the multivariate model instead of its categorization. The goodness-of-fit of the model was assessed with the Hosmer–Lemeshow test (P > 0.05) and the area under the ROC curve was 0.869 (95% CI = 0.845–0.892, P = 0.0001), showing a good ability to predict mortality at 30 days. The addition of the PS for remdesivir (Table S1 and Table S2, available as Supplementary data at JAC Online) did not significantly change the results of the multivariate analysis (Table S3).
Table 1.
Variables associated with 30 day mortality
| Variable | Alive (N = 2328) | Died (N = 279) | P |
|---|---|---|---|
| Age >66 years, n (%) | 963 (41.5) | 251 (90) | 0.001 |
| Male, n (%) | 1339 (57.8) | 167 (59.9) | 0.522 |
| Hypertension, n (%) | 998 (42.9) | 196 (80.3) | 0.001 |
| Diabetes mellitus, n (%) | 423 (18.2) | 84 (30.1) | 0.001 |
| Chronic heart disease, n (%) | 487 (20.9) | 150 (53.8) | 0.001 |
| Chronic pulmonary disease, n (%) | 541 (23.2) | 89 (31.9) | 0.002 |
| Chronic renal failure, n (%) | 201 (8.6) | 97 (34.8) | 0.001 |
| Liver disease, n (%) | 156 (6.7) | 17 (6.1) | 0.799 |
| Haematological malignancy, n (%) | 136 (5.8) | 32 (11.5) | 0.001 |
| Solid neoplasm, n (%) | 306 (13.1) | 65 (23.3) | 0.001 |
| Solid organ transplantation, n (%) | 45 (1.9) | 9 (3.2) | 0.177 |
| HIV, n (%) | 34 (1.5) | 5 (1.8) | 0.602 |
| Pre-admission duration of symptoms, n (%) | |||
| ≤3 days of symptoms | 464 (19.9) | 113 (40.5) | 0.001 |
| 4–6 days of symptoms | 629 (27) | 67 (24) | 0.001 |
| >6 days of symptoms | 1235 (53) | 99 (35.5) | 0.001 |
| Temperature >37°C (N = 2554), n (%) | 1184 (51.4) | 104 (41.4) | 0.003 |
| Respiratory rate >21 bpm at day 1–2 (N = 2128), n (%) | 891 (46.7) | 168 (76.7) | 0.001 |
| Oxygen saturation ≤94% (N = 2546), n (%) | 1203 (52.4) | 188 (74.9) | 0.001 |
| Creatinine >0.92 mg/dL (N = 2593), n (%) | 1017 (43.9) | 211 (77) | 0.001 |
| Lactate dehydrogenase >305 U/L (N = 2524), n (%) | 1096 (48.4) | 171 (66.3) | 0.001 |
| Lymphocyte count ≤800 cells/mm3 (N = 2593), n (%) | 1127 (48.6) | 182 (66.4) | 0.001 |
| C-reactive protein >7.52 mg/dL (N = 2590), n (%) | 1127 (48.7) | 198 (72.3) | 0.001 |
| Remdesivir, n (%) | 419 (18) | 19 (6.8) | 0.001 |
| Tocilizumab within 3 days, n (%) | 140 (6) | 18 (6.5) | 0.790 |
| Corticosteroids within 3 days, n (%) | 264 (11.3) | 27 (9.7) | 0.481 |
| ICU admission, n (%) | 415 (17.8) | 61 (21.9) | 0.101 |
| Invasive mechanical ventilation, n (%) | 214 (9.2) | 50 (17.9) | 0.001 |
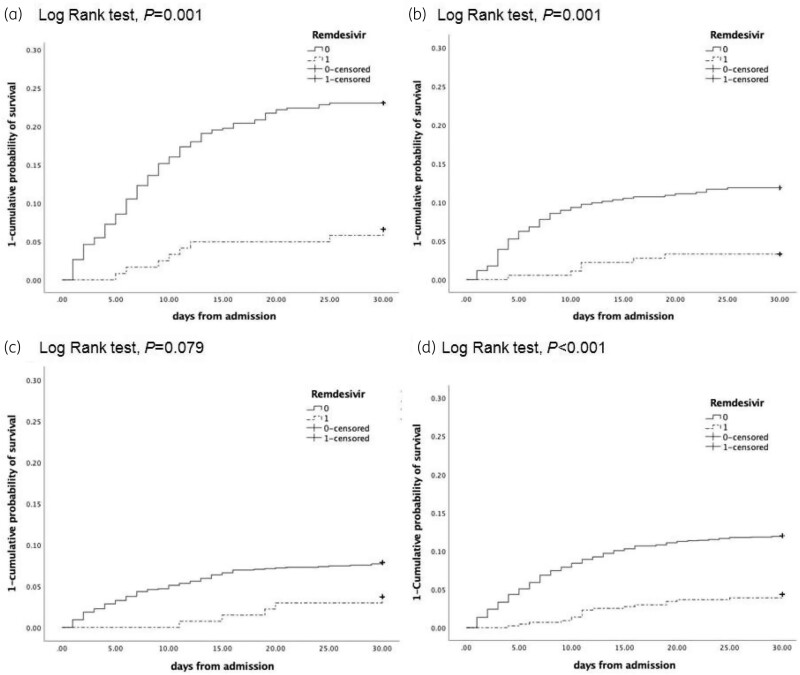
Figure 3.
One minus the cumulative probability of survival by remdesivir treatment. (a) Patients with pre-admission duration of symptoms ≤3 days. (b) Patients with pre-admission duration of symptoms of 4–6 days. (c) Patients with pre-admission duration of symptoms >6 days. (d) The entire population included in the study. Remdesivir treatment corresponds to 1 (continuous line) and no remdesivir corresponds to 0 (broken line).
Table 2.
Independent predictors associated with 30 day mortality
| Variable | OR (95% CI) | P |
|---|---|---|
| Age >66 years | 8.763 (5.232–14.676) | 0.001 |
| Chronic renal failure | 2.442 (1.622–3.677) | 0.001 |
| Pre-admission duration of symptoms | ||
| >6 days | 1 | – |
| 4–6 days | 1.588 (1.042–2.422) | 0.031 |
| ≤3 days | 2.587 (1.722–3.887) | 0.001 |
| Oxygen saturation ≤94% | 1.631 (1.108–2.398) | 0.013 |
| Respiratory rate >21 bpm | 3.068 (2.080–4.525) | 0.001 |
| Mechanical ventilation | 1.820 (1.170–2.829) | 0.008 |
| Creatinine >0.92 mg/dL | 1.803 (1.208–2.693) | 0.004 |
| Lymphocyte count ≤800 cells/mm3 | 1.650 (1.156–2.358) | 0.006 |
| C-reactive protein >7.52 mg/dL | 1.956 (1.353–2.828) | 0.001 |
| Remdesivir | 0.382 (0.218–0.671) | 0.001 |
Discussion
To the best of our knowledge, this is the first report assessing the mortality rate of COVID-19 patients receiving or not receiving remdesivir according to the pre-admission duration of symptoms. Our results show a low mortality rate (4.3% versus 12%, respectively) in hospitalized patients receiving remdesivir. This result is in line with that reported in the ACTT-1 study that randomized patients to remdesivir or placebo.5 Our patients mainly correspond to those in the ACTT-1 study with a baseline ordinal score of 5 (hospitalized patients requiring supplemental oxygen) who had a mortality rate of 4% in the remdesivir arm versus 12.7% in the control arm, perfectly matching with our results. The primary endpoint of the ACTT-1 study was the time to recovery and the authors showed that the major difference was observed in patients with <6 days of symptoms; however, the impact on the mortality rate was not reported. Our results also match with those from a comparative study between patients included in a Phase 3, randomized, open-label trial where patients with severe COVID-19 received remdesivir11 and a parallel study in centres where remdesivir was not available and the authors selected patients with similar characteristics.12 The mortality rate at 14 days was 7.6% versus 12.5%, respectively, with a 62% reduction in the odds of death versus standard-of-care treatment that corresponds to the same benefit observed in our study (OR = 0.382, 95% CI = 0.218–0.671).
The viral load in infections caused by respiratory viruses, including SARS-CoV-2, achieves peak levels at the moment of symptom onset13 and from here the immune system, mainly mediated by type I IFN production, progressively controls viral replication.14 Data from a clinical trial to evaluate the efficacy of a neutralizing monoclonal antibody against SARS-CoV-2 including patients with mild or moderate COVID-19 demonstrated that those requiring hospital admission poorly control viral replication.15 Several mechanisms explain why SARS-CoV-2 evades a type I IFN response, including self-proteins that interfere with IFN activity,16–18 auto-antibodies against type I IFN or inborn errors of type I IFN19 that finally define a pattern of progression to severe disease characterized by low serum levels of type I IFN and a high viral load.20 Indeed, an animal model and three clinical trials (two with inhaled preparations) using IFN at early stages demonstrated clinical and virological improvements.21–24 Accordingly, the Ct of rRT–PCR, as a surrogate marker of the viral load, has been associated with a higher mortality rate in a large cohort of patients.25 It is reasonable to hypothesize that patients with less viral replication control trigger a more potent and earlier inflammatory response leading to a higher mortality. Indeed, recent data support this concept by demonstrating that both the shorter duration of symptoms prior to testing (≤3 days) and a high viral load were associated with a higher mortality.9 Unfortunately, we don’t have data about the viral load, but our results confirm that ≤3 days and 4–6 days from symptom onset to admission are associated with a 2.5- and 1.5-fold higher risk of death, respectively.
Remdesivir is an inhibitor of RNA-dependent RNA polymerase of SARS-CoV-2 with an EC50 of 1.65 μM when measured in Vero6 cells, but 0.01 μM when the analysis is done using human respiratory cells, due to the low capacity of Vero6 cells to metabolize remdesivir.26 In addition, animal models have demonstrated the capacity of remdesivir to reduce the viral load compared with a control.26,27 However, clinical trials showed apparently contradictory results5,7,8,28 and only one study that evaluated the dynamics of viral load did not show benefit in the remdesivir arm.7 Our study shows that the beneficial effect of remdesivir is linked to the number of days from symptom onset. The shorter the pre-admission duration of symptoms the higher the difference in the mortality rate between patients receiving or not receiving remdesivir (Figure 2) that probably represents the subpopulation in whom the expected viral load is higher and the type I IFN production lower. The lack of information on the duration of symptoms prior to admission in the Solidarity trial does not allow us to rule out that most of the patients would have had >6 days of symptoms explaining the negative results. In the case of the Chinese trial, 7 the median number of days from symptom onset was 11 and 19% of the patients included in the study had undetectable viral RNA on the nasopharyngeal swab taken at baseline and so probably did not capture the population that benefits the most from remdesivir treatment.
The main limitation of our study is its retrospective design; however, this is a unicentric study with a common protocol for the management of patients with severe COVID-19. The second limitation is the lack of information about the viral load to better define the efficacy of remdesivir, but unfortunately the Ct of rRT–PCR was not available from the EHRs. The third limitation is the lack of chest X-ray data, but pneumonia is almost universal in patients admitted due to COVID-19 although the extension of the lung infiltrates could not be assessed. Finally, we don’t have data about potential adverse events associated with remdesivir administration.
In conclusion, ≤3 days and 4–6 days from symptom onset to admission are associated with a 2.5- and 1.5-fold higher risk of death, respectively. Remdesivir was associated with 62% reduced odds of death versus standard-of-care treatment, but the difference was observed mainly among patients with ≤6 days of symptoms before being admitted to the hospital. In the future, the impact of baseline viral load should be incorporated in the analysis of remdesivir efficacy and, together with the duration of symptoms prior to hospital admission, both should be included to design future trials involving antivirals.
Supplementary Material
Acknowledgements
Members of the Hospital Clinic of Barcelona COVID-19 Research Group
Infectious Diseases’ Research Group: Blanco JL, Mallolas J, Martínez E, Martínez M, Miró JM, Moreno A, Solá M, Ugarte A, Gonzalez-Cordón A, Laguno M, Leal L, Rojas J, Torres B and all the staff members.
Medical Intensive Care Unit: Fernandez S, Tellez A, Fuentes F and Ayala M.
Department of International Health: Campubri D, de Alba MT, Fernandez M, Ferrer E, Grau B, Marti H, Muelas M, Pinazo MJ, Rodriguez N, Roldan M, Subira C, Vera I, Williams N, Almuedo-Riera A, Muñoz J and all the staff members.
Department of Internal Medicine: Aldea A, Camafort M, Calvo J, Capdevila A, Cardellach F, Carbonell I, Coloma E, Foncillas A, Estruch R, Feliu M, Fernández-Solá J, Fuertes I, Gabara C, Grafia I, Ladino A, López-Alfaro R, López-Soto A, Macaya I, Masanés F, Matas A, Navarro M, Marco-Hernández J, Miguel L, Milisenda JC, Moreno P, Naval J, Nicolás D, Oberoi H, Padrosa J, Prieto-González S, Pellicé M, Ribot J, Rodríguez-Núnez O, Sacanella E, Seguí F, Sierra C, Tomé A, Torres M, Ventosa H, Zamora-Martínez C and all the staff members.
Department of Microbiology: Almela M, Alvarez M, Bosch J, Costa J, Cuesta G, Fidalgo B, Gonzàlez J, Marco F, Narvaez S, Pitart C, Rubio E, Vergara A, Valls ME, Zboromyrska Y and all the staff members.
Department of Pharmacy: López E and all the staff members.
Funding
This work was financed by ad hoc patronage funds for research on COVID-19 from donations from citizens and organizations to the Hospital Clínic de Barcelona-Fundació Clínic per a la Recerca Biomèdica.
Transparency declarations
Carolina Garcia-Vidal has received honoraria for talks on behalf of Gilead Sciences, MSD, Novartis, Pfizer, Janssen and Lilly, as well as grants from Gilead Sciences and MSD. Laura Morata has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer and Angelini. Pedro Puerta-Alcalde has received honoraria for talks on behalf of Gilead Sciences and MSD. Montse Tuset has received grants from Janssen, Gilead, ViiV and Merck Sharp and Dohme. Josep Mensa has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis and Angelini. Alex Soriano has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, Gilead, Menarini and Angelini, as well as grant support from Pfizer and Gilead. All other authors: none to declare.
Supplementary data
Tables S1, S2 and S3 are available as Supplementary data at JAC Online.
References
- 1. Wu Z, McGoogan J.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 2. Cummings MJ, Baldwin MR, Abrams D. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395: 1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia-Vidal C, Moreno-García E, Hernández-Meneses M. et al. Personalized therapy approach for hospitalized patients with COVID-19. Clin Infect Dis 2020; doi:10.1093/cid/ciaa964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beigel JH, Tomashek KM, Dodd LE. et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020; 383: 1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spinner CD, Gottlieb RL, Criner GJ. et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324: 1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhang D, Du G. et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19 — interim WHO Solidarity trial results. New Engl J Med 2021; 384: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller EH, Zucker J, Castor D. et al. Pretest symptom duration and cycle threshold values for severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction predict coronavirus disease 2019 mortality. Open Forum Infect Dis 2021; 8: ofab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Vidal C, Sanjuan G, Puerta-Alcalde P. et al. Artificial intelligence to support clinical decision-making processes. EBioMedicine 2019; 46: 27–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldman JD, Lye DCB, Hui DS. et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; 383: 1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olender SA, Perez KK, Go AS. et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis 2020; doi:10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He X, Lau EHY, Wu P. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26: 672–5. [DOI] [PubMed] [Google Scholar]
- 14. Stetson DB, Medzhitov R.. Type I interferons in host defense. Immunity 2006; 25: 373–81. [DOI] [PubMed] [Google Scholar]
- 15. Chen P, Nirula A, Heller B. et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin D, Mukherjee R, Grewe D. et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020; 587: 657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blanco-Melo D, Nilsson-Payant BE, Liu W-C. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036–45.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee AK, Blanco MR, Bruce EA. et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell 2020; 183: 1325–39.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Q, Bastard P, Liu Z. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020; 370: eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hadjadj J, Yatim N, Barnabei L. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369: 718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoagland DA, Møller R, Uhl SA. et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity 2021; 54: 557–70.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monk PD, Marsden RJ, Tear VJ. et al. Safety and efficacy of inhaled nebulised interferon β-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feld JJ, Kandel C, Biondi MJ. et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir Med 2021; 9: 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hung IF-N, Lung K-C, Tso EY-K. et al. Triple combination of interferon β-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395: 1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magleby R, Westblade LF, Trzebucki A. et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2020; doi:10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pruijssers AJ, George AS, Schäfer A. et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep 2020; 32: 107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williamson BN, Feldmann F, Schwarz B. et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020; 585: 273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalil AC, Patterson TF, Mehta AK. et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021; 384: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.