ABSTRACT
Background
Chronic kidney disease is a recognized risk factor of poor outcomes from coronavirus disease 2019 (COVID-19).
Methods
This retrospective cohort study used the UK Renal Registry database of people on kidney replacement therapy (KRT) at the end of 2019 in England and who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) between 1 March 2020 and 31 August 2020 to analyse the incidence and outcomes of COVID-19 among different KRT modalities. Comparisons with 2015–2019 mortality data were used to estimate excess deaths.
Results
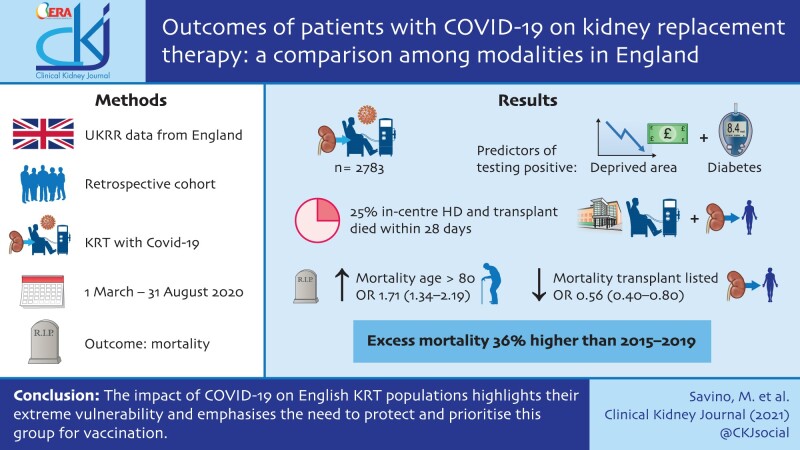
A total of 2783 individuals on KRT tested positive for SARS-CoV-2. Patients from more-deprived areas {most deprived versus least deprived hazard ratio [HR] 1.20 [95% confidence interval (CI) 1.04–1.39]} and those with diabetes compared with those without [HR 1.51 (95% CI 1.39–1.64)] were more likely to test positive. Approximately 25% of in-centre haemodialysis and transplanted patients died within 28 days of testing positive compared with 36% of those on home therapies. Mortality was higher in those ≥80 years of age compared with those 60–79 years [odds ratio (OR) 1.71 (95% CI 1.34–2.19)] and much lower in those listed for transplantation compared with those not listed [OR 0.56 (95% CI 0.40–0.80)]. Overall, excess mortality in 2020 for people on KRT was 36% higher than the 2015–2019 average. Excess deaths peaked in April 2020 at the height of the pandemic and were characterized by wide ethnic and regional disparities.
Conclusions
The impact of COVID-19 on the English KRT population highlights their extreme vulnerability and emphasizes the need to protect and prioritize this group for vaccination. COVID-19 has widened underlying inequalities in people with kidney disease, making interventions that address health inequalities a priority.
Keywords: COVID-19, dialysis, kidney replacement therapy, SARS-CoV-2, transplant
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), a highly contagious zoonosis caused by an RNA betacoronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), started as an epidemic in Wuhan in 2019 and has since become a pandemic [1], imposing a huge burden on public health globally [2]. To date (7 April 2021), the worldwide number of confirmed cases of coronavirus is >100 million and in Europe the number of deaths attributed to COVID-19 is ~800 000 [3].
Chronic kidney disease (CKD) is a recognized risk factor of poor outcomes from COVID-19 [4, 5]. A UK cross-sectional survey of 17 278 392 adults registered with a general practice and who had at least 1 year of follow-up before 1 February 2020 reported a hazard ratio (HR) of COVID-19-related death for people with CKD of 1.57 [95% confidence interval (CI) 1.51–1.64] [6]. Likewise, the recently validated COVID-19 risk calculator has highlighted the importance of reduced kidney function and the very high risk of death for people with Stage 5 CKD and on kidney replacement therapy (KRT) [7]. However, the numbers of people on dialysis and transplantation in this study were too small to investigate differences in risk between KRT modalities. Our previous retrospective cohort analysis in England and Wales [8] focused only on people treated with in-centre haemodialysis (ICHD) and, similar to a small Scottish study [9], reported a survival rate of ∼75% for people with COVID-19 on dialysis.
The aims of this retrospective cohort study are to describe the incidence of COVID-19 infection, compare the outcomes between KRT modalities in England during the first wave of the pandemic between March and August 2020 and estimate the excess mortality related to the pandemic using a comparison with 2015–2019 data.
MATERIALS AND METHODS
Study population and design
This study is a retrospective cohort analysis of all adults in the UK Renal Registry (UKRR) database who were receiving KRT at an English renal centre on 31 December 2019, still alive on 1 March 2020, tested positive for SARS-CoV-2 between 1 March 2020 and 31 August 2020 and had not opted out from using their data.
COVID-19 infection data
SARS-CoV-2 test data held by the UKRR were derived from two sources: weekly returns from renal centres listing test dates for any KRT patients with a positive polymerase chain reaction test and a dataset from Public Health England (PHE) of the dates of all positive laboratory tests for a wider set of patients, including this cohort. After excluding patients with no valid National Health Service (NHS) number, the UKRR patient cohort was linked to PHE data of positive tests for SARS-CoV-2 between 1 March and 31 August 2020.
Covariate data
Demographic data were extracted from the UKRR database, including age, sex, ethnicity, Index of Multiple Deprivation rank quintile from patient postcode [10] and primary renal disease (PRD), whether the patient was on the transplant (Tx) waiting list on 31 December 2019 (as an indicator of general health status) and KRT modality on 31 December 2019.
Outcome data
The NHS Demographics Batch Service was used to obtain dates of death for the cohort through 13 January 2021; mortality analyses considered death up to 28 days after the test date.
Ethics
Data were collected without individual consent. Patients can opt out from data linkage using a national opt-out system. The UKRR holds data on kidney patients under Section 251 of the NHS Act (2006), granted by the Health Research Authority’s Confidentiality Advisory Group. This gives the UKRR permission to carry out analyses on de-identified data without individual patient consent.
Statistical analysis
The cumulative incidence of COVID-19 from 1 March to 31 August 2020 was calculated by KRT modality. Death was treated as a competing event in incidence analyses. For reference, this was compared with the cumulative cases in the English general population across all ages as published by the UK Government per 100 00 population using the 2020 mid-year estimate from the UK Office of National Statistics. Risk factors for SARS-CoV-2 positivity were examined using a multivariable Cox proportional hazards model using a cause-specific model (i.e. censoring deaths before a positive test). The proportionality assumption was checked using graphical methods. In addition to the demographic variables listed above, interactions between treatment and ethnicity and differences by testing period (March–May versus June–August) were checked. Age was grouped into 20-year intervals.
Kaplan–Meier plots were used to show deaths within 28 days of a positive test by KRT modality. Multivariable logistic regression was used to determine risk factors for mortality at 28 days.
Finally, monthly deaths between January and September 2020 among all KRT patients in England alive on 1 March 2020 (regardless of SARS-CoV-2 positivity) were compared with the average number of deaths in the same months for the 2015–2019 cohorts, overall and by KRT modality, as a measure of excess deaths occurring during the first wave of the pandemic. Excess deaths were compared across English regions and demographics, grouping all months together.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA)[11] and 95% CIs are reported throughout this article.
RESULTS
Description of the cohort prior to the pandemic start in March 2020
At the end of 2019 there were 55 799 adult patients on KRT in England not on the national NHS data opt-out register. After excluding those who died before 1 March 2020 (n = 898) and those without a valid NHS number (n = 106), a total of 54 795 patients were included in the study cohort (Supplementary data, Figure S1).
Of the patients included, 43% were on dialysis. The median age of patients on ICHD was higher than that of patients on home therapies [HTs; peritoneal dialysis (PD) and home HD] and with a Tx (67.1 versus 55.6 years). Among dialysis patients, 20% were listed for transplantation at the end of 2019 (Table 1).
Table 1.
Sociodemographic characteristics of patients on KRT in England at the end of 2019 who were alive on 1 March 2020, overall and by modality
| Variables | All | Modality | |||
|---|---|---|---|---|---|
| HHD | ICHD | PD | Tx | ||
| All, n (%) | 54 795 | 1134 (2.1) | 19 541 (35.7) | 2993 (5.5) | 31 127 (56.8) |
| Sex, n (%) | |||||
| Male | 33 583 (61.3) | 697 (61.5) | 12 142 (62.1) | 1781 (59.5) | 18 963 (60.9) |
| Female | 21 212 (38.7) | 437 (38.5) | 7399 (37.9) | 1212 (40.5) | 12 164 (39.1) |
| Age (years), median (IQR) | 59.4 (48.7–70.4) | 54.7 (45.8–64.4) | 67.1 (55.4–77.1) | 63.9 (51.1–74.8) | 55.6 (45.3–64.9) |
| Ethnicity, n (%) | |||||
| Missing | 1094 (2) | 14 (1.2) | 652 (3.3) | 122 (4.1) | 306 (1) |
| Asian | 7894 (14.4) | 78 (6.9) | 3054 (15.6) | 427 (14.3) | 4335 (13.9) |
| Black | 4673 (8.5) | 88 (7.8) | 2358 (12.1) | 248 (8.3) | 1979 (6.4) |
| Mixed | 853 (1.6) | 15 (1.3) | 337 (1.7) | 52 (1.7) | 449 (1.4) |
| Other | 1006 (1.8) | 16 (1.4) | 399 (2) | 61 (2) | 530 (1.7) |
| White | 39 275 (71.7) | 923 (81.4) | 12 741 (65.2) | 2083 (69.6) | 23 528 (75.6) |
| IMD quintile, n (%) | |||||
| Missing | 7 (0.01) | (0) | 1 (0.01) | (0) | 6 (0.02) |
| 1 (least deprived) | 8619 (15.7) | 182 (16) | 2356 (12.1) | 461 (15.4) | 5620 (18.1) |
| 2 | 9743 (17.8) | 215 (19) | 2942 (15.1) | 547 (18.3) | 6039 (19.4) |
| 3 | 10 883 (19.9) | 225 (19.8) | 3709 (19) | 618 (20.6) | 6331 (20.3) |
| 4 | 12 399 (22.6) | 231 (20.4) | 4803 (24.6) | 657 (22) | 6708 (21.6) |
| 5 (most deprived) | 13 144 (24) | 281 (24.8) | 5730 (29.3) | 710 (23.7) | 6423 (20.6) |
| PRD, n (%) | |||||
| Missing | 1523 (2.8) | 38 (3.4) | 796 (4.1) | 140 (4.7) | 549 (1.8) |
| Diabetes | 9779 (17.8) | 161 (14.2) | 5260 (26.9) | 709 (23.7) | 3649 (11.7) |
| Glomerulonephritis | 10 150 (18.5) | 263 (23.2) | 2489 (12.7) | 454 (15.2) | 6944 (22.3) |
| Hypertension | 3599 (6.6) | 52 (4.6) | 1545 (7.9) | 239 (8) | 1763 (5.7) |
| Other | 9735 (17.8) | 225 (19.8) | 3242 (16.6) | 445 (14.9) | 5823 (18.7) |
| Polycystic kidney disease | 5432 (9.9) | 93 (8.2) | 1021 (5.2) | 198 (6.6) | 4120 (13.2) |
| Pyelonephritis | 4942 (9) | 129 (11.4) | 1334 (6.8) | 171 (5.7) | 3308 (10.6) |
| Renovascular disease | 1409 (2.6) | 22 (1.9) | 872 (4.5) | 160 (5.3) | 355 (1.1) |
| Uncertain | 8226 (15) | 151 (13.3) | 2982 (15.3) | 477 (15.9) | 4616 (14.8) |
| Waitlisted for Tx before 31 December 2019, n (%) | |||||
| Not listed | 18 875 (79.7) | 745 (65.7) | 16 094 (82.4) | 2036 (68) | – |
| Listed | 4793 (20.3) | 389 (34.3) | 3447 (17.6) | 957 (32) | – |
HHD, home haemodialysis; IMD, Index of Multiple Deprivation.
Incidence of COVID-19 among KRT modalities
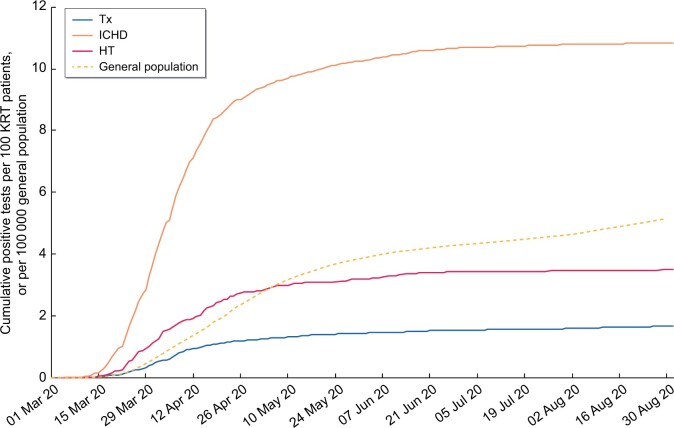
From 1 March to 31 August 2020, 5% (2783) of the study cohort tested positive for SARS-CoV-2 (Table 2). The vast majority became infected in the first 3 months of the pandemic between March and May 2020 (Supplementary data, Table S1) and only 7% became infected between June and August 2020. The cumulative percentage of patients who tested positive for SARS-CoV-2 was much higher among those on ICHD, reaching ∼11% at the end of August 2020. The corresponding percentages for transplanted and HT patients were much lower, at 2% and 4%, respectively (Figure 1).
Table 2.
Sociodemographic characteristics of patients on KRT in England at the end of 2019 who were alive on 1 March 2020 with a positive test for SARS-COV-2, overall and by modality
| Variables | All | Modality | |||
|---|---|---|---|---|---|
| HHD | ICHD | PD | Tx | ||
| All, n (%) | 2783 | 41 (1.5) | 2114 (76) | 104 (3.7) | 524 (18.8) |
| Sex, n (%) | |||||
| Male | 1734 (62.3) | 28 (68.3) | 1316 (62.3) | 67 (64.4) | 323 (61.6) |
| Female | 1049 (37.7) | 13 (31.7) | 798 (37.7) | 37 (35.6) | 201 (38.4) |
| Age (years), median (IQR) | 65.5 (55.4–75.7) | 60 (47.4–64.8) | 68.5 (57.5–77.5) | 61.5 (51.8–73.8) | 58.3 (48.7–66) |
| Ethnicity, n (%) | |||||
| Missing | 70 (2.5) | 2 (4.9) | 58 (2.7) | 4 (3.8) | 6 (1.1) |
| Asian | 663 (23.8) | 1 (2.4) | 497 (23.5) | 19 (18.3) | 146 (27.9) |
| Black | 494 (17.8) | 4 (9.8) | 390 (18.4) | 19 (18.3) | 81 (15.5) |
| Mixed | 59 (2.1) | 0 (0) | 48 (2.3) | 2 (1.9) | 9 (1.7) |
| Other | 80 (2.9) | 2 (4.9) | 54 (2.6) | 4 (3.8) | 20 (3.8) |
| White | 1417 (50.9) | 32 (78) | 1067 (50.5) | 56 (53.8) | 262 (50) |
| IMD quintile, n (%) | |||||
| 1 (least deprived) | 281 (10.1) | 7 (17.1) | 200 (9.5) | 12 (11.5) | 62 (11.8) |
| 2 | 380 (13.7) | 7 (17.1) | 267 (12.6) | 16 (15.4) | 90 (17.2) |
| 3 | 567 (20.4) | 10 (24.4) | 419 (19.8) | 32 (30.8) | 106 (20.2) |
| 4 | 744 (26.7) | 7 (17.1) | 585 (27.7) | 23 (22.1) | 129 (24.6) |
| 5 (most deprived) | 811 (29.1) | 10 (24.4) | 643 (30.4) | 21 (20.2) | 137 (26.1) |
| PRD, n (%) | |||||
| Missing | 83 (3) | 3 (7.3) | 71 (3.4) | 3 (2.9) | 6 (1.1) |
| Diabetes | 949 (34.1) | 6 (14.6) | 800 (37.8) | 48 (46.2) | 95 (18.1) |
| Glomerulonephritis | 360 (12.9) | 10 (24.4) | 236 (11.2) | 10 (9.6) | 104 (19.8) |
| Hypertension | 192 (6.9) | 0 (0) | 139 (6.6) | 7 (6.7) | 46 (8.8) |
| Other | 389 (14) | 8 (19.5) | 288 (13.6) | 14 (13.5) | 79 (15.1) |
| Polycystic kidney disease | 141 (5.1) | 4 (9.8) | 84 (4) | 1 (1) | 52 (9.9) |
| Pyelonephritis | 164 (5.9) | 4 (9.8) | 117 (5.5) | 2 (1.9) | 41 (7.8) |
| Renovascular disease | 109 (3.9) | 3 (7.3) | 91 (4.3) | 6 (5.8) | 9 (1.7) |
| Uncertain | 396 (14.2) | 3 (7.3) | 288 (13.6) | 13 (12.5) | 92 (17.6) |
| Waitlisted for Tx before 31 December 2019, n (%) | |||||
| Not listed | 1902 (84.2) | 27 (65.9) | 1799 (85.1) | 76 (73.1) | – |
| Listed | 357 (15.8) | 14 (34.1) | 315 (14.9) | 28 (26.9) | – |
HHD, home haemodialysis; IMD, Index of Multiple Deprivation.
FIGURE 1:
Kaplan–Meier ‘failure’ plot of cumulative incidence of SARS-CoV-2 infections in patients on KRT and the general population in England from 1 March to 31 August 2020. Presented as cases per 100 people on each modality included in the study cohort for the KRT population and per 100 000 England population.
Risk factors for testing positive for SARS-CoV-2 by KRT modality
Looking at the cohort of people with COVID-19 on KRT, 76% were on ICHD, 24% were of Asian ethnic background and 56% were from the lowest two deprivation quintiles. Only 16% of dialysis patients with COVID-19 were on the Tx list and there was a higher proportion of positive patients with diabetes as the PRD, especially among those on ICHD and PD (Table 2).
Of the positive cases occurring between March and May, only 17% occurred in Tx patients, increasing to 37% between June and August. This increase may be attributable to the discontinuation of the shielding policy in June. Notably, the incidence of COVID-19 remained higher among Asian people and those with higher socio-economic deprivation between June and August (Supplementary data, Table S1).
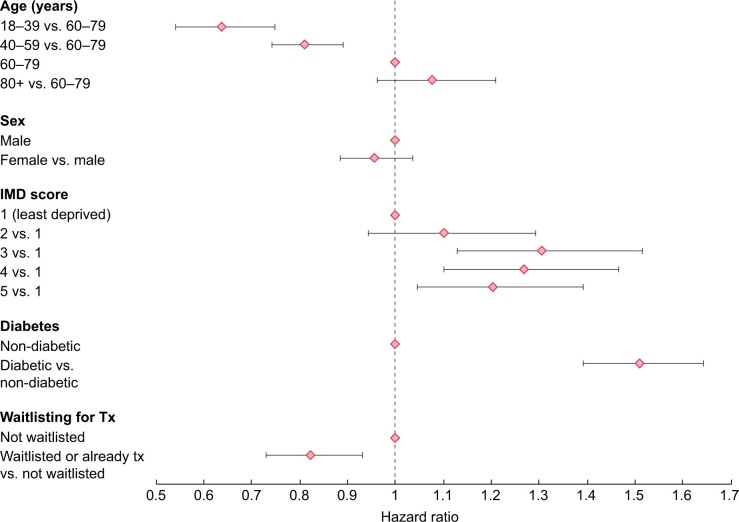
The adjusted analysis showed that the main risk factor for testing positive was diabetic status as the PRD (Figure 2), with a 51% higher risk among patients with diabetes compared with those without. The risk of testing positive also appeared to be higher among patients from more deprived areas. Conversely, it was lower among younger patients [18–39 years age group: HR 0.64 (95% CI 0.54–0.75)] compared with the 60–79 years age group and patients transplanted or listed for transplantation compared with those not listed [listed: HR 0.83 (95% CI 0.73–0.93)] (Figure 2). Modality differences varied across ethnic groups (P-value for ethnicity–modality interaction = 0.0002). Patients of White ethnicity were in general at lower risk of positivity than other ethnic groups, and the differences were larger in magnitude among Tx patients (Supplementary data, Figure S2). People on ICHD were at higher risk of positivity compared with Tx or HT patients, particularly in White patients. A higher risk in HT compared with Tx was only seen in White patients (Supplementary data, Figure S3). In contrast, there were no important differences by sex. Just 2% of the cohort was excluded from the adjusted analyses due to missing ethnicity, deprivation or PRD data.
FIGURE 2:
HRs (with bars showing 95% CIs) for risk of positivity among patients on KRT in England at the end of 2019 who were alive on 1 March 2020, from 1 March to 31 August 2020. Adjusted for treatment modality, ethnicity, modality–ethnicity interaction, age, sex, Index of Multiple Deprivation quintile, diabetes and waitlisting status. Modality and ethnicity parameters are shown in Supplementary data, Figures S3 and S4.
Risk factors for mortality from COVID-19 by KRT modality
The 28-day crude survival among ICHD patients with COVID-19 was ∼76% and for Tx it was 75%. Crude survival of those on HT appeared to be worse, at ∼64% (Figure 3).
FIGURE 3:
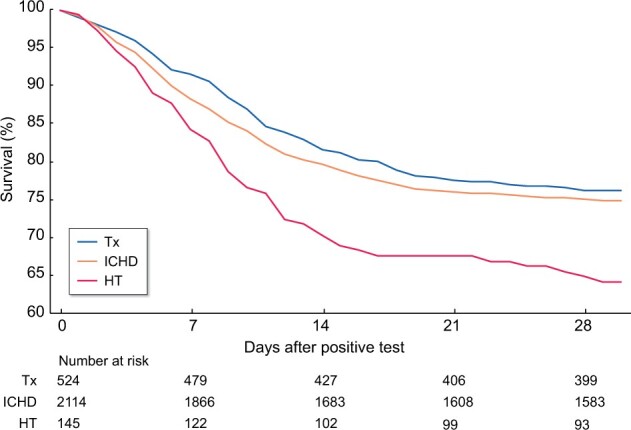
Kaplan–Meier plot of 28-day survival of patients on KRT in England who tested positive for SARS-CoV-2 from 1 March to 31 August 2020.
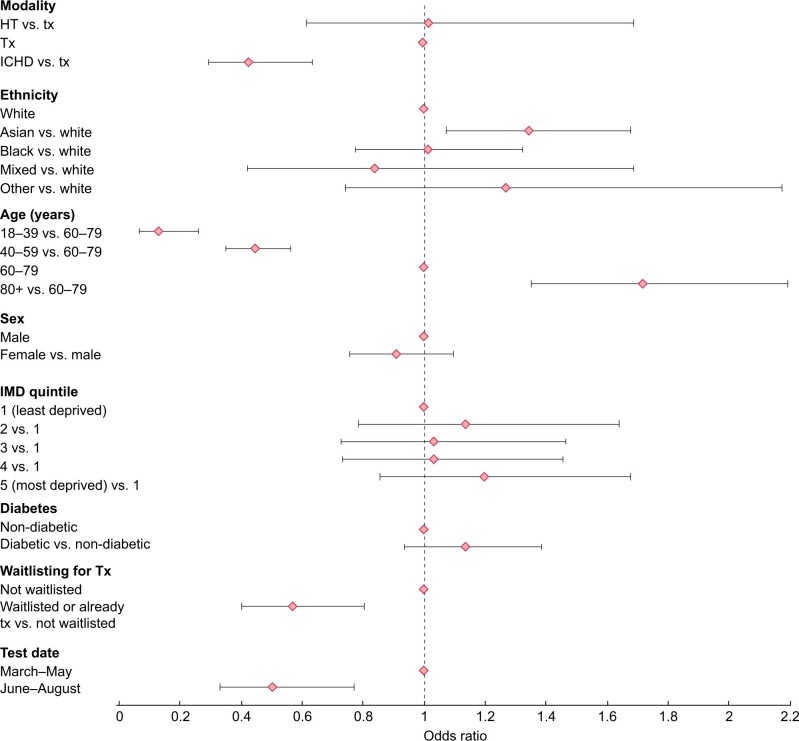
In mutually adjusted models of risk factors of death among those who tested positive, the odds of mortality after infection with SARS-CoV-2 was higher among older patients, in particular those ≥80 years of age compared with those 60–79 years of age [odds ratio (OR) 1.72 (95% CI 1.35–2.19)] (Figure 4). There was also higher odds of mortality among patients of Asian ethnicity compared with patients of White ethnicity [OR 1.34 (95% CI 1.07–1.68)] and borderline higher odds among those with diabetes compared with those without [OR 1.14 (95% CI 0.94–1.38)]. ICHD patients had lower odds of mortality compared with transplanted patients [OR 0.43 (95% CI 0.29–0.63)] and odds were much lower in those transplanted or listed for transplantation compared with those not listed [OR 0.57 (95% CI 0.40–0.81)]. Neither sex nor level of social deprivation was associated with mortality and there was no evidence of an ethnicity–modality interaction.
FIGURE 4:
OR (with bars showing 95% CIs) for risk of mortality among patients on KRT in England at the end of 2019 who were alive on 1 March 2020 and tested positive for SARS-COV-2. Adjusted for treatment modality, ethnicity, age, sex, Index of Multiple Deprivation quintile, diabetes, waitlisting status and test period. Period 1, March–May 2020; Period 2, June–August 2020.
Excess mortality on KRT during the COVID-19 pandemic
Excess deaths among all patients on KRT were particularly high in April 2020, when the number of deaths was more than double the average for the period 2015–2019 (Figure 5). The same analysis by each KRT modality (Supplementary data, Figure S4) showed that in April 2020, excess deaths for Tx patients were almost 3 times higher than the average for the period 2015–2019 and excess deaths for ICHD patients were almost twice the average. For HT patients, excess deaths in April 2020 were up by only a third compared with the 5-year average. After April 2020, excess deaths decreased for patients on all three modalities, with the decrease slower among Tx patients. In England, between March and August 2020, the 2019 KRT population experienced 2984 deaths, an excess of almost 800 compared with the average for 2015–2019 and representing a 36% increase. Of the total number of deaths, 867 (29%) occurred after testing positive for SARS-CoV-2.
FIGURE 5:
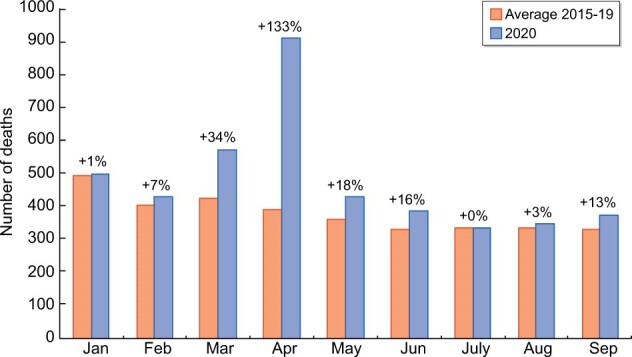
Excess deaths by month during the period January–September 2020 compared with the average between January and September 2015–2019 for the prevalent population on KRT in England at the beginning of each year.
Excess deaths among all patients on KRT between January and September 2020 varied markedly between regions in England and were particularly high in London (Supplementary data, Figure S5). The number of excess deaths was higher for men than women (573 more deaths in males from January to September 2020 than the 2015–2019 average compared with only 298 in females), although proportionally the increase in deaths was similar (26% compared with 24%). Examination of excess deaths across ethnic groups showed a wide disparity. Black patients comprised 9% of the KRT population at the end of 2019, but 20% of the excess deaths. Similarly, Asian patients accounted for 26% of the excess deaths, but only 14% of the KRT population (Supplementary data, Figure S6).
DISCUSSION
Patients on ICHD were disproportionately affected by COVID-19, with a 5-fold infection rate compared with other modalities. Patients from minority ethnic groups and those living in the most socio-economically deprived areas were also more likely to test positive. There was high mortality among those with COVID-19 on all KRT modalities, ranging from 25% to 36%. Excess mortality was highest in April 2020 at the peak of the pandemic. Overall, excess mortality in 2020 was 36% higher than the average of the 5-year period 2015–2019 and was characterized by wide ethnic disparities. In total, 29% of deaths between March and August 2020 among patients in England who were on KRT at the end of 2019 were attributable to COVID-19.
The cumulative incidence of COVID-19 was higher in patients on ICHD than in those with a Tx or on HT. COVID-19 is known to have high intrahospital transmission rates [12], putting patients on ICHD at higher risk, because they need to attend hospital to receive their lifesaving treatment. From May 2020 onwards, some dialysis units in heavily affected areas (primarily London) started testing all ICHD patients, irrespective of whether they were symptomatic, to control transmission.
Our analysis showed that higher socio-economic deprivation is an independent risk factor for testing positive for COVID-19 and proportionally more people of Asian background tested positive compared with other ethnic groups. Also, the risk of testing positive was much higher for those on KRT with diabetes as the PRD. Transmission of COVID-19 is facilitated by overcrowded housing and occupations that require close contact with others. In England, the 2011 census showed that Asians, in particular the Bangladeshi group, live in the most deprived neighbourhoods and are most likely to work in at-risk occupations [13]. There were also significant interactions between treatment modality and ethnicity. In particular, the risk of testing positive for COVID-19 was especially significant for White patients on ICHD and for Black patients with Txs.
Our findings are in line with studies in the general population. For example, in a meta-analysis, the pooled adjusted relative risk for Asian people was 1.50 (95% CI 1.24–1.83) of White people [14]. Possible explanations include the higher incidence of diabetes [15] and greater socio-economic deprivation among people of Asian ethnicity.
Mortality at 28 days following a positive test was similarly high for patients with a Tx or on ICHD, at ∼25%, but was even higher for those on HT, at almost 36%. In March and April 2020, people on HT or with a Tx would only have been tested if they had severe symptoms requiring hospital admission; this was due to a national shortage of test capacity. After adjusting for other risk factors, we found no difference in mortality between people on HT and Tx patients. Additionally, when we analysed excess deaths by modality, we found that the excess mortality in HT patients was relatively small compared with other modalities. It is possible that the small number of HT patients included in our analysis constituted a subpopulation that had more severe COVID-19 that required hospitalization and they were therefore more likely to be tested.
Older age was the most important risk factor for mortality, with the highest risk being in those ≥80 years of age. The risk was also higher among those on dialysis not listed for kidney Tx at the end of 2019. Despite being important risk factors for testing positive, diabetes as the PRD and Asian ethnicity compared with White ethnicity were associated with only a borderline higher risk for mortality. Our results are in line with data from the European Renal Association (ERA)–European Dialysis and Transplant Association Registry, showing that mortality among patients on KRT was high, especially in the elderly [16].
Recent data published by the ERA COVID-19 Database [17] showed that in dialysis patients, the risk of mortality from COVID-19 was correlated to a more general status of frailty rather than independent risk factors [18]. Frailty is a term widely used to denote a multidimensional syndrome of loss of reserves (energy, physical ability, cognition and health) that give rise to vulnerability. It generally affects between 25% and 50% of those >85 years of age [19] and is especially prevalent among those on dialysis, affecting between 14% and 82% of patients [20]. Older age and frailty status are both recognized as contraindications to transplantation [21].
The OR of mortality from COVID-19 in Tx patients was higher than for individuals on ICHD. As for HT patients, those with a Tx would have only been tested for COVID-19 when requiring hospitalization, while ICHD patients were tested when they developed symptoms, facilitating infection control in dialysis units.
According to the latest available Intensive Care National Audit and Research Centre report, until 31 August 2020, 1.7% of patients admitted to critical care with COVID-19 were patients previously treated with KRT for end-stage kidney disease (ESKD), of which 42% were invasively ventilated during the first 24 h of admission and 50% were treated with advanced respiratory support during admission. Additionally, until the end of August 2020, the 28-day in-hospital survival of those with COVID-19 admitted to critical care with a previous severe comorbidity, including ESKD treated with KRT, was reported to be ∼53% [22].
The peak in excess deaths for people on KRT occurred in April 2020, which coincided with excess deaths in the general population [23]. The excess mortality then began to increase again from September onwards, when COVID-19 cases began to increase again, as they did in the general population. However, in the general population, COVID-19 did not feature in the top 10 leading causes of death registered in September 2020. The leading cause of death in England in September 2020 was dementia [23]. In January to September 2020, COVID-19 was the underlying cause of death in 11.5% of all deaths that occurred in England in the general population. Among patients on KRT treatment, deaths were particularly high for those with a Tx or on ICHD, at more than double the 5-year average. Excess deaths among KRT patients varied widely according to region, which also mirrored trends in the general population. For the general population, this level of regional inequality was much greater than the inequalities in all-cause mortality rates in previous years [24].
Strengths and limitations
This is the first nationwide analysis to compare COVID-19 outcomes between people on the three KRT modalities in England. In addition, it is the first analysis to compare excess deaths in the KRT population during the first wave of the pandemic by specific modality, demographics and region in one of the countries most severely affected by the pandemic. Unlike published previous analyses, in which data capture was dependent on clinicians submitting data to a central platform, our data represent the entire KRT population in England and all COVID-19 tests that were recorded in these patients.
We were unable to adjust the analyses for comorbidities and link our data with hospital data to confirm whether our Tx and HT cohorts were mainly composed of a hospitalized subgroup with worse outcomes. Instead, we used baseline diabetic status for all those on KRT and Tx waitlisted status for those on dialysis as surrogates to inform the general health status at baseline of patients.
CONCLUSION
In conclusion, the impact of COVID-19 on the English KRT population has been severe across all three KRT modalities. Patients on KRT are extremely vulnerable and require protection from exposure to infection and urgent vaccination. Similar to the general population, the results also suggest that COVID-19 has widened underlying inequalities in patients with kidney disease. It is therefore important to ensure that interventions that address health inequalities for patients with kidney disease become a priority for the whole renal community.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff at all adult renal centres in the UK who submit data to the UKRR and who care for kidney patients affected by COVID-19.
FUNDING
The authors received no specific funding for this work.
AUTHORS’ CONTRIBUTIONS
M.S. was responsible for the conception of the work, data interpretation, writing the draft of the manuscript and critical revision before submission. S.S. was responsible for data analyses and interpretation, drafting of the Methods section and critical revision before submission. K.E. was responsible for writing the draft of the manuscript, data interpretation and critical revision before submission. R.S. and F.B.D. were responsible for data curation and interpretation and critical revision before submission. J.M. and D.N. were responsible for supervision and critical revision before submission.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
ETHICAL STATEMENT
On behalf of the Renal Association, the UKRR collects patient data without consent under section 251 of the NHS Act (2006), granted by the Health Research Authority’s Confidentiality Advisory Group (ref 16/CAG/0064). Data are always pseudonymized prior to being analysed. The UKRR database has approval for research studies from the North East Newcastle and North Tyneside 1 Research Ethics Committee (16/NE/0042).
DATA AVAILABILITY STATEMENT
The data underlying this article are available from the UKRR through the UKRR’s data application process (see https://renal.org/audit-research/how-access-data/ukrr-data). For any data access queries, contact ukrr-research@renalregistry.nhs.uk.
Contributor Information
Manuela Savino, UK Renal Registry, Bristol, UK.
Shalini Santhakumaran, UK Renal Registry, Bristol, UK.
Katharine M Evans, UK Renal Registry, Bristol, UK.
Retha Steenkamp, UK Renal Registry, Bristol, UK.
Fran Benoy-Deeney, UK Renal Registry, Bristol, UK.
James F Medcalf, UK Renal Registry, Bristol, UK; Department of Cardiovascular Sciences, University of Leicester, Leicester, UK; Leicester General Hospital, Leicester, UK.
Dorothea Nitsch, UK Renal Registry, Bristol, UK; London School of Hygiene and Tropical Medicine, London, UK; Royal Free London NHS Foundation Trust, London, UK.
REFERENCES
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323: 709–710 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. COVID-19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. 2021. https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (20 January 2021, date last accessed)
- 3. Johns Hopkins Center for Systems Science and Engineering. COVID-19 Dashboard. https://coronavirus.jhu.edu/map.html (27 January 2021, date last accessed)
- 4. Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID 2019) outbreak in China: summary of a report of 72,314 cases from the Chinese centre for disease control and prevention. JAMA 2020; 323: 1239–1242 [DOI] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clift AK, Coupland CAC, Keogh RH et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ 2020; 371: m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savino M, Casula A, Santhakumaran S et al. Sociodemographic features and mortality of individuals on haemodialysis treatment who test positive for SARS-CoV-2: a UK renal registry data analysis. PLoS One 2020; 15: e0241263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell S, Campbell J, McDonald J et al. COVID-19 in patients undergoing chronic kidney replacement therapy and kidney transplant recipients in Scotland: findings and experience from the Scottish Renal Registry. BMC Nephrol 2020; 21: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ministry of Housing, Communities & Local Government. English Indices of Deprivation. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (27 January 2021, date last accessed)
- 11. SAS Institute. SAS 9.4 SQL Procedure User’s Guide, 4th edn. Cary, NC: SAS Institute, 2016 [Google Scholar]
- 12. Naicker S, Yang CW, Hwang SJ et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; 97: 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Office for National Statistics. Census. https://www.ons.gov.uk/census/2011census. (2 February 2021, date last accessed)
- 14. Sze S, Pan D, Clareece R et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine 2020; 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon AN, Raymond NT, Mughal S et al. Prevalence of microalbuminuria and hypertension in South Asians and white Europeans with type 2 diabetes: a report from the United Kingdom Asian Diabetes Study (UKADS). Diab Vasc Dis Res 2006; 3: 22–25 [DOI] [PubMed] [Google Scholar]
- 16. Jager KJ, Kramer A, Chesnaye NC et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020; 98: 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilbrands LB, Duivenvoorden R, Vart P et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020; 35: 1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harhay MN, Rao MK, Woodside KJ et al. An overview of frailty in kidney transplantation: measurement, management and future considerations. Nephrol Dial Transplant 2020; 35: 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. Lancet 2013; 381: 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vleut R, Abramowicz D, Hellemans R. Frailty: a new comorbidity in kidney transplant candidates? Nephrol Dial Transplant 2020; 35: 1085–1087 [DOI] [PubMed] [Google Scholar]
- 21. Tennankore KK, Gunaratnam L, Suri RS et al. Frailty and the kidney transplant wait list: protocol for a multicenter prospective study. Can J Kidney Health Dis 2020; 7: 2054358120957430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Intensive Care National Audit and Research Centre. ICNARC Case Mix Programme Database. https://www.icnarc.org/Our-Audit/Latest-News/2020/04/10/Report-On-3883-Patients-Critically-Ill-With-Covid-19 (20 June 2021, date last accessed) [DOI] [PMC free article] [PubMed]
- 23. Office for National Statistics. Provisional Weekly Death Registrations and Percentage of Excess Deaths for Selected Causes, Deaths Registered in England and Wales. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/11821provisionalweeklydeathregistrationsandpercentageofexcessdeathsforselectedcausesdeathsregisteredin2020englandandwales (20 February 2020, date last accessed)
- 24. Public Health England. Disparities in the Risk and Outcomes of COVID-19. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908434/Disparities_in_the_risk_and_outcomes_of_COVID_August_2020_update.pdf (20 February 2020, date last accessed)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from the UKRR through the UKRR’s data application process (see https://renal.org/audit-research/how-access-data/ukrr-data). For any data access queries, contact ukrr-research@renalregistry.nhs.uk.