Abstract
Objective
To elaborate on possible cognitive sequelae related to COVID-19, associated cerebrovascular injuries as well as the general consequences from intensive care. COVID-19 is known to have several, serious CNS-related consequences, but neuropsychological studies of severe COVID-19 are still rare.
Methods
M., a 45-year-old man, who survived a severe COVID-19 disease course including Acute Respiratory Distress Syndrome (ARDS), cerebral microbleeds, and 35 days of mechanical ventilation, is described. We elaborate on M’s recovery and rehabilitation process from onset to the 8-month follow-up. The cognitive functions were evaluated with a comprehensive screening battery at 4 weeks after extubation and at the 8-month follow-up.
Results
Following extubation, M. was delirious, reported visual hallucinations, and had severe sleeping difficulties. At about 3 months after COVID-19 onset, M. showed mild to moderate deficits on tests measuring processing speed, working memory, and attention. At assessments at 8 months, M. performed better, with results above average on tests measuring learning, memory, word fluency, and visuospatial functions. Minor deficits were still found regarding logical reasoning, attention, executive functioning, and processing speed. There were no lingering psychiatric symptoms. While M. had returned to a part-time job, he was not able to resume previous work-tasks.
Conclusion
This case-study demonstrates possible cognitive deficits after severe COVID-19 and emphasizes the need of a neuropsychological follow-up, with tests sensitive to minor deficits. The main findings of this report provide some support that the long-term prognosis for cognition in severe COVID-19 may be hopeful.
Keywords: COVID-19, Case Report, Assessment, Rehabilitation, ARDS, Executive functions
Introduction
Coronaviruses commonly cause mild upper respiratory tract infections, but some strains may cause severe acute respiratory syndrome (SARS), Middle East respiratory syndrome, and coronavirus disease-19 (COVID-19), which may result in severe respiratory tract infections and multi-organ failure (Cheng, To, Tse, Hung, & Yuen, 2012; Lu et al., 2020). Age (>45 years) is one of the strongest predictors for hospitalization due to COVID-19 (Kass, Duggal, & Cingolani, 2020). Other factors that may lead to a more severe disease course in younger patients include obesity, presumably by reducing the respiratory function and lung volume, (for an extensive review, see Yu, Rohli, Yang, & Jia, 2020), diabetes mellitus (Lim, Bae, Kwon, & Nauck, 2020), hypertension, and cerebrovascular disorders (Yu et al., 2020). Although COVID-19 usually does not lead to hospitalization, there is evidence suggesting direct neuroviral and neuroinvasive harm to the central nervous system (CNS), especially in severe cases (Helms et al., 2020; Ritchie, Chan, & Watermeyer, 2020). However, the neurological manifestations could also be the result of postinfectious immune–mediated processes (Almeria, Cejudo, Sotoca, Deus, & Krupinski, 2020).
It has been shown that a large number of Intensive Care Unit (ICU) patients show long-term psychiatric, motor, and cognitive deficits after hospital discharge; something currently known as Post Intensive Care Syndrome (PICS) (Desai, Law, & Needham, 2011; Sasannejad, Ely, & Lahiri, 2019). The incidence of PICS is estimated to 25%–75% of all ICU-treated patients and the cognitive consequences associated include memory impairment, attention, and executive functioning deficits (Korupolu, Francisco, Levin, & Needham, 2020; Merbitz, Westie, Dammeyer, Butt, & Schneider, 2016; Needham et al., 2012). PICS is especially common after surviving Acute Respiratory Distress Syndrome (ARDS) which in turn has an incidence estimated to 33% of all patients hospitalized with COVID-19 and ~66% of all COVID-19 patients requiring ICU care (Tzotzos, Fischer, Fischer, & Zeitlinger, 2020). It has even been shown that one year after hospitalization, one-third of the survivors of ARDS show neuropsychological test scores equivalent to those after moderate traumatic brain injury (Pandharipande et al., 2013).
The long-term sequelae after severe COVID-19 is still unknown, but we do know that critically ill COVID-19 patients frequently present with ARDS and require prolonged mechanical ventilation in ICU, which is a potent risk factor for long-term cognitive decline (Bulic et al., 2017; Jayaswal, Sampath, Soohinda, & Dutta, 2019). Studies including comprehensive neuropsychological evaluations of patients who have suffered severe COVID-19 are still scarce. Zhou and colleagues (2020) found a significant positive correlation between higher c-reactive protein, a common marker of inflammation, and higher reaction time and more errors in the Continuous Performance Test (a measure of sustained attention and inhibition) for the COVID-19 group compared with healthy matched controls. Almeria and colleagues (2020) concluded in a single center cohort study that the presence of neurological symptoms during COVID-19, such as headache, anosmia, and dysgeusia, were risk factors for later display of cognitive deficits regarding memory, executive functions, and attention. Need for oxygen therapy and reports of digestive problems were also connected to the deficits of attention and executive functions in Almeria and colleagues (2020) study. To our knowledge, apart from these two retrospective studies, no extensive cognitive work-up has been assessed in patients with COVID-19.
As we now see increasing evidence of direct or indirect impacts of COVID-19 on the CNS, it is most likely that health care workers in post-intensive care and rehabilitation facilities will face more cognitive complaints from patients once the respiratory syndrome is in remission. Hence, it is of substantial scientific and clinical relevance to describe the cognitive consequences of COVID-19. Therefore, we present a case of a 45-year-old man, M., with obesity and diabetes mellitus who survived severe COVID-19 after 35 days of critical care. The objective of the following case report was to elaborate on possible cognitive sequelae related to COVID-19 as well as associated cerebrovascular injuries.
Case Presentation
Patient Background and Medical History
The patient (M.) described is a 45-year-old man with a Bachelor’s degree. M. had a total of 16 years of education, in which 4 years of studies was at university level. Premorbid cognitive function was estimated to be at least “high average” due to educational background and biography reports from the patient history. This estimate is consistent with M’s later result in the Block Design Subtest from Wechsler Adult Intelligence Scale IV (WAIS-IV), in which the performance was approximately one standard deviation (SD) above average. In an anamnestic interview, M. mentioned that he considered himself as a bit impulsive and restless compared with others, but these traits had not been causing any known functional impairment. By the time of the SARS-CoV-2 infection, the patient was living with his family in a Swedish suburb, working full-time as a senior financial consultant. Prior to COVID-19, the patient reported feeling physically healthy, although with a weight of 150 kg and a height of 186 cm resulting in a Body Mass Index of 43.36 kg•m−2 (clinical obesity). He also suffered from type 2 diabetes, mellitus ulcerative proctitis, and hypertension. Twelve months prior to the hospitalization, M. reported minor symptoms of anxiety, stress, and dysthymia, for which he received sertraline (100 mg•day−1) and a few sessions of cognitive behavioral therapy, which he found helpful. In summary, there were no reports of cognitive decline, serious psychiatric symptoms, or any other noticeable cognitive impairment before the patient was sickened with COVID-19.
M. became critically ill with COVID-19, requiring intubation and mechanical ventilation at Day 14 after confirmed illness (Table 1). Initially, the prognosis was poor, and despite intense attempts to treat the severe respiratory distress, the situation escalated with bacterial infections, acute renal damage, and multi-organ failure. Contact was established with the extracorporeal membrane oxygenation (ECMO) department as the situation deteriorated, but his obesity disqualified him from such interventions. At this point, the situation was so dire that the family was called in to the ICU to say their final farewell. However, M. slowly began to respond to treatments, and after 4 weeks in the ICU, M. stabilized, was extubated, and transferred to an intermediate care department (ICD). During the first two weeks after critical care, M. presented as confused and disoriented and was diagnosed with delirium. Signs of delirium were reported from extubation, Day 46, to approximately Day 58, two days before M. was moved to the rehabilitation department. The delirium was categorized as a mixed variant with fluctuations between a state of irritability and visual hallucinations and periods of drowsiness and slowed motor function. M. reported severe nightmares, had disturbing visual hallucinations, and experienced severe sleeping disorders the first two weeks after extubation. In the ICU, he was administered orally clonidine (maximum of 600 μg•day−1) and olanzapine (maximum of 20 mg•day−1), which were both gradually tapered and discontinued within the first week of intermediate care. At about Day 51 after COVID-19 onset, a rehabilitation specialist physician performed a short cognitive screening assessment with Confusion Assessment Method-Short (Item Ia, acute change in mental status: yes; Item Ib, fluctuation of abnormal behavior: yes; Item II, inattention: yes, Item III, disorganized thinking: yes; Item IV: no [alert]) and selections from the Montreal Cognitive Assessment (MoCa), which indicated confusion and deficits in the areas of working memory, orientation, arithmetics, immediate and delayed memory retention, and verbal abstraction. Only a targeted selection of MoCa was performed in which M’s score was 9 out of a maximum of 18 (Delayed Memory: 2 of 5, Attention: 2 of 5, Abstraction: 1 of 2, Orientation: 4 of 6). At this time, M. reported symptoms of increased anxiety. M. received an ICU-diary from the nurses, an intervention that has proven efficient for minimizing symptoms of trauma (McIlroy, King, Garrouste-Orgeas, Tabah, & Ramanan, 2019).
Table 1.
Timeline
| Day | Timeline of important clinical events, symptoms, and assessments |
|---|---|
| 1 | M. is at home, onset of COVID-19 symptoms (fever, dry cough, and nausea) |
| 6 | First visit at the Emergency department. M. is diagnosed with COVID-19 but sent home because he did not need supplemental oxygen therapy (oxygen saturation 93% in activity), with instructions to return if his condition deteriorated |
| 12 | Emergency department visit number two. M. presents with worsen dyspnea and hypoxia (oxygen saturation of 70%) and is sent to ICD |
| 12 | ICD. M. is treated with noninvasive ventilation but is not able to maintain an oxygen saturation over 90%. He is therefore admitted to the ICU. |
| 13 | Admission to ICU |
| 14 | Intubated during the first day of ICU. Discussions of treatment escalation (ECMO), but was denied due to obesity |
| 15 | Renal failure, start of dialysis. Bacterial infections, treated with antibiotics |
| 46 | Extubated. Taken out of sedation. M. is delirious, disoriented, and reports visual hallucinations. Reports of severe sleeping disorders. |
| 48 | Back to ICD. M. is still delirious and confused |
| 51 | Consultation and assessment by rehabilitation medicine specialist. M. is disoriented and MoCA-screening shows cognitive deficits (9 p out of maximum 18). CAM-S indicate confusion |
| 53 | Head MRI. CMBs are discovered |
| 60 | M. is moved to the rehabilitation In Care department. Delirium and confusion are now in full remission |
| 72 | First neuropsychological assessment |
| 77 | Oxygen therapy is discontinued |
| 95 | Discharged from hospital and in care rehabilitation |
| 156 | Follow-up out care rehabilitation |
| 227 | Second neuropsychological assessment |
Diagnostic Assessment
M. was diagnosed with COVID-19 at Day 6 after developing symptoms through a reverse transcriptase-polymerase chain reaction positive for SARS-CoV-2-RNA obtained from a nasopharyngeal swab. On Day 12, when admitted to the ICD, a computed tomography of the chest revealed bilateral, extensive pulmonary infiltrates, consistent with COVID-19. At Day 15, the patient tested positive for the presence of SARS-CoV-2 IgG in serum. Repeated testing for SARS-CoV-2-RNA in serum from Day 13 and onward came back negative.
Physical Examination
At the physical examination (PE) performed at the ICD at about Day 51 after first symptoms, M. was awake but disoriented, with a Glasgow Coma Scale score of 14 out of 15. An Oxygen saturation of 98% was noted with 2 l•min−1 of oxygen flow through an open mask. Arterial blood gas analysis revealed a mild hypercapnia due to hypoventilation. At a bedside neurological examination, there were no abnormalities found on examination of the cranial nerves. A general muscular weakness was detected without focal deficits. Deep tendon reflexes were normal in the upper extremities but symmetrically absent in the lower extremities (knee and ankle). The clinical pattern raised a suspicion of a possible critical illness polyneuropathy, but no confirmatory neurophysiological examination was conducted. M. was transferred to an inpatient rehabilitation department 9 days later, still in need of supplementary oxygen. At the PE, the first day after moving M. to the rehabilitation ward (Day 60), he was no longer in a confusional state and was fully oriented. As before, he suffered from a general weakness, now with the addition of postural and intention tremor. Laboratory findings suggested a mild anemia with Hemoglobin 101 g•L−1 (reference: 134–170 g•L−1) but otherwise interpreted as nonsignificant with regard to complete blood cell count, thyroid function test, and basic metabolic panel.
Clinical follow-up was performed 5 months after onset of COVID-19. At follow-up, M. reported remaining physical and cognitive symptoms and recurring nausea when coughing, resulting in some difficulty eating. M. had no remaining need of oxygen but suffered from dyspnea when walking at a slow pace. The lowest saturation during the 6-min walk test was estimated to 89% after 2 min. M. also suffered from low-grade intermittent fever after physical activity and myalgia. Neurological examination only showed sensory deficit on the upper part of the left leg. There were no objective signs of heart failure and chest X-ray showed minor residual linear opacities. M. reported no lingering anosmia or dysgeusia.
Imaging
The Department of Radiology at Danderyds’ Hospital uses the following pulse sequences in detecting cerebral lesions following ICU care on magnetic resonance imaging (MRI): T1, T2, susceptibility weighted intensity (suscepibility weighted imaging [SWI], sensitive for cerebral microbleeds [CMBs]), fluid attenuated inversion recovery, and diffusion weighted imaging (sensitive for ischemic lesions). An MRI of the head performed on Day 53 showed a few supra- and infratentorial CMBs located in the right temporal lobe (Fig. 1A), left basal ganglia (Fig. 1B), and right cerebellar hemisphere (Fig. 1C).
Fig. 1.
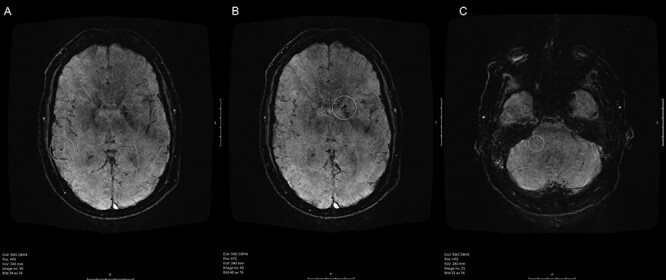
Susceptibility weighted imaging (SWI) MRI of the patient’s brain. (A) Highlights (white ring) a CMB in the right temporal lobe, (B) shows a CMB in the basal ganglia in the left hemisphere, whereas (C) shows an infratentorial CMB.
Clinical Neuropsychological Findings
In the first session with the clinical neuropsychologist, M. reported mental fatigue, short-term memory deficits, and an overall feeling of being less vigilant and not as attentive as before. The nightmares, visual hallucinations, and disorientation had gradually subsided the first two weeks following extubation. At the time of the assessment, 72 days after being diagnosed, M. was no longer troubled by symptoms of anxiety. By administering the Post Traumatic Checklist-5, Post Traumatic Stress Disorder (PTSD) was ruled out. Cognition was assessed using a local protocol for neuropsychological screening and follow-up used at the current rehabilitation clinic. The protocol for the early assessment consisted of a selection of tests from the screening battery Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) A. To test in-depth executive functioning, we used Key Search and Rule Shift Cards from Behavioral Assessment of the Dysexecutive Syndrome (BADS), Trail Making Test 1–5 (TMT), and Word Fluency Test from the test battery Delis Kaplan Executive Functioning System (D-KEFS). The test protocol for the follow-up consisted of partly corresponding tests (Matrixes, Digit Span, Block Design, Coding) from WAIS-IV, TMT (retest of the same version of TMT 1–5 as in the first assessment), Color Word Inhibition Test, and Verbal Fluency Test from D-KEFS with Zoo Map from BADS. Learning, immediate, and delayed memory were assessed with Rey Auditory Verbal Learning Test (RAVLT) and visuospatial/constructional ability, visual attention, and visual memory with Rey Complex Figure Copying Test (RCFT) and Rey Complex Figure Test–Delayed Recall.
The performance levels on both assessments were generally high, with a few exceptions, demonstrating a good cognitive recovery. To interpret the results from Table 2, it is important, particularly in cases like this, to emphasize that an average or low average performance in tests may be a representation of a minor or moderate cognitive decline, if the premorbid cognitive function, like in this particular case, was considered to be “high average” or above. The existence of change in neuropsychological tests is harder to document in cases in which the patient has a high premorbid functioning in combination with suspicion of minor or more subtle deficiencies (Franzen, Burgess, & Smith-Seemiller, 1997). Comparing an individual’s performance to a population average score is appropriate only when the performance is not related to education (Lezak, Howieson, Loring, & Fischer, 2004). Even minor cognitive deficits can be of great occupational importance, if the premorbid occupation is intellectually demanding. For example, a small difference in processing speed and decision-making may affect productivity, notably in M’s occupation where quick decisions could be crucial. It is also important to note that RBANS is a battery known for notable ceiling effect, especially on the subtests of visuospatial functioning, visual memory, and naming. Maximum raw score on these tests translated to a scaled score does not attain higher levels than average or upper average. In tests with ceiling effects, it is easy for a high performing individual to attain a maximum score, despite having for her/himself subtle, but noticeable, difficulties in everyday life.
Table 2.
Results on neuropsychological assessment Day 72 and Day 227
| Neuropsychological investigation | Normative average score and (SD) | Assessment 1. Day 72 | Assessment 2. Day 227 | |||||
|---|---|---|---|---|---|---|---|---|
| Tests | Functions | Reference group, age 40–49 | Patient raw score | T-valuea | Discrepancy from estimated premorbid level | Patient raw score | T-value | Discrepancy from estimated premorbid level |
| RBANSb List learning | Verbal learning immediate memory | 27.6 (4.4) | 36 | 63 | no | — | — | — |
| RBANS Figure Copy | Visuospatial/constructional | 18.4 (1.4) | 20 | 57 | no | — | — | — |
| RBANS Line Orientation | Visuospatial/constructional | 17 | 20 | 60 | no | — | — | — |
| RBANS Picture Naming | Naming | 9.4 (1.1) | 10 | 50 | no | — | — | — |
| RBANS Coding | Attention and processing speed | 49.8 (8.1) | 41 | 33 | yes, moderate | — | — | — |
| RBANS List Recall | Delayed verbal memory | 6.3 (1.9) | 8 | 57 | no | — | — | — |
| RBANS List Recognition | Delayed memory recognition | 20 | 20 | 55 | no | — | — | — |
| RBANS Figure Recall | Delayed visual memory | 13.5 (3.3) | 17 | 50 | no | — | — | — |
| D-KEFSc TMT 1 Visual Scanning | Visual scanning and visuomotor speed | 22–44 | 15 | 60 | no | 28 | 43 | yes |
| D-KEFS TMT 2 Number Sequencing | Visual motor speed | 31–34 | 23 | 57 | no | 20 | 60 | no |
| D-KEFS TMT 3 Letter Sequencing | Alphabetical automation | 31–34 | 42 | 43 | yes, mild | 19 | 60 | no |
| D-KEFS TMT 4 Number-Letter Sequencing | Visual motor speed, flexibility and switching | 74–84 | 62 | 57 | no | 69 (12 errors) | 57 | yes |
| D-KEFS TMT 5 Motor Speed | Motor speed | 0 | 21 | 57 | no | 11 | 63 | no |
| D-KEFS Color-Word Color Naming | Naming | 30–38 | — | — | — | 28 | 50 | no |
| D-KEFS Color-Word Word Reading | Reading | 22 | — | — | — | 20 | 53 | no |
| D-KEFS Color-Word Inhibition | Inhibition | 57–53 | — | — | — | 49 | 53 | possible |
| D-KEFS Color-Word Inhibition/Switching | Flexibility and switching | 64–60 | — | — | — | 48 | 60 | no |
| D-KEFS 1 Word Fluency FAS | Verbal letter fluency | 38–40 | 57 | 67 | no | 57 | 67 | no |
| D-KEFS 2 Category Fluency | Verbal category fluency | 39–41 | — | — | 60 | 80 | no | |
| D-KEFS 3 Category Switching | Verbal fluency category switching | 14 | 17 | 66 | no | 19 | 70 | no |
| WAIS-IIId Digit Span | Working memory | — | 15 (F7R4)i | 47 | mild (R4)i | — | — | — |
| WAIS-IVe Digit span | Working memory | 24–25 | — | — | — | 29 (F6R5S7)j | 57 | possible in reversed |
| WAIS-IV Block Design | Visuospatial/constructional | 43–47 | — | — | — | 56 | 60 | no |
| WAIS-IV Coding correct 0–30 seconds 31–60 seconds 61–90 seconds 91–120 seconds |
Attention and processing speed | 64–69 | — | — | — | 56 17 14 13 14 |
47 | yes |
| WAIS-V Matrix Reasoning | Perceptual reasoning | 18–19 | — | — | — | 15 | 43 | yes |
| BADSf Key Search | Visual planning and problem solving | 2.60 (1.32) | 4 | 66 | no | — | — | — |
| BADS Rule Shift | Inhibition and switching | 3.56 (0.78) | 4 | 55 | no | — | — | — |
| BADS Zoo Map version 1. | Planning, complex condition | 2.44 (1.13) | — | — | — | Sequence 4 (6 errors) | 46 | yes |
| BADS Zoo Map version 2. | Planning, structured condition | — | — | — | — | Sequence 8 (0 error) | — | no |
| BADS Zoo Map (1 and 2) | See above | 2.44 (1.13) | — | — | — | Profile total 2 | 46 | yes |
| Rey AVLTg learning | Verbal learning/immediate learning | 49.0 (8.0) | — | — | — | 65 | 70 | no |
| Rey AVLT 30 min | Delayed verbal memory | 10.0 (2.9) | — | — | — | 14 | 63 | no |
| Rey CFh Copy | Visuospatial/constructional | 34.5 | — | — | — | 33 | 43 | yes |
| Rey CF immediate memory | Delayed visual memory | 18–18.5 | — | — | — | 23 | 57 | no |
a T-value has a mean of 50 and an SD of 10.
bRBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
cD-KEFS = Delis-Kaplan Executive Function System.
dWAIS III = Wechsler Adult Intelligence Scale Third Edition.
eWAIS IV = Wechsler Adult Intelligence Scale Fourth Edition.
fBADS = Behavioral Assessment of the Dysexecutive Syndrome.
gRey AVLT = Rey Auditory Verbal Learning Test.
hRey CF = Rey-Osterrieth Complex Figure Test.
i(F7R4) = Maximal number of digits Forward 7, Reversed 4.
j(F6R5S7) = Maximal number of digits Forward 6, Reversed 5, Sequencing 7.
Cognitive Domains with Results Corresponding to Estimated Premorbid Level
Verbal Learning, Immediate, and Delayed Verbal Memory
M’s performance in the early assessment showed high average performance on tests measuring verbal learning and high average performance on tests of delayed verbal recall (RBANS List Learning and List Delayed Recall). In the follow-up after 8 months, performance levels on similar tests were even higher. Immediate Word List recall scores were estimated as very superior and delayed recall was at a high average level (RAVLT). This probably reveals a recuperation.
Visuospatial/Constructional Ability
Aside from a small possible reduction on the RCFT at the follow-up, M. performed at a high average level in more complex and demanding tests sensitive to visuospatial/constructional ability (RBANS Line Orientation, WAIS-IV Block Design). M. reached the maximum score on RBANS Line Orientation, so the modest normed score result (high average) should probably represent a ceiling effect.
Verbal Fluency
Semantic, category, and switching word fluency measured with D-KEFS Verbal Fluency Test were estimated as above average to very superior both at the initial assessment and at the follow-up.
Executive Functions
Regarding the executive function, the results were mixed. No deficits were present in the early assessment which contained a test selection that was only partly comprehensive: BADS Key Search (visual planning and problem solving) and Rule Shift Cards (inhibition and switching). Possible deficits regarding executive functioning as seen on the follow-up assessment are discussed in the following subsection.
Possible Deficits—Results from Both Assessments Showing Discrepancy Compared with Estimated Premorbid Level
Processing Speed, Attention, and Working Memory
The results from the early assessment showed a significant, moderate (at least ∼2 SD) deficit on the subtest Coding from RBANS A. At follow-up, the result on the corresponding test (WAIS-IV Coding) was significantly higher (now average), but still considered a minor decline compared with the estimated premorbid profile. At the WAIS Coding test, a decrease in performance over time was observed, which is notable because an increase in symbol production is expected at a normal performance (Möller, Bartfai, Nygren de Boussard, Rådestad, & Calissendorff, 2014). Thus, the result indicated cognitive fatigability. Additionally, small deficits were suspected on the subtest Trail Making Test 3 (sensitive to attention, speed, visual scanning) and Digit Span Reversed (a measure of auditory working memory) on the early assessment. Results on Trail Making Test 1 (visual scanning, processing speed, attention) and Digit Span Reversed were lower than expected at follow-up assessment. The suspected cognitive decline seen on a selection of tests at the early assessment (Coding, Digit Span, and Trail Making Test 3) was comparatively smaller at the follow-up assessment, but results on these tests were still estimated as a relative shortcoming in the profile.
Logical/Perceptual Reasoning
On the neuropsychological evaluation at the 8-month follow-up (Day 227), the test results showed mild to moderate deficits compared with estimated premorbid level on measures of nonverbal logical reasoning (Matrix reasoning). The early assessment did not include tests of perceptual logical problem solving, so we do not know if this reduction was present then, but it could be assumed.
Executive Functions
Regarding executive functioning, there were as mentioned above mixed findings. A possible mild reduction was seen on a selection of tests measuring inhibition, planning, and switching at the follow-up assessment. M. had difficulties with performance on BADS Zoo Map (a measure of planning and goal orientation) and a relatively poor performance with more errors than expected (12) on Trail Making Test 4, a test sensitive to attention, flexibility/switching and alternating attention. On Color Word Inhibition Test, measuring primarily verbal inhibition, the result shows a possible mild deficit compared with estimated premorbid level.
Delayed Visual Memory
Tested initially with RBANS Figure Recall and at the follow-up with delayed recall of the similar test Rey Complex Figure, show average results on both early and follow-up assessment. Both of these tests are sensitive to deficits in spatial working memory, but the RBANS Figure Recall test also has a noticeable ceiling effect; therefore, it is primarily the RCFT-result that possibly could be interpreted to reveal a small cognitive reduction.
Subjective Cognitive Complaints and Occupational Status
At the 8-month follow-up, M. reported subjectively as being slower, more easily fatigued and having troubles with decision-making in his daily life. He had not yet been able to return to his former occupational level as a full-time employment in an academic field; M. was working only 2 hours a day, currently with a less intellectually demanding work.
Discussion
The main finding of this case study was that the neuropsychological assessment did not disclose any serious cognitive deficiencies, despite that M. had gone through a severe COVID-19 infection, including multi-organ failure, prolonged intubation, delirium, and presented structural brain injuries on a head-MRI. According to both the early and the 8-month follow-up neuropsychological assessment, M’s mental abilities were generally preserved with the exception of mild to moderate deficits on tests sensitive to processing speed, working memory, attention, and aspects of executive functioning. The deficits seen on the later follow-up assessment were, compared with the results of the early assessment, minor and were considered deficits mainly in comparison to assumed premorbid level which was estimated to be “high average.” It is important to emphasize that a relatively small reduction of cognitive functions, like the one shown here, could lead to serious impact on everyday life functioning. At the 8-month follow-up, M. had not yet been able to return to his former level of occupational functioning. He reported feeling less sharp and attentive and was subjectively easily fatigued.
How can we understand M’s mild and selective but lingering cognitive deficits, resulting in a significant occupational dysfunction?
It is widely known among clinicians that the Wechsler and RBANS processing speed subtest (including Coding) has an overall sensitivity to most clinical conditions such as autism, learning difficulties, hormonal disturbances, and neurological conditions such as concussion. According to Holdnack, Prifitera, Weiss, and Saklofske (2016), a result below expected level in tests sensitive to processing speed, such as the deficit presented in both neuropsychological assessments here, should always be of clinical interest and require further testing and monitoring. Difficulties in Coding could in rare cases be a result of perfectionism. In Coding, perfectionism can clinically manifest as a preoccupation with writing the characters as accurately as possible, at the expense of speed, even though it is emphasized to conduct the test as fast as possible. In M’s observed test performance, there were neither noticeable signs of perfectionism in Coding nor in other tests, for example, during RBANS Figure Copy.
It seems likely to assume that M’s deficits on tests are caused by the severe COVID-19, but we do not fully comprehend the underlying pathophysiological mechanisms. There are many possible explanations. One partial explanation to the cognitive decline may be the occurrence of postcovid-fatigue, which has been recognized as one of the most common presenting complaints affecting an estimate of 50% of COVID-19 survivors 10 weeks after symptom remission (Townsend et al., 2020). However, Townsend and colleagues (2020) found no association between the severity of the infection (need for inpatient care, oxygen, or critical care) and postinfection fatigue. Interestingly in the work by Townsend et al., a pre-existing diagnosis of depression and anxiety were overrepresented in patients reporting fatigue. Fatigue is one of the more debilitating symptoms following respiratory collapse, after ICU-care and generally after acquired brain injury. More than two-thirds of ARDS survivors reported clinically significant fatigue symptoms during the first year (Neufeld et al., 2020). Researchers are now starting to examine the role of the so-called Post-infectious Fatigue Syndrome in lingering COVID-19. This syndrome has previously been described after different types of infectious agents such as SARS and herpes simplex infections, and the lingering chronic fatigue following each illness appears to be quite similar (Komaroff & Bateman, 2021). An objection that could be made by an attentive clinician is that the results of the assessments in some ways are self-contradictory. Despite minor deficits on tests measuring processing speed, attention, and working memory, as seen in both assessments, the results also include average or superior performance on other tests sensitive to corresponding domains. This uneven performance level may be explained by irregular mental/cognitive fatigue due to fluctuating attention or simply test order in the administration of tests. Interestingly, M. complained both about fatigue and showed cognitive fatigability. Hypothetically, this could be related to altered connectivity as CMB and especially those that affect thalamocortical connectivity have been shown to cause cognitive impairment (Wang et al., 2019). In previous studies, but in patients with, for example, multiple sclerosis or traumatic brain injury, thalami-cortical connectivity has been shown to be linked to fatigue (Dobryakova, DeLuca, Genova, & Wylie, 2013) and fatigability (Möller, Nordin, Bartfai, Julin, & Li, 2017).
It is likely to assume that the CMBs located supra- and infratentorially seen on M’s MRI-scans occurred during the hospitalization period. It has been shown that CMB occurs in other patients that receive ICU care (Fanou et al., 2017), not making it specific for COVID-19. In a large MRI-study (n = 3,979), Poels and colleagues (2012) disclose an association between a higher number of CMB with lower scores and worse performance on tests of processing speed and motor speed in neuropsychological tests. Due to the limited number of studies having performed post-ICU head MRI, it is unknown how widespread CMBs are following ICU care. Neither do we know if CMBs, together with other ICU related factors, are independently associated with cognitive symptoms (Neufeld et al., 2020). However, in an MRI-study of nine patients diagnosed with COVID-19 and ARDS (Fitsiori, Pugin, Thieffry, Lalive d'Epinay, & Vargas Gomez, 2020), neurological manifestations were connected to unusual CMBs seen on MRI. According to a French study by Lersy and colleagues (2020), the etiology of the CMBs in COVID-19 survivors is believed to be a blood–brain barrier dysfunction, secondary to hypoxemia and high concentrations of uremic toxins (caused by renal failure). The occurrence and number of CMBs was also higher among patients, like M., that were in need of endotracheal intubation. The presence of five or more CMB (in contrast to three that M. had) was associated with worse performance in all cognitive domains, except memory. Similarly, Agarwal and colleagues (2020) defined the presence of five or more CMB as a critical threshold and noted that these patients had a significantly worse outcome compared with patients with fewer CMBs. However, while M. did not suffer from any detectable focal neurological deficits as a result of CMB, it is plausible that even fewer than five CMBs could result in cognitive deficits, as the location can be more crucial to how much cognitive functions are affected (Warren et al., 2014). In summary, CMBs affecting cognition are presumably more common than what is previously known following ICU care, and have been seen following COVID-19 ICU treatment, such as in M in our case-study.
Mechanical ventilation is a potent risk factor for long-term cognitive decline (Bulic et al., 2017; Jayaswal et al., 2019). Acute hypoxia, which could be suspected after ARDS, is also well known to have a negative effect on global cognition (McMorris, Hale, Barwood, Costello, & Corbett, 2017). In addition, the occurrence of delirium in the postacute phase, which affects roughly 80% of survivors from ICU, is associated with adverse outcomes including long-term cognitive impairment (Brummel et al., 2014). The patient in this case report did show several of these risk factors known to predict long-term cognitive and psychiatric consequences: for example, 35 days in mechanical ventilation, ARDS, multi-organ failure in addition to delirium and psychiatric symptoms after anesthesia. Because of his pre-existing obesity and diabetes mellitus, M. had an even higher risk of developing a severe disease trajectory. With this in mind, we may have expected more pervasive and severe cognitive decline in the areas of memory, sustained attention, and executive functioning (Almeria et al., 2020; Zhou et al., 2020). The protective factors present, which may partly explain this recovery, were M’s relatively young age, high education (Marra et al., 2018), and the fact that he had no prior cognitive deficits, indicating a good cognitive reserve. We know from the research on PICS that the consequences after ICU have more impact on the cognition of already vulnerable patients, for example, those who suffer from dementia (Lee, Kang, & Jeong, 2020).
After undergoing a life-threatening episode at ICU, like M., lingering psychiatric symptoms would be expected, particularly symptoms of anxiety and PTSD. Fortunately, M’s psychiatric symptoms: hallucinations, nightmares, flashbacks, and anxiety, did withdraw within 2 weeks after sedation. Early on, M. received an ICU-diary, which is an evidence-based intervention aimed at minimizing the occurrence of PTSD (McIlroy et al., 2019). According to Almeria and colleagues (2020), newly developed psychiatric symptoms were associated with later subjective cognitive complaints, but not linked to deficits in tests. It is possible that M’s mild but pre-existing anxiety and depression could have affected cognitive ability, but the fact that M. did not experience any occupational dysfunction before sickened with COVID-19 speaks against that hypothesis. In summary, it does not seem likely that the psychiatric symptoms could have caused the neurocognitive decline seen in the assessments.
Limitations of the Study and Future Research
As a case report, the study suffers from the limitation of not automatically being generalizable to a larger sample. The optimum would have been to conduct a controlled, prospective study with a larger, representative sample. However, during a pandemic that in Sweden has only lasted for just over a year, the possibilities of conducting planned, controlled studies are extremely limited. Another limitation is that not all tests were applied during both assessments. Despite these limitations, we believe that it is important to shed some light on the neuropsychological consequences of COVID-19, and we believe M’s case well represents the disease course and rehabilitation of seriously ill COVID-19 patients.
The assessments of mild cognitive symptoms in this study largely depend on comparisons with the estimated premorbid level. The fact that this estimation was not made with any formal instrument can, of course, be questioned. The approach used instead was the best performance level (on the WAIS), which is an established method for making estimations of the premorbid cognitive level (Lezak, Bigler, & Tranel, 2012), which guarantees a certain reliability in the present estimation. The validity of the best performance level as “high average” was also strengthened by the fact that M. had completed an academic Bachelor’s degree and performed an intellectually demanding job. Using this kind of demographic information as estimates of premorbid level is in line with clinical practice when exact measures of premorbid level are unavailable.
Neuropsychological assessment is very much about identifying typical test profiles for different clinical conditions. When it comes to COVID-19, the question of typical test profiles is complicated by that fact that it is necessary to discriminate between different stages of the disease course: acute phase, early postacute phase, and late postacute phase, as well as to pay attention to the degree of severity of the infection (Kamal, Abo Omirah, Hussein, & Saeed, 2021). One problem is that there do not appear to exist any definitions of the duration in time of the various disease phases. One way to circumvent this problem is to indicate how long after the illness the examination was performed (Mattioli et al., 2021). With this in mind, the test profile of M. in an estimated late postacute phase revealed mild deficits in logical reasoning, different aspects of executive functioning, and processing speed. Thus, the primary contribution that the current study makes to the knowledge about the neuropsychological consequences of COVID-19 is to present a late postacute test profile of a severely ill patient. A second contribution is that the study raises awareness of the need to apply test batteries that capture the typical test profile of COVID-19. Such batteries must also take into account the different phases of the disease and be sensitive to both mild and moderate cognitive abnormalities, not just extensive ones. Future research will tell to what extent these tests used in the present study catches what could be considered as a typical postacute neuropsychological symptomatology of COVID-19, and if the pattern of selective cognitive decline observed in this study coincides with postacute cognitive sequelae in other COVID-19 patients, other related infectious diseases, or just from general ICU care.
A third contribution is that this case study emphasizes the need to monitor the patient’s cognition after the acute phase (Kamal et al., 2021). In addition to closing the above-mentioned gaps in current research, a proposal to future research is thus to conduct longitudinal neuropsychological studies aimed at identifying early predictors of long-term cognitive problems after COVID-19.
Conclusion
Neuropsychologists and rehabilitation specialist physicians in postintensive care and rehabilitation facilities are now starting to realize that recovering from severe COVID-19 does not always guarantee a full return to the previous functional or occupational level. For M., the recovery from COVID-19 has been more challenging than expected, even though he survived a severe disease trajectory against all odds. One important conclusion of this report is that despite the subtle but lingering cognitive decline, M. had an extraordinary convalescence. The recovery can probably partly be explained by the powerful protective factors of young age and a high premorbid functioning. For a more vulnerable patient with a similar disease trajectory, including prolonged intensive care, the cognitive sequelae would probably have been much more severe.
We still do not know which aspects of severe COVID-19 that represent the causal connection to the cognitive decline seen after respiratory symptom remission. Severe cognitive and physical fatigue, affecting at least 50% of COVID-19 survivors, may be one factor that partly could explain the deficiencies, but what is the mechanism behind postinfectious fatigue? As mentioned in the discussion, the cognitive deficits and fatigue shown in this case are mainly in line with the previous research on sequelae after PICS and ARDS, which indicate that the decline could also be regarded as a general ICU-care consequence, not making it specific for COVID-19.
Another conclusion is that the cognitive decline in M’s case is presumably not caused by, or is secondary to, lingering symptoms of anxiety or depression but that they are a result of the long and complicated hospital stay, likely exacerbated by the CMBs seen on MRI. In summary, we lack knowledge about how COVID-19 is connected to the CNS-related consequences seen in COVID-19 survivors around the world. This case cannot provide additional insight into ulterior pathways, mechanisms, or causes of the deficits discovered, which of course is one of the weaknesses of this study. More research on the underlying mechanism and etiology of the possible cognitive decline of COVID-19 is warranted.
This case-report may contribute to the research field by serving as one example of a quite adequate recovery, despite several unfavorable conditions, stressing that withdrawal of care even in desperate cases should not be performed lightly. There is an almost total scarcity of published case reports that could shed light on the illness phase coming after the recovery from the severe respiratory symptoms. Initially, case descriptions may also guide clinicians in rehabilitation regarding neuropsychological follow-up after ICU-care. The main contribution of this case report is that it could serve as a hopeful contribution regarding prognosis for the severely ill, relatively young COVID-19 patients, but this case also emphasizes the need of a neuropsychological follow-up, with tests sensitive to minor deficits.
Patient Perspective
After the hospital discharge, M. was pleased with the rehabilitation staff, training, and the progress made. With respect to the severe delirium symptoms at the ICU, he was anticipating a long-term disability regarding cognitive and psychiatric sequelae. At the follow-up, M. was notably less optimistic about his chances of returning to his former occupational level. He was appreciative regarding continued rehabilitation follow-up, which is still ongoing.
Informed Consent
The patient has given us informed consent and therefore permission to publish this case report.
Conflict of Interest
None declared.
This article is a thorough rework of a minor case report examination paper from a neuropsychology specialist course given by the Psychology Department of Stockholm University.
Contributor Information
Linda Backman, Department of Rehabilitation Medicine, Danderyd University Hospital, Stockholm, Sweden; Department of Clinical Sciences, Karolinska Institutet, Stockholm, Sweden.
Marika C Möller, Department of Rehabilitation Medicine, Danderyd University Hospital, Stockholm, Sweden; Department of Clinical Sciences, Karolinska Institutet, Stockholm, Sweden.
Eric P Thelin, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Neurology, Karolinska University Hospital, Stockholm, Sweden.
Daniel Dahlgren, Department of Rehabilitation Medicine, Danderyd University Hospital, Stockholm, Sweden.
Catharina Deboussard, Department of Rehabilitation Medicine, Danderyd University Hospital, Stockholm, Sweden; Department of Clinical Sciences, Karolinska Institutet, Stockholm, Sweden.
Gunilla Östlund, Department of Rehabilitation Medicine, Danderyd University Hospital, Stockholm, Sweden.
Maria Lindau, Department of Psychology, Stockholm University, Stockholm, Sweden.
References
- Almeria, M., Cejudo, J. C., Sotoca, J., Deus, J., & Krupinski, J. (2020). Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity-Health, 9, 100163. 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, S., Jain, R., Dogra, S., Krieger, P., Lewis, A., Nguyen, V., et al. (2020). Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke, 51(9), 2649–2655. 10.1161/strokeaha.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel, N. E., Jackson, J. C., Pandharipande, P. P., Thompson, J. L., Shintani, A. K., Dittus, R. S., et al. (2014). Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Critical Care Medicine, 42(2), 369–377. 10.1097/ccm.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulic, D., Bennett, M., Rodgers, H., Nourse, M., Rubie, P., Looi, J. C., et al. (2017). Delirium after mechanical ventilation in intensive care units: The cognitive and psychosocial assessment (CAPA) study protocol. JMIR research protocols, 6(2), e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, V. C., To, K. K., Tse, H., Hung, I. F., & Yuen, K. Y. (2012). Two years after pandemic influenza a/2009/H1N1: What have we learned? Clinical Microbiology Reviews, 25(2), 223–263. 10.1128/CMR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova, E., DeLuca, J., Genova, H., & Wylie, G. (2013). Neural correlates of cognitive fatigue: Cortico-striatal circuitry and effort–reward imbalance. Journal of the International Neuropsychological Society: JINS, 19(8), 849–853. 10.1017/S1355617713000684. [DOI] [PubMed] [Google Scholar]
- Desai, S. V., Law, T. J., & Needham, D. M. (2011). Long-term complications of critical care. Critical Care Medicine, 39(2), 371–379. 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- Fanou, E. M., Coutinho, J. M., Shannon, P., Kiehl, T.-R., Levi, M. M., Wilcox, M. E., et al. (2017). Critical illness-associated cerebral microbleeds. Stroke, 48(4), 1085–1087. 10.1161/strokeaha.116.016289. [DOI] [PubMed] [Google Scholar]
- Fitsiori, A., Pugin, D., Thieffry, C., Lalive d'Epinay, P., & Vargas Gomez, M. I. (2020). Unusual microbleeds in brain MRI of Covid-19 patients. Journal of Neuroimaging, 30(5), 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen, M. D., Burgess, E. J., & Smith-Seemiller, L. (1997). Methods of estimating premorbid functioning. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists, 12(8), 711–738. [PubMed] [Google Scholar]
- Helms, J., Kremer, S., Merdji, H., Clere-Jehl, R., Schenck, M., Kummerlen, C., et al. (2020). Neurologic features in severe SARS-CoV-2 infection. New England Journal of Medicine, 382(23), 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdnack, J. A., Prifitera, A., Weiss, L. G., & Saklofske, D. H. (2016). WISC-V and the personalized assessment approach. WISC-V and the personalized assessment approach. In L. G. Weiss, D. H. Saklofske, J. A. Holdnack & A. Prifitera (Eds.), WISC-V assessment and interpretation: Scientist-practitioner perspectives (pp. 373–413). Academic Press. [Google Scholar]
- Jayaswal, A. K., Sampath, H., Soohinda, G., & Dutta, S. (2019). Delirium in medical intensive care units: Incidence, subtypes, risk factors, and outcome. Indian Journal of Psychiatry, 61(4), 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal, M., Abo Omirah, M., Hussein, A., & Saeed, H. (2021). Assessment and characterisation of post-COVID-19 manifestations. International Journal of Clinical Practice, 75(3), e13746. 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, D. A., Duggal, P., & Cingolani, O. (2020). Obesity could shift severe COVID-19 disease to younger ages. Lancet (London, England), 395(10236), 1544–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff, A. L., & Bateman, L. (2021). Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Frontiers in Medicine, 7, 1132. 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korupolu, R., Francisco, G. E., Levin, H., & Needham, D. M. (2020). Rehabilitation of critically ill COVID-19 survivors. The Journal of the International Society of Physical and Rehabilitation Medicine, 3(2), 45. [Google Scholar]
- Lee, M., Kang, J., & Jeong, Y. J. (2020). Risk factors for post–intensive care syndrome: A systematic review and meta-analysis. Australian Critical Care, 33(3), 287–294. 10.1016/j.aucc.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Lezak, M., Bigler, E., & Tranel, D. (2012). Neuropsychological assessment (5th ed.). Oxford University Press. [Google Scholar]
- Lezak, M. D., Howieson, D. B., Loring, D. W., & Fischer, J. S. (2004). Neuropsychological assessment (4th ed.). Oxford University Press. [Google Scholar]
- Lim, S., Bae, J. H., Kwon, H. S., & Nauck, M. A. (2020). COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nature Reviews Endocrinology, 17(1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersy, F., Willaume, T., Brisset, J.-C., Collange, O., Helms, J., Schneider, F., et al. (2020). Critical illness-associated cerebral microbleeds for patients with severe COVID-19: Etiologic hypotheses. Journal of Neurology, 268(8), 2676–2684. 10.1007/s00415-020-10313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395(10224), 565–574. 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli, F., Stampatori, C., Righetti, F., Sala, E., Tomasi, C., & De Palma, G. (2021). Neurological and cognitive sequelae of Covid-19: A four month follow-up. Journal of Neurology. Advance online publication. 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra, A., Pandharipande, P. P., Girard, T. D., Patel, M. B., Hughes, C. G., Jackson, J. C., et al. (2018). Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Critical Care Medicine, 46(9), 1393–1401. 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris, T., Hale, B. J., Barwood, M., Costello, J., & Corbett, J. (2017). Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neuroscience & Biobehavioral Reviews, 74, 225–232. 10.1016/j.neubiorev.2017.01.019. [DOI] [PubMed] [Google Scholar]
- McIlroy, P. A., King, R. S., Garrouste-Orgeas, M., Tabah, A., & Ramanan, M. (2019). The effect of ICU diaries on psychological outcomes and quality of life of survivors of critical illness and their relatives: A systematic review and meta-analysis. Critical Care Medicine, 47(2), 273–279. [DOI] [PubMed] [Google Scholar]
- Merbitz, N. H., Westie, K., Dammeyer, J. A., Butt, L., & Schneider, J. (2016). After critical care: Challenges in the transition to inpatient rehabilitation. Rehabilitation Psychology, 61(2), 186. [DOI] [PubMed] [Google Scholar]
- Möller, M. C., Bartfai, A., Nygren de Boussard, C., Rådestad, A. F., & Calissendorff, J. (2014). High rates of fatigue in newly diagnosed Graves' disease. Fatigue: Biomedicine, Health & Behavior, 2(3), 153–162. 10.1080/21641846.2014.935279. [DOI] [Google Scholar]
- Möller, M. C., Nordin, L. E., Bartfai, A., Julin, P., & Li, T. Q. (2017). Fatigue and cognitive fatigability in mild traumatic brain injury are correlated with altered neural activity during vigilance test performance. Frontiers in Neurology, 8(496), 1–13. 10.3389/fneur.2017.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham, D. M., Davidson, J., Cohen, H., Hopkins, R. O., Weinert, C., Wunsch, H., et al. (2012). Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders' conference. Critical Care Medicine, 40(2), 502–509. [DOI] [PubMed] [Google Scholar]
- Neufeld, K. J., Leoutsakos, J.-M. S., Yan, H., Lin, S., Zabinski, J. S., Dinglas, V. D., et al. (2020). Fatigue symptoms during the first year following ARDS. Chest, 158(3), 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandharipande, P. P., Girard, T. D., Jackson, J. C., Morandi, A., Thompson, J. L., Pun, B. T., et al. (2013). Long-term cognitive impairment after critical illness. New England Journal of Medicine, 369(14), 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels, M. M., Ikram, M. A., van derLugt, A., Hofman, A., Niessen, W. J., Krestin, G. P., et al. (2012). Cerebral microbleeds are associated with worse cognitive function: The Rotterdam scan study. Neurology, 78(5), 326–333. [DOI] [PubMed] [Google Scholar]
- Ritchie, K., Chan, D., & Watermeyer, T. (2020). The cognitive consequences of the COVID-19 epidemic: Collateral damage? Brain Communications, 2(2), Article fcaa069. 10.1093/braincomms/fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasannejad, C., Ely, E. W., & Lahiri, S. (2019). Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Critical Care, 23(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, L., Dyer, A. H., Jones, K., Dunne, J., Mooney, A., Gaffney, F., et al. (2020). Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One, 15(11), e0240784. 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzotzos, S. J., Fischer, B., Fischer, H., & Zeitlinger, M. (2020). Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Critical Care, 24(1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Jiang, Y., Suo, C., Yuan, Z., Xu, K., Yang, Q., et al. (2019). Deep/mixed cerebral microbleeds are associated with cognitive dysfunction through thalamocortical connectivity disruption: The Taizhou imaging study. NeuroImage: Clinical, 22, 101749. 10.1016/j.nicl.2019.101749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, D. E., Power, J. D., Bruss, J., Denburg, N. L., Waldron, E. J., Sun, H., et al. (2014). Network measures predict neuropsychological outcome after brain injury. PNAS Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14247–14252. 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Lu, S., Chen, J., Wei, N., Wang, D., Lyu, H., et al. (2020). The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research, 129, 98–102. 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W., Rohli, K. E., Yang, S., & Jia, P. (2020). Impact of obesity on COVID-19 patients. Journal of Diabetes and its Complications, 35(3), 107817. [DOI] [PMC free article] [PubMed] [Google Scholar]


