Abstract
Objective:
Utilize a random sample to estimate the prevalence, child traits, and maternal risk for fetal alcohol spectrum disorders (FASD) in a Southeastern United States county.
Methods:
From all first-grade students (n=1073) a simple random sample was drawn, and 32% (n=231) were consented. All 231 children were examined for dysmorphology and growth, 84 were tested and rated on neurobehavior, and 72 mothers were interviewed for maternal risk.
Results:
Significant differences (α=.05) between the physical traits of children diagnosed with FASD and the entire sample were: height, weight, head circumference, body mass index, and total dysmorphology scores, and all three cardinal features of fetal alcohol syndrome, palpebral fissure length, smooth philtrum, and narrow vermilion. Intellectual function and inhibition were not significantly different between FASD and typically-functioning children, but two executive function measures and one visual/spatial measure approached significance (α=.10). Three behavioral measures were significantly worse for the FASD group: parent-rated problems of communication, daily living, and socialization. Significant maternal risk factors reported were postpartum depression, frequency of drinking, and recovery from problem drinking. The prevalence of FASD was 71.4 per 1,000 or 7.1%. This rate falls clearly within the prevalence range identified in eight larger samples of other communities in the Collaboration on FASD Prevalence (CoFASP) study in four regions of the United States.
Conclusion:
Careful and detailed clinical evaluation of children from small random samples can be useful for estimating the prevalence and traits of FASD in a community.
Keywords: Fetal Alcohol Spectrum Disorders, Prevalence, Alcohol Use, Women, Prenatal Alcohol Use, Children with FASD, Maternal Risk Traits for FASD
1. Introduction
Determining or estimating the prevalence of fetal alcohol syndrome (FAS), or any of the specific disorders of the fetal alcohol spectrum disorders (FASD), has challenged researchers since the diagnosis of FAS was first described (Jones and Smith, 1973). The four most common approaches to determining the prevalence of FASD are: 1.) surveillance record systems (Bower et al., 2000; Centers for Disease Control and Prevention, 1995, 1993; Chávez et al., 1988); 2.) individual studies in existing prenatal clinics (Sokol et al., 2003, 1981); 3.) meta-analyses of multiple individual studies utilizing multiple methods (Abel and Sokol, 1987; Lange et al., 2017; Roozen et al., 2016); or 4.) active case ascertainment in a circumscribed population. Of these four, the most effective has been active case ascertainment (ACA) employed in certain well delineated and receptive populations (May et al., 2009; May and Gossage, 2001; Roozen et al., 2018; Stratton et al., 1996). Once a population has been identified for an ACA Study, the two most common methods have been active recruitment to a centralized clinical venue (May et al., 1983) or ACA employed via field studies among school children (Burd et al., 1999; Chambers et al., 2019; May et al., 2006, 2000, 2021, 2020b, 2020a, 2020c, 2018, 2014, 2011, 2007; Okulicz-Kozaryn et al., 2017; Petkovic and Barisic, 2013, 2010; Poitra et al., 2003; Popova et al., 2019; Viljoen et al., 2005). Within these school-based field studies, there have been three common methods of sampling employed (May et al., 2018): 1) Employing a behavioral/developmental screening tool for stand alone observations of individual children to directly estimate the prevalence of FASD (Poitra et al., 2003), or for screening prior to full examinations and testing (Burd et al., 1999; Chambers et al., 2019), 2) preliminary screening of all (a census) small children (generally ≤10th or ≤25th on height, weight, or head circumference) prior to physical/dysmorphology exams and developmental testing (May et al., 2021, 2020a, 2020b, 2020c); and 3) from a simple random sample (May et al., 2020a, 2020b). Recently a combination of ACA methods have been used simultaneously in some populations in independent samples of school cohorts and they have yielded similar results, although the random samples have generally yielded higher overall FASD rates (May et al., 2020a, 2020b). These higher rates were due to a greater capture of children with alcohol-related neurodevelopmental disorder (ARND), who are by definition (Hoyme et al., 2016), not required to have growth deficiency or cardinal FASD dysmorphia. ARND cases, therefore, are more likely to be identified in simple random samples due to no preliminary screen for size or dysmorphic traits.
1.1. This Study
Described here is a study carried out from a simple random sample among first grade children attending public schools in a single county in the southeastern region of the United States (USA). Initially there were no plans for pre-screening of children by size, dysmorphology, or developmental trait assessment for entry into the study. Entry into the full study was to be completely by random numbers. However, due to pressing limitations of time and budget, dysmorphology examinations were ultimately used to determine entry into Tier II of the study. Therefore, the sample, although small, should represent a relatively accurate cross-section of the first-grade population in this county, or at least of the consented population.
2. Methods
2.1. Sampling
Following university IRB approvals, a presentation to the County Board of Education, and the Board’s approval, enrollment data lists were obtained from the County Superintendent of Public Education for all enrolled students in first grade schools in the county. The 16 public elementary schools had 1,073 first grade students enrolled (Figure 1). Using the computer program Research Randomizer, a first random sample was drawn for 400 children (without replacement). Each of those children chosen was sent home with a study program description and consent form for their parents to read and sign if their child was given permission to participate. After a second set of information materials and forms were again sent to the same 400 families, and the response yielded only a little over 100 children, a second set of random numbers of unduplicated children was drawn (again without replacement), and materials and consent forms were sent home with these newly chosen children. After a second set of requests from this second group was pursued, the selection process was ended and physical/dysmorphology exams were scheduled and carried out in each of the schools. From the total of 721 selected participants, 231 children were provided consent from their parents to participate. This consented sample represented 21.5% of the enrolled students, and 32% of the randomly-selected students (Figure 1).
Figure 1.
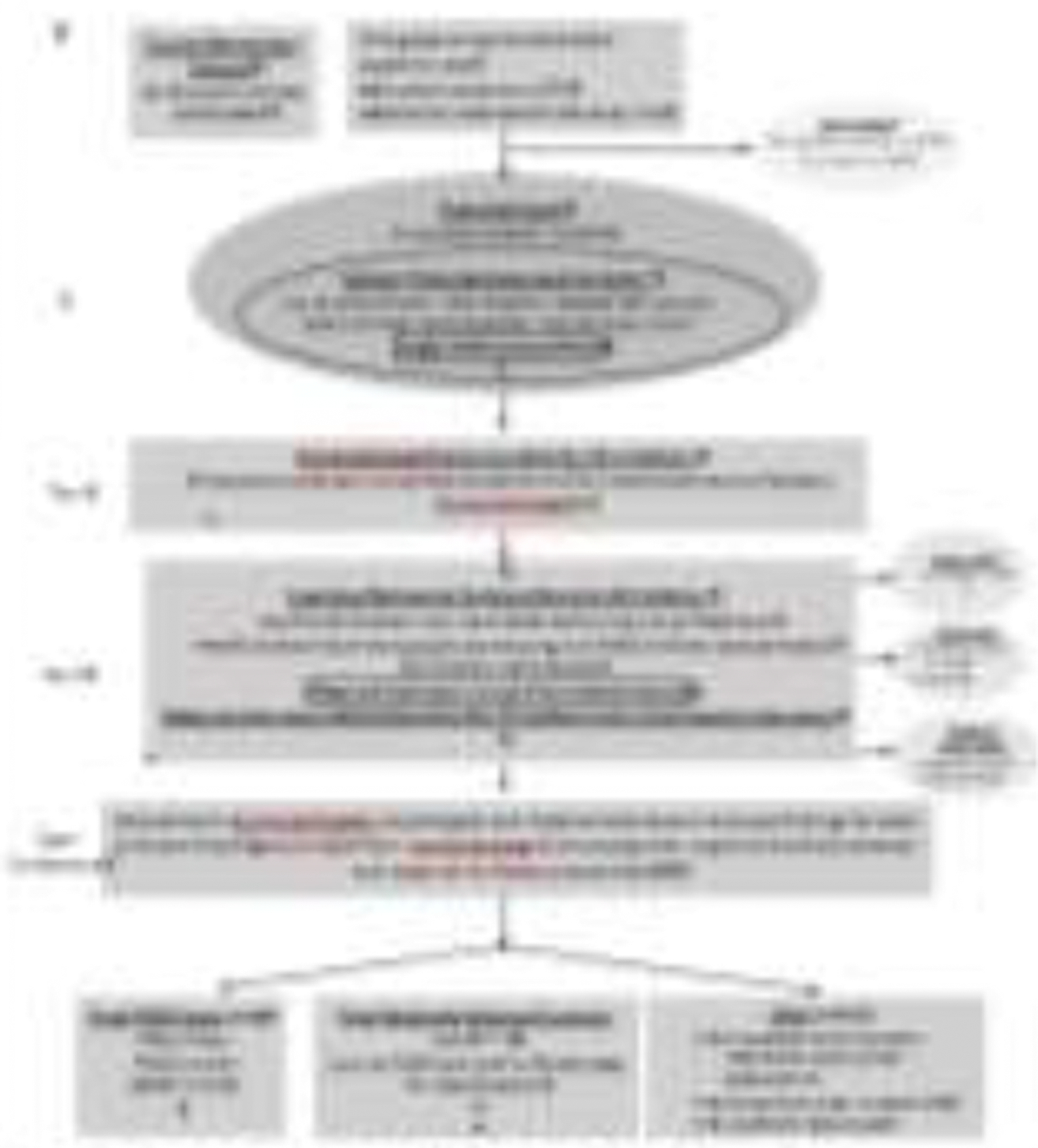
Sampling Methodology for Prevalence of FASD in a County (II) in the Southeastern Region *if a child was randomly selected and found to have an FASD or another known genetic or teratogenic disorder, he/she was classified appropriately and not eligible as a control. **3 children were not FASD and found to have another genetic disorder at dysmorphology examination. They did not advance to Tier III, but were referred to clinics for other assessment.
2.2. Diagnostic Criteria
The Revised Institute of Medicine (IOM) diagnostic guidelines for FASD (Hoyme et al., 2005) were used along with revised cut-off values established by the NIAAA-funded, Collaboration on FASD Prevalence (CoFASP) advisory group (Hoyme et al., 2016). The domains assessed for all study participants who completed the entire study were: (1) physical growth, (2) dysmorphology; (3) cognitive tests and behavioral assessments, and (4) maternal risk factors impacting the index pregnancy (see Figure 2). The continuum of FASD has four specific diagnoses: fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (PFAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD) (Hoyme et al., 2016). Criteria for each diagnostic category were utilized in this study (see Figure 2), yet ARBD has been found to be rare in any population (May et al., 2016a, 2016b, 2015, 2014, 2011). The diagnosis of FAS without a confirmed history of alcohol exposure can be made according to the original IOM criteria (Stratton et al., 1996), and revised criteria (Hoyme et al., 2016, 2005). Revised criteria also permit diagnosis of PFAS without evidence of prenatal drinking reported directly by the mother. However, the diagnosis of FASD in epidemiology studies is rarely made without direct maternal reports of alcohol use prior to pregnancy recognition, during pregnancy, or collateral reports. An ARND diagnosis always requires direct confirmation of alcohol use in the index pregnancy. Final diagnoses were assigned by a multidisciplinary team headed by the dysmorphologists in formal, structured, data-driven case conferences after the examiners of the individual domains presented detailed findings and assessments for each child.
Figure 2.

Institute of Medicine Diagnostic Guidelines for Specific Fetal Alcohol Spectrum Disorders (FASD) as clarified by Hoyme (2016). Reproduced with permission from Pediatrics, Vol. 138, Pages 3–4, Copyright © 2016 by the AAP
2.3. Tier I - Assessment of Physical Traits and Growth
All randomly-selected, consented children were then engaged into Tier I of the two-tiers of the diagnostic process. In Tier I, 231 children were provided a physical examination by one member of our team of pediatric dysmorphologists at their school. All medical examiners were fellowship-trained pediatricians in medical genetics/dysmorphology, all were unfamiliar with the children in the study, and were blinded from any medical/school records or other previous information on the children and their mothers. Multiple measurements of growth and development traits were taken, two-dimensional photographs were taken, and a complete, standard dysmorphology assessment for the full complement of known birth defects/anomalies was completed on each child. A research team member assisted each physician by recording the exam information on a project-specific standardized form. At the end of each examination day, the dysmorphologists and scribes completed each child’s form with age and sex specific growth centiles for each child from pediatric growth charts of the Centers for Disease Control and Prevention (CDC) and from clinical trait distribution charts (Nellhaus, 1968; Thomas et al., 1987). Once completed, the forms yielded the specific number of dysmorphology traits and a total dysmorphology score specific to the FASD-linked traits identified for each child (Hoyme et al., 2005). Once the forms had been tabulated for all children seen that day, the findings for each child were reviewed by the entire clinical team. Over the course of all the clinic exam days, 48 children were assessed by the dysmorphologists to be preliminary candidates for one of the diagnoses on the FASD continuum; but final diagnose were deferred until neurobehavioral tests and maternal risk interviews were completed. Because of the random selection process and the research plan to test all consented children and interview all mothers, all 231 selected and consented children were referred on to Tier II for neurobehavioral assessment and their mothers to be interviewed about the index pregnancy.
2.4. Tier II - Neurobehavioral Assessment and Maternal Interviews
The initial plan was for all 231 children to be assessed for neurobehavior, and all mothers interviewed for complete coverage of the consented sample. Therefore, the study would accommodate discovery of all cases of FASD and provide a large number of verified, typically-developing controls. However, time and budgetary limitations intervened, and complete coverage for all 231 children was not possible. A team decision was made, and cleared with school officials, to limit full testing and assessment beyond the dysmorphology exam to the 48 FASD suspects and the first 48 children who were all contacted and found to be present in the county and available for testing. And the teachers and parents of these 48 children were believed to be available for behavioral assessments. Ultimately, not all mothers consented to a maternal risk interview, and some failed to make an appointment or failed to show up for an interview. Full or sufficient data (e.g., Teacher Report Forms and/or Parent Reports) for neurobehavioral assessments were completed on 84 children; 12 of the final 96 were either not located, could not be scheduled for an interview, or had moved before the end of the study.
Of the 84 mothers of the Tier II child participants who were tested, 72 mothers were either interviewed (n=69) or maternal data were obtained from a co-lateral source familiar with the mother during the gestational period of the index child (n=3). Other mothers (n=12) either refused consent to the interview or were impossible to schedule for the in-person interview.
2.5. Tier II - Neurobehavioral Testing and Maternal Risk Questionnaires
Development and behavior were assessed by professional staff and/or graduate students from the Carolina Institute for Developmental Disabilities with the CoFASP-endorsed battery (Figure 3). The battery was designed to evaluate the following domains: cognitive development, executive functioning, academic achievement, behavior, and adaptive skills. Instruments included were: Differential Abilities Scale (DAS-II) (Elliott, 2007) to assess general intelligence; NEPSY-II (Korkman et al., 2007) to assess executive functioning, memory, and visual/spatial integration; Developmental Test of Visual-Motor Integration (VMI) (Beery and Beery, 2004) to assess eye-hand coordination; Bracken Basic Concepts Scale (Bracken, 1998) to assess basic concept development in math, reading, and spelling; the Achenbach (Achenbach and Rescorla, 2001) Child Behavior Checklist (CBCL) and Teachers Report Form (TRF) to assess behavior; and Vineland Adaptive Behavior Scales (Sparrow et al., 2005) to examine activities of daily living and adaptive skills.
Figure 3.

CoFASP Cut-Off Criteria: Neurobehavioral Testing Battery
All consenting mothers of children in Tier II (potential cases and controls) who could be scheduled successfully, were provided face-to-face interviews by grant-funded project staff. Sequencing of questions was designed to maximize accurate self-reporting of: general health, reproduction, nutrition, alcohol and drug use, and socioeconomic status (SES). Maternal height, weight, and OFC were directly measured. Drinking questions employed a timeline, follow-back sequence (Sobell et al., 2001, 1988) and Vessels alcohol quantity methodology for accurate calibration of standard alcohol units (Kaskutas and Graves, 2001, 2000; Kaskutas and Kerr, 2008). The American “Standard Drink” was used, where one drink was equal to consuming 14 grams of absolute alcohol: 12oz. (350mL at 5% alcohol by volume) of beer; 5oz. (150mL) of wine (12% by volume); and 1.5oz. (44mL of 40% alcohol by volume) of liquor (NIAAA, n.d.). Current alcohol consumption for the week preceding the interview was embedded into dietary intake questions (King, 1994) to aid accurate calibration of quantity, frequency, and timing of alcohol use before and during the index pregnancies (Alvik et al., 2006; May et al., 2013, 2008, 2005). Retrospective reports of alcohol use have been found to be highly accurate in some populations when designed and administered properly (Czarnecki et al., 1990; Fortin et al., 2017; Hannigan et al., 2010; May et al., 2018).
Maternal risk data gathered from the mothers directly, or from knowledgeable collateral sources (relative or close associates), indicated that drinking prior to pregnancy recognition or during the index pregnancy was confirmed with the CoFASP criteria (Hoyme et al., 2016) if at least one of these measures were reported: a) six or more standard drinks per week for two or more weeks during pregnancy; b) a binge of 3 or more drinks per occasion on two or more occasions during pregnancy; or c) documentation of social or legal problems in proximity to the index pregnancy (e.g. treatment of alcohol abuse or infractions of driving under the influence). These criteria were not intended to reflect a threshold for damage associated with FASD. Rather, cut-off levels were established based on previous experience with responses in prior self-reported drinking surveys that were associated with dysmorphology and neurobehavioral impairment characteristic of an FASD.
2.6. Multidisciplinary Case Conferences for Final Diagnoses
Following data collection and aggregation, final diagnoses were made in confidential, multidisciplinary case conferences. The findings for each child in each domain were discussed in a structured manner where summary results were presented by the research team members who produced them. While findings were being presented and discussed, two-dimensional, digital photos of the child’s face (frontal and profile views) were projected to contextualize the discussion. Findings from each domain and examiner were weighed throughout the presentation, and the final diagnosis was made by the examining dysmorphologists with the consensus of the group. In rare cases, where there was lack of agreement among participants, the final diagnosis was delayed until clarification or additional details were brought to the table from the child’s file for the group to weigh. In classifying children, consistency and quality assurance were enhanced by strict application of the CoFASP criteria when preparing for and during case conferences. After the conference was completed, final diagnoses and data were double-checked for consistency and accuracy by the data management team and examiners.
2.7. Data Analysis and Final Prevalence Rates
Data analyses were performed with SPSS (IBM, 2020). Child physical, cognitive/behavioral, and maternal risk findings were compared across diagnostic groups using one-way analysis of variance, t-tests, and chi square. Statistical significance was determined with alpha of .05 and a significance level between >0.05 and ≤ 0.10 was considered to be approaching significance if the direction of the relationship was consistent with other studies of FASD (one-tailed significance).
3. Results
3.1. The Study Community
The county that hosted this study is characterized by several small towns in a primarily rural area in the Southeastern Region of the USA. As detailed in Appendix Table A1, the county population was 91,810 and had declined approximately 3% in the last decade. This compares to 6% growth overall in the USA (United States Census Bureau, 2019). The county has approximately the same percentage of Whites, slightly more Blacks, and fewer Hispanics and other minorities than the USA general population. The median household value is approximately 52% that of the general USA, and education levels are lower than the overall USA, with only half as many college graduates and 7% fewer high school graduates (United States Census Bureau, 2019). The county has lost many of its wage labor jobs (textiles, furniture, and small businesses) over the past 30 years. The county’s per capita and household incomes ($24,209 and $43,579) fall well below national averages (71% and 69% respectively for US averages), and 18% of the population lives below the poverty line compared to 10.5% of the USA. Health behavior is ranked lower in this county than the general population of at least 35 other states (United Health Foundation, 2020). But alcohol use data from both the CDC (Centers for Disease Control and Prevention, 2020) and NIAAA (Lavallee and Yi, 2011) indicate fewer drinking problems overall in this county and region from binge drinking, excessive drinking, and per capita ethanol consumption than in the USA. Only one drinking measure used by the CDC, heavy drinking (females having one or more drinks per day and males 2 or more) is high at 7.3% in this region compared to 5.0–5.6% elsewhere in the USA.
3.2. Child Physical Traits
Table 1 presents data on the key physical features of all children. In columns 1 and 2 are data on all children who received the full evaluation of physical traits, sufficient evaluation and testing of neurobehavior, and sufficient maternal risk information to be diagnosed either as cases or as controls (developing within the normal age-appropriate range). The third column provides data for the remainder of the random sample that were not evaluated or their mothers interviewed. Statistically significant differences across the three groups (α≤.05) were found for the following traits, each representing a characteristic typical of a child with FAS or PFAS: depressed height, weight, OFC (head circumference) ≤10th centile, Body Mass Index (BMI), and inter pupillary distance (IPD). Furthermore, the three cardinal features of FAS were significantly different between the three groups: short palpebral fissure length (PFL), smooth philtrum, and narrow vermilion of the upper lip as was the average total dysmorphology score across categories (see Figure 4). Approaching significance (≤0.10) in the expected direction for children with an FASD were: three other common features found with FAS and PFAS, decreased outer canthal distance (OCD) and maxillary and mandibular arc measurements. The children with FASD had a higher total dysmorphology score (9.2 vs. 4.3 for the control/comparison groups.
Table 1.
Demographic, Physical Growth, Cardinal FAS Features, Other Minor Anomalies, and Total Dysmorphology Score for Southeastern County II
| Children with FASD (n=6) | Randomly-Selected Control Children (n=71) | All Other Randomly-Selected Children (n=154) | Test-score | p-value | |
|---|---|---|---|---|---|
| Growth and Cardinal Features | |||||
| Sex (% Male) | 50.0 | 50.7 | 46.8 | .313 | .855 |
| Current Age (in months) – Mean (SD) | 83.7 (3.0) | 85.7 (4.9) | 85.1 (5.2) | .682 | .507 |
| Race/Ethnicity (%) | |||||
| White | 66.7 | 64.8 | 64.3 | ||
| Hispanic | 0.0 | 9.9 | 7.8 | ||
| Black | 33.3 | 21.1 | 24.7 | ||
| Other | 0.0 | 4.2 | 3.2 | 1.605 | .952 |
| Height Percentile – Mean (SD) | 31.5 (33.6) | 44.5 (29.7) | 56.6 (29.0) | 5.672 | .004C |
| Weight Percentile – Mean (SD) | 30.0 (34.2) | 49.2 (29.6) | 64.0 (29.0) | 9.099 | <.001C |
| Occipitofrontal Circumference (OFC) Centile – Mean (SD) | 16.7 (21.6) | 50.4 (33.0) | 63.1 (26.8) | 11.082 | <.001A,B,C |
| OFC centile ≤3rd centile | 16.7 | 11.3 | 1.3 | 12.574 | .002 |
| OFC centile ≤10th centile | 66.7 | 21.1 | 3.2 | 37.642 | <.001 |
| Child’s BMI Percentage – Mean (SD) | 38.7 (33.4) | 55.7 (28.0) | 65.7 (28.3) | 5.098 | .007C |
| Palpebral Fissure Length (PFL) Centile – Mean (SD) | 21.8 (18.7) | 37.4 (22.0) | 42.4 (18.1) | 4.371 | .014 |
| Smooth Philtrum (% Yes) | 50.0 | 25.4 | 14.9 | 7.247 | .027 |
| Narrow Vermilion (% Yes) | 66.7 | 25.4 | 19.5 | 7.846 | .020 |
| Other Minor Anomalies | |||||
| Inter Pupillary Distance (IPD) Centile – Mean (SD) | 41.7 (19.2) | 60.5 (24.9) | 67.0 (24.7) | 4.284 | .015 |
| Outer Canthal Distance (OCD) Centile – Mean (SD) | 32.2 (14.4) | 43.2 (20.2) | 47.8 (21.6) | 2.444 | .089 |
| Maxillary Arc (cm) – Mean (SD) | 24.1 (.9) | 25.0 (1.3) | 25.2 (1.2) | 2.455 | .088 |
| Mandibular Arc (cm) – Mean (SD) | 24.9 (1.4) | 26.1 (1.5) | 26.3 (1.4) | 2.974 | .053 |
| Total Dysmorphology Score – Mean (SD) | 9.2 (2.6) | 5.4 (3.9) | 3.8 (3.0) | 12.339 | <.001A,B,C |
Bonferroni adjusted significance level for Growth and Cardinal Features = 0.004; for other minor anomalies = .010 Post-hoc Dunnett C Comparisons were significantly different (p<.05) between:
FASD & Randomly-Selected Control Children;
FASD & All Other Randomly-Selected Children;
Randomly-Selected Control Children & All Other Randomly-Selected Children.
Figure 4.

Total Dysmorphology Score, Occipitofrontal Circumference (OFC), and Narrow Vermilion by FASD Diagnosis, Southeastern II County Sample
The dysmorphology exams performed on the entire sample of 231 children provide an additional description of significant FASD traits versus the physical traits of all of the children who were randomly selected for the study. The physical trait findings comparing the six children with FASD to the rest of the entire sample (n=225) are presented in the Appendix (Table A2). In this comparison most of the traits commonly associated with FAS and PFAS are also significantly different between the children with FASD and the rest of the sample. Children with FASD were smaller than others on weight centile, OFC centile, percent with OFC ≤10th centile, BMI centile, PFL centile, smooth philtrum, narrow vermilion, IPD centile, maxillary and mandibular arcs, and total dysmorphology score.
3.3. Child Neurobehavioral Traits
Results from selected neurobehavioral traits of the children are found in Table 2, and these measures are illustrated in Figures 5 and 6. Table 2 includes all intellectual domain variables tested, two executive function summary measures, one visual/spatial measure, and all behavioral checklist measures that significantly discriminated the children with FASD from controls. None of the four intellectual domain variable means were statistically different between groups, two executive function measure means approached significance, and the visual/spatial domain variable (VMI Standard Score) also approached significance. The behavioral measures obtained from the CBCL and the Vineland Adaptive Behavior Scale (VABS) demonstrated significantly greater discrimination of the groups. Three measures of impulse control (aggressive behavior, oppositional defiant behavior, and conduct problems) were all rated by the children’s teachers as less characteristic of the children with FASD, although these scores did fall within the average range for both groups. Parents of children with FASD rated their children on the VABS as having significantly more problems with communication, daily living skills, and socialization than did the parents of controls.
Table 2.
Neurobehavioral Traits Differentiating Diagnostic Groups in Southeastern County II: Children with FASD vs. Controls
| Children with FASD | Control Children | t-score | p-value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Intellectual Domain | (n=5) | (n=68) | ||
| General Abilities Percentile | 37.4 (34.2) | 45.7 (27.1) | −.50 | .518 |
| Verbal Cluster Percentile | 36.2 (34.2) | 50.6 (29.1) | −1.059 | .293 |
| Nonverbal Reasoning Cluster Percentile | 34.4 (26.7) | 44.1 (27.3) | −.766 | .446 |
| Spatial Cluster Percentile | 39.4 (35.8) | 45.0 (23.2) | −.507 | .614 |
| Executive Function | (n=5) | (n=68) | ||
| INN vs. INI Contrast Scaled Score | 7.0 (1.9) | 9.5 (3.2) | −1.679 | .098 |
| INI (Inhibition) combined scaled score | 6.4 (1.8) | 9.1 (3.2) | −1.854 | .068 |
| Visual Spatial | (n=5) | (n=68) | ||
| VMI Standard Score | 86.4 (9.9) | 92.2 (6.2) | −1.925 | .058 |
| Impulse Control – Child Behavior Checklist (CBCL) | (n=4) | (n=50) | ||
| TRF Aggressive behavior t-score | 50.3 (.5) | 53.6 (5.7) | −3.973 | <.001 |
| TRF Oppositional defiant problems t-score | 50.0 (.0) | 53.5 (6.6) | −3.723 | .001 |
| TRF Conduct problems t-score | 50.5 (1.0) | 53.3 (5.1) | −3.155 | .004 |
| Adaptive Function – Vineland (VABS) | (n=4) | (n=50) | ||
| Parent Communication Standard Score | 85.5 (7.7) | 103.7 (16.1) | −2.228 | .030 |
| Parent Daily Living Skills Standard Score | 83.5 (15.5) | 106.3 (14.8) | −2.957 | .005 |
| Parent Socialization Standard Score | 78.3 (13.4) | 101.1 (15.9) | −2.792 | .007 |
Figure 5.

Selected Cognitive and Executive Function Measures by FASD Diagnoses, Southeastern II County Sample
Figure 6.

Significant Behavioral Traits for Children with FASD vs. Controls Southeastern II County Sample
3.4. Maternal Risk Findings
Selected maternal risk factors identified from maternal interviews of the mothers of both groups are presented in Table 3 where it can be seen that there were few statistically significant differences registered between groups. The sample size significantly limited statistical power, especially on the large variety of the maternal risk variables considered. Significant maternal risk factors reported by the mothers of children with FASD were post-partum depression, less frequent breastfeeding of the index child, and a higher pre-pregnancy frequency of drinking (drinking every day or 1–4 times per week on average). Additionally, 20% of the mothers of children with FASD reported that they were “recovering drinkers.” Approaching significance was that a higher percentage of mothers of children with FASD reported lifetime misuse of prescription medication/pain killers. There were many other variables addressed in the maternal interviews, but no others were found to be significantly different between maternal groups.
Table 3.
Selected Maternal Risk Variables: Southeastern County II
| Children with FASD Mean (SD) |
Control Children Mean (SD) |
χ2 | p-value | |
|---|---|---|---|---|
| Physical | (n=5) | (n=61) | ||
| Age at pregnancy (yrs) | 30.4 (7.8) | 28.3 (6.4) | .689 | .494 |
| Height at interview (cm) | 159.4 (8.8) | 164.4 (6.8) | −1.517 | .136 |
| Weight at interview (kg) | 74.3 (25.2) | 83.7 (23.1) | −.860 | .394 |
| Demographic | (n=5) | (n=61) | ||
| Years of Education completed | 12.6 (.9) | 14.2 (2.5) | −1.414 | .163 |
| Household yearly income - during pregnancy | 25666 (12423) | 45230 (29692) | −1.121 | .270 |
| Household yearly income – at interview | 22529 (28227) | 51168 (38265) | −1.652 | .212 |
| Marital Status – current | ||||
| Married | 40.0 | 63.3 | ||
| Divorced/Widowed/Separated/Single | 60.0 | 32.7 | ||
| Living with Partner | 0.0 | 4.1 | 1.570 | .456 |
| Health Status | (n=5) | (n=59) | ||
| Postpartum depression (% Yes) | 60.0 | 16.9 | 5.278 | .022 |
| Postnatal Environment | (n=5) | (n=58) | ||
| Breastfed (% Yes) | 0.0 | 59.3 | 4.249 | .011 |
| Partner ever had a drinking problem^ | ||||
| Never | 50.0 | 82.8 | ||
| In the past, but not currently | 0.0 | 6.9 | ||
| Currently | 0.0 | 0.0 | ||
| Both past and currently | 50.0 | 10.3 | 3.839 | .129 |
| Alcohol Use – Before and During Pregnancy | (n=5) | (n=60) | ||
| Drank before pregnancy (% Yes) | 80.0 | 48.3 | 2.115 | .146 |
| # of drinks consumed on usual drinking day before pregnancy1 | 2.8 (1.5) | 1.9 (1.4) | 1.017 | .319 |
| Usual frequency – before pregnancy1 | ||||
| Everyday or almost everyday | 50.0 | 0.0 | ||
| 3–4 times per week | 0.0 | 3.6 | ||
| 1–2 times per week | 25.0 | 14.3 | ||
| 2–3 times per month | 25.0 | 17.9 | ||
| 1 time per month or less | 0.0 | 64.3 | 17.067 | .002 |
| Drank in 1st trimester (% Yes) | 20.0 | 4.0 | 2.405 | .300 |
| Alcohol Use - Current | (n=5) | (n=58) | 11.787 | .001 |
| Recovering drinker at interview (% Yes) | 20.0 | 0.0 | 9.384 | .002 |
| Drug Use | (n=5) | (n=60) | ||
| Used tobacco – in lifetime^ | ||||
| Yes, within last 30 days | 50.0 | 21.7 | ||
| Yes, in lifetime | 50.0 | 31.7 | ||
| Never | 0.0 | 46.7 | .921 | .631 |
| Used any drug in lifetime (% Yes) | 20.0 | 47.9 | 1.424 | .233 |
| Used marijuana – in lifetime (% Yes) | 20.0 | 43.8 | 1.052 | .305 |
| Used crack/cocaine – in lifetime (% Yes) | 20.0 | 8.3 | .721 | .396 |
| Abused pain killers – in lifetime (% Yes) | 20.0 | 3.4 | 2.846 | .092 |
Among those who reported drinking in the given time period.
Only four respondents answered this question.
3.5. Prevalence of FASD
The prevalence of FASD in this population, based on this randomly-selected, active case ascertainment sample, is presented in Table 4. The rates were: two children or 23.8 per 1,000 children qualified for a diagnosis of FAS, 2 children or 23.8 per 1,000 were diagnosed as PFAS, and 2 children or 23.8 per 1,000 were diagnosed with ARND. Based on the six children diagnosed with an FASD, the total FASD rate was 71.4 per 1,000, or stated as a percentage, 7.1% of first grade children.
Table 4.
Final Prevalence Estimates of FASD (with CoFASP criteria) Utilizing Random Selection Only and 95% Conference Intervals by Specific Diagnoses: Southeastern County II
| Diagnosis | n | Proportion | Rate per 1,000 | 95% Confidence Intervals |
| FAS | 2 | 0.02381 | 23.8 | 0.0 to 56.4 |
| PFAS | 2 | 0.02381 | 23.8 | 0.0 to 56.4 |
| ARND | 2 | 0.02381 | 23.8 | 0.0 to 56.4 |
| Total FASD | 6 | 0.07143 | 71.4 | 16.4 to 126.5 |
Total school enrollment n=1073; children picked from random sampling n =721 (67.2% of enrolled students); dysmorphology exams were completed for all consented children, n=231 (21.5% of enrolled students; 32.0% of those selected randomly); 39 of 44 of children suspected as FASD cases completed both dysmorphology exams and neurobehavioral testing + 45 of 48 controls = 84 (7.8% of enrolled students; 11.7% of randomly-selected students).
4. Discussion
4.1. Feasibility of Using Random Samples to Study FASD
As the demographic and economic data indicated, this study took place in a community highly impacted by economic retraction and stagnation, combined with low education and income levels. This created challenges for the data collection process due to transience of families and children and lack of availability of working mothers for interviews, but even in this environment the use of established methods and study protocol resulted in information that can inform prevention and treatment efforts. In other words, given the challenges of conducting studies like these in highly-economically-impacted communities, having the option of doing a smaller scale, but well-developed study that is grounded in established research methods and protocols can lessen barriers to determining community prevalence of FASD, and therefore estimate the need for prevention and intervention.
The data presented here provide evidence that even a small random sample of school children can be utilized as a stand-alone method to produce an estimate of the prevalence of FASD in a population even when not all children receive complete, gold standard evaluation. Comparative information on multiple, selected, FASD-linked, physical and behavioral traits in a population of elementary school children can be used to determine average traits for both children developing in the normal range and children with an FASD and as a validation of accurate application of the diagnostic criteria of FASD. But, one can ask, are these prevalence findings creditable? To answer this question, we compared the final prevalence rate of 7.1% of children with FASD established in this study to findings from eight other regional samples in the CoFASP study. The findings in this community fit squarely in the middle of the range of prevalence estimates from the larger CoFASP samples which used a combination of ACA methods. The range of the weighted estimated prevalence rates in CoFASP was 3.1% to 9.9% (May, Chambers et al., 2018). Furthermore, the mean of these weighted CoFASP prevalence estimates from the eight CoFASP regional samples was 6.5% with a median=6.7%, very similar to the 7.1% prevalence yielded here (May, Chambers et al., 2018). In the two CoFASP samples carried out in a different county of the Southeastern region, the estimated FASD rates were 3.1% (95% CI: 1.6–3.8) and 6.7% (95% CI: 3.8–10.6) using a larger sample and ACA study entry techniques via two methods: 1) all consented children who screened small (≤25th centile on height, weight, or head circumference) and 2) provided entry via random selection (May et al., 2020c). Therefore, it appears that this study yielded enough complete and creditable data and results that compared favorably with larger samples utilizing other ACA methods. Certainly, the study could have benefitted from more time and resources to collect all data from all domains of the study from all of the 231 randomly-selected children and their mothers. If so, the data would have been far more creditable, more significant in the case control comparisons, and probably yielded a slightly higher prevalence. But we suspect that all of the prevalence rates that we have produced in the US studies represent undercounts or minimal prevalence. Since the CoFASP consent rate average was less than 60%, many people might suspect that those families with a history of heavy drinking were less likely to provide consent for their children to participate in a FASD study. At this site, because only 32% of the children who were asked to participate received consent from their parents, one might suspect that this study is also likely to be an underestimate.
4.2. Comparison of Trait Findings with Previous Studies
The child physical differences between the children with FASD and not FASD were consistent with all other studies using ACA methods. That is, children with FASD were significantly smaller and less developed physically than the confirmed typically-developing controls in Table 1 and in the comparison of the diagnosed FASD cases with the average traits of all of the rest of the children chosen randomly. In the neurobehavioral domain, the children with FASD had executive function and visual/spatial traits that approached significance, and negative behavioral traits that were quite significantly different. Aggressive behavior, oppositional/defiant, and conduct problems were rated by teachers significantly more common among controls. Parents rated communication, daily learning, and socialization as problems of children with FASD. In the maternal risk data, frequency of pre-pregnancy drinking differentiated the study groups as in other USA studies of FASD (Chambers et al., 2019; May et al., 2020a, 2020b, 2020c). Furthermore, postpartum depression and problem drinking history were confirmed to be greater among the mothers of children with FASD.
4.3. Limitations and Strengths
There were obvious limitations to this study. First, the sample size overall was small with only 21.5% of the first-grade children sampled and complete data collected on all three domains (physical, neurobehavioral, and maternal risk) on 7.8% of the students. Second, even pursuing complete information on this small sample, the study was labor intensive and expensive due to the logistics of in-person examinations, testing, maternal interviews, and the multidisciplinary nature of the study. The extended period of time and the expenses required for completion caused us to limit the final study numbers; but, the findings still attained similar prevalence results to earlier ACA studies in the U.S. with larger samples. Third, fewer children were fully tested on neurobehavior and fewer women were interviewed than originally planned, but comparisons of key findings with other studies seemed to indicate reliability of findings on the traits of the children and the prevalence estimates. More time and money could have facilitated validation of the efficacy of this simple random sampling method. Fourth, the final control group was generally representative of children who were developing within the normal range for this community. But since it also contained 36 children who had originally been deferred as suspects for an FASD diagnosis after the initial dysmorphology exam and growth measurements, it may not have been as random as desired, and may not have yielded as many significant differences between children with FASD and controls. The data in Table 1 and Appendix Table A2 confirm that the control group was intermediate between the FASD cases and the other children in the random sample.
One strength of this study was the utilization of the established, revised IOM diagnostic methods with cutoff criteria designed and formulated for the CoFASP studies. This allowed comparison of the results from this study to that of other CoFASP sites in the USA. Secondly, the full dysmorphology exams completed on all of the consented students proved to be an especially valuable contribution to this otherwise small sample study.
5. Conclusion
Utilizing proven clinical methods to diagnose children with FASD drawn from a relatively small random sample in a defined population of first grade school children proved to be efficacious. Findings compared favorably to other studies on overall prevalence results, family characteristics, physical traits, and neurocognitive abilities, and indicate that previously undiagnosed children can still be identified as early as their entry into formal schooling. Such early identification, in turn, can enables and facilitate early interventions to assist the development of those affected by FASD.
Highlights.
The prevalence of FASD in this random sample was 7.1%.
The prevalence estimates are consistent with current US estimates.
Microcephaly and total dysmorphology score were typical of FASD.
Adaptive function was significantly worse in children with FASD.
Random samples can be useful for estimating the prevalence of FASD.
Acknowledgements
Our deepest thanks are extended to the Superintendent of schools, Board of Education, administrators, principals, guidance counselors, and teachers of the local, county school system in this study community who hosted and assisted in the research process. The professional support, guidance, and facilitation of all these people have been vital to the success of this study. We are also grateful for the advice and participation in the planning and implementation of the project by the NIAAA-funded CoFASP Advisory Committee members who were led by Marcia Scott, Ph.D., NIAAA Program Officer, Judith Arroyo, Ph.D., Michael Charness, M.D., William Dunty, Ph.D., Daniel Falk, Ph.D., Dale Herald, M.D., Ph.D., and Edward Riley, Ph.D.
The authors and research team are also grateful to our institutional partner in this research community, the University of North Carolina at Greensboro, especially Terri Shelton, the Vice Chancellor for Research and Engagement. Furthermore, the support and facilitation of Dr. Rodney Shotwell and Dr. Cindy Corcoran were vital to our efforts at all phases of this endeavor.
Role of the funding source:
This project was funded by the National Institutes of Health (NIH), the National Institute on Alcohol Abuse, and Alcoholism (NIAAA), grant UO1 AA019894 as part of the Collaboration on Fetal Alcohol Spectrum Disorders Prevalence (CoFASP) consortium. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Table Al.
Demographic Indicators for Southeastern County II compared to the United States.
| Demographic Indicator | SE Study County | United States |
|---|---|---|
| Population (7/2019)1 percentage of US population | 91,810 (0.028%) |
328,239,523 (100%) |
| Population change (%) since 20101 | −2.8% | 6.3% |
| Race and Hispanic Origin (2010)1 | ||
| White alone | 77.5% | 76.3% |
| Black alone | 19.0% | 13.4% |
| American Indian and Alaskan Native alone | 0.6% | 1.3% |
| Asian alone | 0.7% | 5.9% |
| Two are more races | 2.1% | 2.8% |
| Hispanic or Latino | 6.3% | 18.5% |
| Foreign born persons1 | 3.8% | 13.6% |
| Age – years (median) | 44.5 | 38.3 |
| Housing1 | ||
| Median household value | $112,800 | $217,500 |
| Education1 | ||
| High School graduate or higher, % ages ≥25 years | 82.7% | 88.0% |
| Bachelor’s degree or higher, % ages ≥25 years | 15.1% | 32.1% |
| Economy1 | ||
| Per capita income in past 12 months (2014 dollars) | $24,209 | $34,103 |
| Median household income | $43,579 | $62,843 |
| Persons in poverty | 18.4% | 10.5% |
| Health Behavior State Rank in U.S.2 | 35–40 | Median 25 |
| Overall State Health Rank in U.S.2 | 30–34 | (Range 1–50) |
| Alcohol Use | ||
| Excessive drinking2 | 15.4% | 18.6% |
| Binge drinking*, region % | 15.6% | 16.8% |
| Heavy drinking#, local region3 | 7.3% | 5.0–5.6% |
| State per capita ethanol consumption (2009), volume per person 14 years and older4 | 2.02 gallons(8th decile) 7.65 liters |
2.30 gallons 8.71 liters |
Sources:
US Census, Quick Facts for the Southeastern II County, Calendar year 2019.
United Health Foundation, America’s Health Rankings, 2020; comprised of scores on behaviors, community and environment, policy and clinical care; scores are ranked for each of the 50 states with better scores resulting in a higher rank among the 50 states; ranges indicate that different rankings are provided for each of the four domains named above.
BRFSS (Behavioral Risk Factor Surveillance System Survey) data of the CDC. Reported in local regional statistical reports.
La Valle and Yi, NIAAA Surveillance Report #92.
Binge drinking defined as: during the past 30 days, the consumption of 5 or more drinks for men or 4 or more drinks for females on an occasion.
Heavy drinking is defined as males having more than two drinks pes day and females having more than one drink per day.
Excessive drinking of alcohol is defined as both binge drinking (above) and chronic drinking also referred to as heavy drinking (above).
Table A2.
Child Physical Traits for the Total Sample of Randomly Selected Children: Southeastern County II.
| Children with FASD (n = 6) |
All Other Randomly Selected Children (n = 225) |
||||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | P | |
| Sex (% Male) | 50.0 | 48.0 | .923 | ||
| Current Age (in months) | 83.7 | (3.0) | 85.3 | (5.1) | .440 |
| Height Percentile | 31.5 | (33.6) | 52.8 | (29.7) | .085 |
| Weight Percentile | 30.0 | (34.2) | 59.3 | (29.9) | .019 |
| OFC Percentile | 16.7 | (21.6) | 59.1 | (29.5) | .001 |
| OFC centile <3rd centile | 16.7 | 4.4 | .165 | ||
| OFC centile <10th centile | 66.7 | 8.9 | <.001 | ||
| Child’s BMI Percentage | 38.7 | (33.4) | 62.5 | (28.5) | .045 |
| PFL Percentile | 21.8 | (18.7) | 40.8 | (19.5) | .019 |
| Smooth Philtrum (% Yes) | 50.0 | 18.2 | .050 | ||
| Narrow Vermillon (% Yes) | 66.7 | 21.3 | .009 | ||
| ICD Percentile | 68.7 | (17.3) | 61.2 | (22.5) | .422 |
| IPD Percentile | 41.7 | (19.2) | 65.0 | (24.9) | .024 |
| OCD Percentile | 32.2 | (14.4) | 46.3 | (21.2) | .106 |
| Maxillary Arc (in cm) | 24.1 | (0.9) | 25.1 | (1.2) | .048 |
| Mandibular Arc (in cm) | 24.9 | (1.4) | 26.2 | (1.4) | .028 |
| Total Dysmorphology Score | 9.2 | (2.6) | 4.3 | (3.4) | .001 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no financial relationship relevant to this article to disclose. The authors have no conflict of interest to declare.
References
- Abel EL, Sokol RJ, 1987. Incidence of fetal alcohol syndrome and economic impact of FAS-related anomalies. Drug Alcohol Depend. [DOI] [PubMed]
- Achenbach T, Rescorla L., 2001. Manual For The ASEBA School-Age Forms And Profiles. University of Vermont, Research Center for Children, Youth, and Families, Burlington, VT. [Google Scholar]
- Alvik A, Haldorsen T, Lindemann R., 2006. Alcohol consumption, smoking and breastfeeding in the first six months after delivery. Acta Paediatr. Int. J. Paediatr 95, 686–693. [DOI] [PubMed] [Google Scholar]
- Beery KE, Beery NA, 2004. The Beery-Buktenica Development Test of Visual-Motor Integration, Fifth Edit. ed. Pearson Assessment, San Antonio, TX. [Google Scholar]
- Bower C, Silva D, Henderson TR, Ryan A, Rudy E., 2000. Ascertainment of birth defects: The effect on completeness of adding a new source of data. J. Paediatr. Child Health 36, 574–576. [DOI] [PubMed] [Google Scholar]
- Bracken BA, 1998. Bracken Basic Concept Scale - Revised.
- Burd L, Cox C, Poitra B, Wentz T, Ebertowski M, Martsolf JT, Kerbeshian J, Klug MG, 1999. The FAS Screen: a rapid screening tool for fetal alcohol syndrome. Addict. Biol 4, 329–336. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2020. Behavioral Risk Factor Surveillance System [WWW Document]. URL https://www.cdc.gov/brfss/index.html
- Centers for Disease Control and Prevention, 1995. Update: Trends in Fetal Alcohol Syndrome—United States, 1979–1993. Morb. Mortal. Wkly. report. MMWR 44, 249–251. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 1993. Fetal alcohol syndrome--United States, 1979–1992. MMWR. Morb. Mortal. Wkly. Rep 42, 339–341. [PubMed] [Google Scholar]
- Chambers CD, Coles C, Kable J, Akshoomoff N, Xu R, Zellner JA, Honerkamp-Smith G, Manning MA, Adam MP, Jones KL, 2019. Fetal Alcohol Spectrum Disorders in a Pacific Southwest City: Maternal and Child Characteristics. Alcohol. Clin. Exp. Res 43, 2578–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez GF, Cordero JF, Becerra JE, 1988. Leading major congenital malformations among minority groups in the United States, 1981–1986. MMWR. CDC Surveill. Summ 37, 17–24. [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D., 1990. Five-year reliability of self-reported alcohol consumption. J. Stud. Alcohol 51, 68–76. [DOI] [PubMed] [Google Scholar]
- Elliott CD, 2007. Differential Ability Scales-II (DAS-II).
- Fortin M, Muckle G, Jacobson SW, Jacobson JL, Belanger RE, 2017. Alcohol use among Inuit pregnant women: Validity of alcohol ascertainment measures over time. Neurotoxicol. Teratol 64, 73–78. [DOI] [PubMed] [Google Scholar]
- Foundation UH, 2020. America’s Health Rankings [WWW Document]. URL https://www.americashealthrankings.org/
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, Delaney-Black V., 2010. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol 44, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA, 2016. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138, e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku PW, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK, 2005. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 115, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM, 2020. IBM SPSS Statistics for Windows.
- Jones KL, Smith DW, 1973. Recognition of the fetal alcohol syndrome in early infancy. Lancet (London, England) 302, 999–1001. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K., 2001. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction 96, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K., 2000. An alternative to standard drinks as a measure of alcohol consumption. J. Subst. Abuse 12, 67–78. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Kerr WC, 2008. Accuracy of Photographs to Capture Respondent-Defined Drink Size. J. Stud. Alcohol Drugs 69, 605–610. [DOI] [PubMed] [Google Scholar]
- King AC, 1994. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am. J. Public Health 84, 294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S., 2007. NEPSY (NEPSY-II), Second. ed. Pearson Assessment, San Antonio, Texas. [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, Popova S., 2017. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr. 171, 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavallee RA, Yi H., 2011. Surveillance Report #92: Apparent Per Capita Alcohol Consumption: National, State, and Regional Trends, 1977–2009. Rockville, MD. [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE, 2014. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134, 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Joubert B, Cloete M, Barnard R, De Vries M, Hasken J, Robinson LK, Adnams CM, Buckley D, Manning M, Parry CDH, Hoyme HE, Tabachnick B, Seedat S., 2013. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend. 133, 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D., 2000. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am. J. Public Health 90, 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Daniel F, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE, 2018. Prevalence of fetal alcohol spectrum disorders in 4 US communities. J. Am. Med. Assoc 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, de Vries MM, Marais AS, Kalberg WO, Adnams CM, Hasken JM, Tabachnick B, Robinson LK, Manning MA, Jones KL, Hoyme D, Seedat S, Parry CD, Hoyme HE, 2016a. The continuum of fetal alcohol spectrum disorders in four rural communities in south africa: Prevalence and characteristics. Drug Alcohol Depend. 159, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Coriale G, Kalberg WO, Eugene Hoyme H, Aragón AS, Buckley D, Stellavato C, Phillip Gossage J, Robinson LK, Jones KL, Manning M, Ceccanti M., 2011. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: New estimated rates are higher than previous estimates. Int. J. Environ. Res. Public Health 8, 2331–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Phillip Gossage J, Kalberg WO, Eugene Hoyme H, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M., 2006. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol. Clin. Exp. Res 30, 1562–1575. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, 2001. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Heal. 25, 159–167. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-SS, Hendricks LS, Croxford JA, Viljoen DL, 2005. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am. J. Public Health 95, 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE, 2009. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev 15, 176–192. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL, 2007. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 88, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL, 2008. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol. Clin. Exp. Res 32, 738–753. [DOI] [PubMed] [Google Scholar]
- May PA, Hasken JM, Baete A, Russo J, Elliott AJ, Kalberg WO, Buckley D, Brooks M, Ortega MA, Hedrick DM, Tabachnick BG, Abdul-Rahman O, Adam MP, Jewett T, Robinson LK, Manning MA, Hoyme HE, 2020a. Fetal Alcohol Spectrum Disorders in a Midwestern City: Child Characteristics, Maternal Risk Traits, and Prevalence. Alcohol. Clin. Exp. Res 44, 919–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Bozeman R, Jones JV, Burns MK, Goodover J, Kalberg WO, Buckley D, Brooks M, Ortega MA, Elliott AJ, Hedrick DM, Tabachnick BG, Abdul-Rahman O, Adam MP, Jewett T, Robinson LK, Manning MA, Hoyme HE, 2020b. Fetal Alcohol Spectrum Disorders in a Rocky Mountain Region City: Child Characteristics, Maternal Risk Traits, and Prevalence. Alcohol. Clin. Exp. Res 44, 900–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Stegall JM, Mastro HA, Kalberg WO, Buckley D, Brooks M, Hedrick DM, Ortega MA, Elliott AJ, Tabachnick BG, Abdul-Rahman O, Adam MP, Robinson LK, Manning MA, Jewett T, Hoyme HE, 2020c. Fetal Alcohol Spectrum Disorders in a Southeastern County of the United States: Child Characteristics and Maternal Risk Traits. Alcohol. Clin. Exp. Res [DOI] [PMC free article] [PubMed]
- May PA, Hymbaugh KJ, Aase JM, Samet JM, 1983. Epidemiology of fetal alcohol syndrome among American Indians of the Southwest. Soc. Biol 30, 374–387. [DOI] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Gossage JP, Robinson LK, Manning M, Hoyme HE, 2015. Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend. 155, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Marais AS, De Vries MM, Buckley D, Kalberg WO, Hasken JM, Stegall JM, Hedrick DM, Robinson LK, Manning MA, Tabachnick BG, Seedat S, Parry CD, Hoyme HE, 2021. The prevalence, child characteristics, and maternal risk factors for the continuum of fetal alcohol spectrum disorders: A sixth population-based study in the same South African community. Drug Alcohol Depend. 218, 108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Marais AS, de Vries MM, Kalberg WO, Buckley D, Hasken JM, Adnams CM, Barnard R, Joubert B, Cloete M, Tabachnick B, Robinson LK, Manning MA, Jones KL, Bezuidenhout H, Seedat S, Parry CDH, Hoyme HE, 2016b. The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend. 168, 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA, n.d. What Is A Standard Drink? [WWW Document]. URL https://www.niaaa.nih.gov/alcohols-effects-health/overview-alcohol-consumption/what-standard-drink
- Nellhaus G., 1968. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics 41, 106–114. [PubMed] [Google Scholar]
- Okulicz-Kozaryn K, Borkowska M, Brzozka K., 2017. FASD Prevalence among Schoolchildren in Poland. J. Appl. Res. Intellect. Disabil 30, 61–70. [DOI] [PubMed] [Google Scholar]
- Petkovic G, Barisic I., 2013. Prevalence of fetal alcohol syndrome and maternal characteristics in a sample of schoolchildren from a rural province of Croatia. Int. J. Environ. Res. Public Health 10, 1547–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic G, Barisic I., 2010. FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod. Toxicol 29, 237–241. [DOI] [PubMed] [Google Scholar]
- Poitra BA, Marion S, Dionne M, Wilkie E, Dauphinais P, Wilkie-Pepion M, Martsolf JT, Klug MG, Burd L., 2003. A school-based screening program for fetal alcohol syndrome. Neurotoxicol. Teratol 25, 725–729. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Poznyak V, Chudley AE, Shield KD, Reynolds JN, Murray M, Rehm J., 2019. Population-based prevalence of fetal alcohol spectrum disorder in Canada. BMC Public Health 19, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L., 2016. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol. Clin. Exp. Res 40, 18–32. [DOI] [PubMed] [Google Scholar]
- Roozen S, Peters GJY, Kok G, Townend D, Nijhuis J, Koek G, Curfs L., 2018. Systematic literature review on which maternal alcohol behaviours are related to fetal alcohol spectrum disorders (FASD). BMJ Open 8, e022578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M., 2001. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst. Use Misuse 36, 313–331. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI, 1988. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol 49, 225–232. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B., 2003. Fetal alcohol spectrum disorder. Jama 290, 2996–2999. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Miller SI, Debanne S, Golden N, Collins G, Kaplan J, Martier S., 1981. The Cleveland NIAAA prospective alcohol-in-pregnancy study: The first year. Neurobehav. Toxicol. Teratol. 3, 203–209. [PubMed] [Google Scholar]
- Sparrow SA, Cicchetti DV, Balla DA, 2005. Vineland Adaptive Behavior Scales, Second. ed. Bloomington, Minnesota. [Google Scholar]
- Stratton K, Howe C, Battaglia F., 1996. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington DC. [Google Scholar]
- Thomas IT, Gaitantzis YA, Frias JL, 1987. Palpebral fissure length from 29 weeks gestation to 14 years. J. Pediatr. 111, 267–268. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau, 2019. US Census [WWW Document]. URL https://www.census.gov/data.html
- Viljoen DL, Gossage JP, Brooke L, Adnams CM, Jones KL, Robinson LK, Hoyme HE, Snell C, Khaole NCO, Kodituwakku P, Asante KO, Findlay R, Quinton B, Marais A-S, Kalberg WO, May PA, 2005. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J. Stud. Alcohol 66, 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]


