Abstract
Aims
We aim to assess changes in routine echocardiographic and longitudinal strain parameters in patients recovering from Coronavirus disease 2019 during hospitalization and at 3-month follow-up.
Methods and results
Routine comprehensive echocardiography and STE of both ventricles were performed during hospitalization for acute coronavirus disease 2019 (COVID-19) infection as part of a prospective pre-designed protocol and compared with echocardiography performed ∼3 months after recovery in 80 patients, using a similar protocol. Significantly improved right ventricle (RV) fractional area change, longer pulmonary acceleration time, lower right atrial pressure, and smaller RV end-diastolic and end-systolic area were observed at the recovery assessment (P < 0.05 for all). RV global longitudinal strain improved at the follow-up evaluation (23.2 ± 5 vs. 21.7 ± 4, P = 0.03), mostly due to improvement in septal segments. Only eight (10%) patients recovering from COVID-19 infection had abnormal ejection fraction (EF) at follow-up. However, LV related routine (E, E/e′, stroke volume, LV size), or STE parameters did not change significantly from the assessment during hospitalization. A significant proportion [36 (45%)] of patients had some deterioration of longitudinal strain at follow-up, and 20 patients (25%) still had abnormal LV STE ∼3 months after COVID-19 acute infection.
Conclusion
In patients previously discharged from hospitalization due to COVID-19 infection, RV routine echocardiographic and RV STE parameters improve significantly concurrently with improved RV haemodynamics. In contrast, a quarter of patients still have LV systolic dysfunction based on STE cut-offs. Moreover, LV STE does not improve significantly, implying subclinical LV dysfunction may be part and parcel of recovering from COVID-19 infection.
Keywords: strain, COVID-19, myocardial dysfunction
Graphical Abstract
Graphical Abstract.
Introduction
There has been an upsurge in the number of publications concerning acute coronavirus disease 2019 (COVID-19) infection.1–3 In contrast, less is known about the short- and long-term cardiac consequences of COVID-19. First, researchers in Italy described a high incidence of symptoms among recovered COVID-19 patients.4 Then, researchers in Germany, China, and the USA described the extent of cardiac injury in recovered patients using cardiac magnetic resonance imaging.5–7 These studies, however, lacked baseline imaging. Lately, two longitudinal echocardiographic studies have analysed the occurrence of persisting cardiac pathology following COVID-19 infection.8,9 However, these studies had opposing results, and were subjected to selection bias because echocardiography was performed during hospitalization only secondary to clinical deterioration. Recently, we have described our experience in consecutive patients with COVID-19 infection undergoing routine and 2D speckle-tracking echocardiography (STE) and lung ultrasound during hospitalization for acute COVID-19 infection.10,11 2D-STE is a major advancement in the understanding of myocardial function and provides additive value regarding prognosis and disease progression to the traditional echocardiographic imaging. It is more robust and sensitive in evaluating RV and LV dysfunction than other routine echocardiographic parameters in specific populations, such as patients with chemotherapy induced cardiac injury12,13 and heart failure.14 Therefore, our primary aim was to evaluate RV and LV myocardial function and haemodynamics using routine and STE parameters in patients recovering from COVID-19 infection. Furthermore, we compared these parameters with those assessed during the hospitalization to analyse whether RV and LV function improve or deteriorate after recovering from the acute stage of the disease.
Methods
Demographic data, comorbid conditions, medications, physical examination, laboratory findings, electrocardiogram (ECG), and echocardiography were collected in consecutive patients hospitalized in one hospital (Tel Aviv Medical Center), with acute COVID-19 from 23 March 2020 to 20 June 2020. In this study, we enrolled only those patients who attended the follow-up visits at the COVID-19 recovery clinic, ∼3 months after discharge.
All patients were diagnosed with COVID-19 confirmed by a polymerase-chain-reaction-assay for SARS-CoV-2, and were risk stratified according to their COVID-19 Modified-Early-Warning-Score (MEWS) and sequential organ failure assessment (SOFA) score.15,16 The cohort underwent routine echocardiographic and STE examination within 48 h of hospitalization for acute infection, as part of a prospective registry, irrespective of clinical severity of disease. All eight certified sonographers in our laboratory participated in echocardiographic data collection at hospitalization and follow-up. Patients recovering from infection, were summoned for follow-up visits, and underwent a similar echocardiographic exam. The cut-off values for RV and LV function, including STE, were based on recent cardiac chamber quantification.17 Twenty controls were selected from a pool of 388 patients undergoing comprehensive echocardiography with similar comorbidities, to create two patient groups that are balanced in their baseline characteristics: age, gender, and major co-morbidities.
The ethics committee of the Tel Aviv Medical Center approved the study.
Echocardiography
Follow-up echocardiographic examinations were performed using the same pre-designed prospective protocol, personnel, and equipment (CX 50, Philips Medical Systems, Bothell, WA, USA) as during the hospitalization for acute infection. During hospitalization for acute infection, and in accordance to present guidelines,18 the following measures were undertaken to minimize the risk of inadvertent infection: (i) All echocardiographic studies were bedside performed at the designated COVID-19 units; (ii) all echocardiographic exams were performed with small dedicated scanners, because their disinfection is easier; and (iii) routine measurements and STE analyses were performed offline to reduce exposure and contamination.
Routine LV echocardiographic parameters included LV diameters and ejection fraction (EF).19 Measurements of mitral inflow included the peak early (E-wave) and late (A-wave) diastolic filling velocities, and calculation of E/A ratio. Early diastolic mitral septal and lateral annular velocities (e’) were measured in the apical four-chamber view.20 Routine RV assessment included the following: end-systolic and end-diastolic RV areas from four-chamber views, tricuspid annular plane systolic excursion (TAPSE), fractional area change (FAC),19,21 assessment of right atrial pressure, and measurement of pulmonary acceleration time.22 RA pressure was evaluated based on inferior vena cava size and collapsibility. Pulmonary vascular resistance is based on the formula22–24: PVR = 80 × ([48 − (0.28 × PAT)]−[4.9+ (0.62 × E/e′)]/CO).
2D-STE image acquisition and offline analysis
Speckle-tracking analysis was performed in accordance with the Consensus Document of the EACVI/ASE/Industry Task Force to Standardize RV and LV myocardial Deformation Imaging.19,25 All STE analyses were performed offline to reduce exposure and contamination. Peak RV4CLS and peak LV four-chamber longitudinal strain (LV4CLS) were obtained using greyscale images of apical four-chamber view of one heart cycle, which was the most feasible during the acute phase and therefore performed in the exact same way at the follow-up clinic. Furthermore, each wall was separated into three segments, which included the basal, middle and apical regions. Although all strain values are negative values, they were presented as absolute values where a decrease in strain (hence, lower absolute value) is observed when RV or LV function deteriorates. Analyses were done using commercial feature-tracking software (2D CPA TomTec Imaging Systems, Unterschleissheim, Germany).
We recently showed that myocardial strain imaging with 2D echocardiography with Tomtec vendor can produce scores with good inter-observer and intra-observer reliability.11
Statistical analysis
Continuous normally distributed parameters were presented as means ±SD and compared using the Student’s t-test. Non-normally distributed data were presented by median, first and third quartiles and compared using the Wilcoxon rank-sum test. Normality was assessed using the Shapiro–Wilk test and visual inspection of quantile–quantile plots. Categorical data were compared between groups using the χ2 test or Fisher’s exact test and expressed as numbers and/or percentage. Normally distributed echocardiography parameters in consecutive echocardiography exams were compared using the paired t-test. Non-normally distributed data in consecutive echocardiographic examinations were analysed by use of the signed Wilcoxon rank sums test. P values of <0.05 were considered to indicate statistical significance. All data were analysed with the JMP System software version 12.0 (SAS Institute, Inc., Cary, NC, USA). All authors participated in designing the study, collecting and analysing data, drafting, and revising the manuscript.
Inter-observer and intra-observer variability
Inter-observer variability for peak RV4CLS and peak LV4CLS were determined by a second independent blinded observer who measured these variables in 15 randomly selected patients. Intra-observer variability was determined by having the second observer who measured the data in all patients re-measure the echo variables in 15 patients one week apart. Inter- and intra-observer variabilities were assessed using the within-subject coefficient of variation. The within-subject coefficient of variation (calculated as the ratio of the standard deviation of the measurement difference to the mean value of all measurements) provides a scale-free, unitless estimate of variation expressed as a percentage.
Results
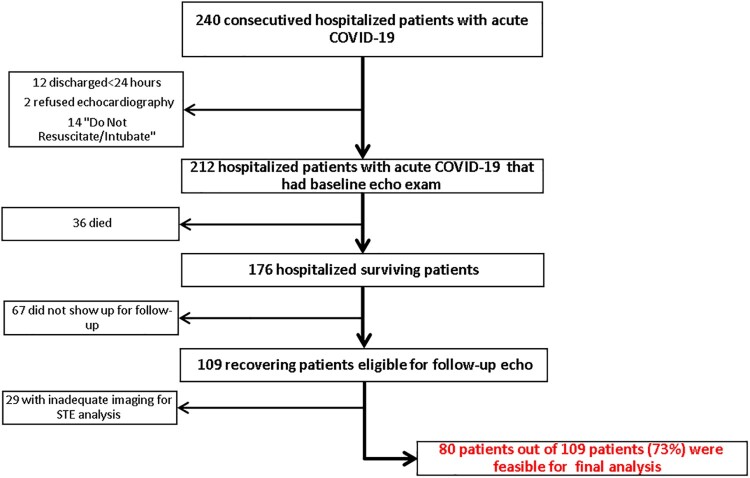
Demographic, medical, and echocardiographic data of 240 consecutive patients hospitalized with acute COVID-19 from 23 March 2020 to 20 June 2020 were recorded. In this study, 28 patients were excluded because they did not undergo baseline echocardiographic assessment during the hospitalization with acute infection. The reasons for not performing the echocardiogram included hospital discharge ≤24 h (12 patients), patient refusal (2 patients), and death shortly after hospitalization (14 patients, all >80 years old and with a ‘Do Not Resuscitate/Intubate’ status). Thirty-six patients died during the hospitalization. Sixty-seven patients did not show up at the follow-up visits, and another 29 patients were excluded because echocardiographic images were inadequate for STE analysis either at baseline or during follow-up visit (see Figure 1).
Figure 1.
Flowchart showing patient selection for the final cohort.
Thus, the final study group included 80 patients (age 57.7 ± 14.9 years, 68% male) recovered from COVID-19 infection who underwent routine and STE evaluation during both acute infection and a mean of 88.2 ± 33 days after baseline evaluation. From the final cohort, seven patients had myocarditis during their hospitalization but only two on these patients were treated with angiotensin-converting enzyme (ACE-inhibitors)/beta-blockers. All other five patients were treated just for their lung disease. From the eight patients with reduced EF, only four were put on ACE-inhibitors/beta-blockers during the hospital stay. From the 26 patients with reduced LV STE, only 9 were put on ACE-inhibitors/beta-blockers during the hospital stay. Baseline characteristics of the study cohort, including laboratory exams taken and analysed 24 h from baseline ‘hospitalization’ echo exam, stratified by clinical severity of acute disease [mild (low clinical severity) vs. moderate or severe or critical ([high clinical severity)], are presented in Table 1. Patients without pneumonia were classified as mild. Patients with pneumonia who had an oxygen level of ≥94% without oxygen supplementation were listed as moderate. Patients with an oxygen level <94% were grouped as severe, whereas intubation or need for extracorporeal membrane oxygenation as critical.
Table 1.
Baseline characteristics
| Demographics and medical history | Entire (n = 80) | Low clinical severity (n = 52) | High clinical severity (n = 28) | P-value |
|---|---|---|---|---|
| Age, mean (±SD) | 56.7 (14.9) | 53.9 (14.9) | 62 (13.5) | 0.008 |
| Male gender, n (%) | 54 (67.5) | 31 (59.6) | 23 (82.1) | 0.04 |
| Body mass index (±SD) | 28.1 (6.3) | 27.1 (5.6) | 30.8 (7.2) | 0.026 |
| Ischaemic heart disease, n (%) | 8 (10) | 6 (11.5) | 2 (7.1) | 0.71 |
| Congestive heart failure, n (%) | 4 (5) | 1 (1.9) | 3 (10.7) | 0.12 |
| S/P coronary artery bypass graft, n (%) | 1 (1.3) | 1 (1.9) | 0 | 1 |
| Atrial fibrillation/flutter, n (%) | 3 (3.8) | 1 (1.9) | 2 (7.1) | 0.28 |
| Transient ischaemic attack/stroke, n (%) | 2 (2.5) | 2 (3.8) | 0 | 0.54 |
| Peripheral artery disease, n (%) | 3 (3.8) | 1 (1.9) | 2 (7.1) | 0.28 |
| Chronic obstructive pulmonary disease, n (%) | 4 (5) | 1 (1.9) | 3 (10.7) | 0.12 |
| Asthma, n (%) | 6 (7.5) | 2 (3.8) | 4 (14.3) | 0.18 |
| Chronic kidney disease, n (%) | 4 (5) | 1 (1.9) | 3 (10.7) | 0.12 |
| Diabetes, n (%) | 16 (20) | 7 (13.5) | 9 (32.1) | 0.047 |
| Smoking, n (%) | 10 (12.5) | 6 (11.5) | 4 (14.3) | 0.73 |
| Hypertension, n (%) | 33 (41.3) | 18 (34.6) | 15 (53.6) | 0.1 |
| Hyperlipidaemia, n (%) | 24 (30) | 14 (26.9) | 10 (35.7) | 0.41 |
| Obesity, n (%) | 19 (23.8) | 10 (19.2) | 9 (32.1) | 0.2 |
| Past malignancy, n (%) | 8 (10) | 6 (11.5) | 2 (7.1) | 0.71 |
| Present malignancy, n (%) | 2 (2.5) | 2 (3.8) | 0 | 0.54 |
| Medications | ||||
| Aspirin, n (%) | 14 (17.5) | 7 (13.5) | 7 (25) | 0.23 |
| P2Y12 inhibitor, n (%) | 2 (2.5) | 2 (3.8) | 0 | 0.54 |
| Direct oral anticoagulant, n (%) | 4 (5) | 1 (1.9) | 3 (10.7) | 0.12 |
| Angiotensin-converting enzyme inhibitor, n (%) | 15 (18.8) | 8 (15.4) | 7 (25) | 0.29 |
| Angiotensin receptor blocker, n (%) | 8 (10) | 4 (7.7) | 4 (14.3) | 0.44 |
| Diuretics, n (%) | 4 (5) | 4 (7.7) | 0 | 0.29 |
| Beta-blocker, n (%) | 15 (18.8) | 9 (17.3) | 6 (21.4) | 0.65 |
| Systemic corticosteroids, n (%) | 1 (1.3) | 1 (3.6) | 0.35 | |
| Other anti-inflammatories, n (%) | 2 (2.5) | 1 (1.9) | 1 (3.6) | 1 |
| Laboratory | ||||
| Haemoglobin (g/dL), mean (±SD) | 13.7 (1.6) | 13.7 (1.4) | 13.6 (2) | 0.94 |
| White blood cells (103/μL), mean (±SD) | 8.2 (5.5) | 7.2 (2.9) | 10 (8.2) | 0.26 |
| Neutrophils (103/μL), mean (±SD) | 6.2 (5.1) | 5 (2.6) | 8.2 (7.4) | 0.027 |
| Lymphocytes (103/μL), mean (±SD) | 1.2 (0.7) | 1.4 (0.7) | 1 (0.7) | 0.004 |
| Platelets (103/μL), mean (±SD) | 214.4 (87.3) | 220 (96.5) | 204.4 (68.9) | 0.63 |
| Sodium (mmol/L), mean (±SD) | 135.8 (4.2) | 136 (3.7) | 135.5 (5.1) | 0.22 |
| Potassium (mmol/L), mean (±SD) | 4 (0.4) | 4 (0.5) | 4.1 (0.4) | 0.5 |
| Glucose (mg/dL), mean (±SD) | 114 (39.6) | 107.8 (36) | 125.2 (43.8) | 0.018 |
| Creatinine (mg/dL), mean (±SD) | 1 (0.2) | 0.9 (0.3) | 1.2 (0.6) | 0.004 |
| BUN (mg/dL), mean (±SD) | 16.5 (6.4) | 14.9 (5.5) | 19.5 (6.8) | 0.002 |
| LDH (U/L), mean (±SD) | 512.8 (265.5) | 447.7 (201.6) | 632.9 (326.1) | 0.003 |
| Bilirubin (mg/dL), mean (±SD) | 0.6 (0.4) | 0.7 (0.5) | 0.6 (0.2) | 0.86 |
| AST (U/L), mean (±SD) | 44.5 (42.8) | 47.1 (51.2) | 41.1 (20.9) | 0.91 |
| ALT (U/L), mean (±SD) | 46.4 (50.7) | 52.3 (59.6) | 35.3 (24.5) | 0.23 |
| Albumin (g/L), mean (±SD) | 39.5 (5) | 41.6 (4) | 36 (4.6) | <0.001 |
| CRP (mg/L), mean (±SD) | 84.2 (87.3) | 53.6 (63) | 140 (98.7) | <0.001 |
| Troponin-I (ng/L), mean (±SD) | 106.4 (788.4) | 7.8 (9.6) | 284.7 (1318.6) | <0.001 |
| Brain natriuretic peptide (BNP) (pg/mL), mean (±SD) | 53.2 (109.5) | 29.5 (67.7) | 110.5 (162.3) | <0.001 |
| D-Dimer, (mg/L), mean (±SD) | 1.7 (3.4) | 0.9 (1.6) | 3.1 (5) | <0.001 |
| Fibrinogen (mg/dL), mean (±SD) | 550.3 (172.4) | 519.6 (162.5) | 595.5 (180.1) | 0.12 |
| Baseline ECG findings | ||||
| ST-segment elevation, n (%) | 2 (2.5) | 2 (3.8) | 0 | 0.54 |
AST, aspartate transaminase; ALT, alanine transaminase; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram;LDH, lactic dehydrogenase;CRP, C-reactive protein
At the time of the evaluation at the COVID-19 recovery clinic, none of the patients had fever or any signs or symptoms of acute illness. Only 30 (37%) patients were completely free of any post-COVID-19-related symptoms. Twenty-one (26%) of patients had persistent dyspnoea, 19 (24%) had persistent chest pain, 28 (35%) had persistent fatigue or effort intolerance, 10 (13%) had persistent anosmia, and 4 (5%) patients had hoarseness.
Sequential routine echocardiography
Echocardiographic exams during the acute infection were compared with exams performed at the COVID-19 recovery clinic. By the time they were evaluated in the clinic, significantly higher RVFAC, longer pulmonary acceleration time, and smaller RV end-diastolic and end-systolic area were observed (P < 0.05 for all). Interestingly, although most echocardiographic RV imaging and haemodynamic parameters had improved at the time of evaluation at the COVID-19 recovery clinic in comparison to values recorded during the acute infection, most of the LV related parameters did not change apart from a small increase in EF. Furthermore, LV size remained small, stroke volume index remained low, and LV relaxation remained abnormal in a large proportion of patients (Table 2).
Table 2.
Evolution of routine RV and LV parameters from acute disease to recovery
| Acute disease N = 80 3 mechanical ventilation 1 ECMO | Recovery period N = 80 0 mechanical ventilation 0 ECMO | Paired P-value | Normal values | Threshold deviating, N (%) | |
|---|---|---|---|---|---|
| LV parameters | |||||
| Ejection fraction (EF) (%) | 57.6 ± 6 | 58.9 ± 5 | 0.02 | M ≥ 52% | M < 52%; F < 54% |
| F ≥ 54% | 8 (10) | ||||
| LVEDD index (mm/m2) | 23 ± 3 | 23.7 ± 4 | 0.16 | M 22–30 | M < 22; F < 23 |
| F 23–31 | 25 (31) | ||||
| LVESD index (mm/m2) | 15.2 ± 3 | 15.3 ± 3 | 0.50 | M 13–21 | M < 13; F < 13 |
| F 13–21 | 17 (21) | ||||
| E wave (m/s) | 60.1 ± 15 | 62.0 ± 15 | 0.46 | 0.67 ± 0.14 | 11 (14) |
| A wave (m/s) | 59.9 ± 16 | 58.7 ± 16 | 0.24 | 0.60 + 0.17 | 15 (19) |
| E/A ratio | 1.07 ± 0.4 | 1.11 ± 0.4 | 0.26 | 1.3 (0.8–2.0) | 0.8–2.0 |
| 12 (15) | |||||
| e′ septal (cm/s) | 6.9 ± 3 | 6.8 ± 2 | 0.64 | 10.3 + 3.0 | <7 |
| 40 (50) | |||||
| e′ lateral (cm/s) | 9.2 ± 4 | 8.6 ± 3 | 0.20 | 13.5 + 4.0 | <10 |
| 39 (49) | |||||
| E/e′ average | 8.0 ± 3 | 8.6 ± 3 | 0.41 | 6.8 + 2.1 | >14 |
| 4 (5) | |||||
| LA volume index | 29.4 ± 9 | 28.7 ± 13 | 0.91 | M 25.1 ± 7 | ≥34 |
| F 24.5 ± 6.4 | 10 (12) | ||||
| Stroke volume index | 32.7 ± 9 | 31.4 ± 8 | 0.31 | 33–47 | ≤35 |
| 42 (53) | |||||
| Cardiac index | 2.4 ± 0.6 | 2.2 ± 0.6 | 0.17 | 2.5–4 | <2.5 |
| 43 (54) | |||||
| RV parameters | |||||
| Right atrium (RA) pressure (mmHg) | 7.6 ± 4 | 6.2 ± 2 | 0.01 | >5 | >5 |
| 12 (15) | |||||
| RVEDA index (cm2/m2) | 11.6 ± 2 | 10.3 ± 2 | 0.0007 | M 8.8 ± 1.9 | M > 12.6; F > 11.5 |
| F 8.0 ± 1.7 | 11 (14) | ||||
| RVESA index (cm2/m2) | 6.7 ± 2 | 5.7 ± 2 | 0.0002 | M 4.7 ± 1.3 | M > 7.4; F > 6.4 |
| F 4.0 ± 1.2 | 6 (8) | ||||
| RVFAC (%) | 41.6 ± 11 | 46.2 ± 8 | 0.002 | 49 ± 7 | <35 |
| <35 | 4 (5) | ||||
| TAPSE (mm) | 22 ± 4 | 22 ± 4 | 0.3 | 24 ± 3.5 | <17 |
| 4 (5) | |||||
| Pulmonary acceleration time (ms) | 93.0 ± 26 | 107.2 ± 27 | 0.0001 | 137 ± 24 | <100 21 (26) |
| Pulmonary vascular resistance (dynes×s/cm5/m2) | 381 ± 161 | 171 ± 136 | P < 0.0001 | 255–285 | >285 11 (13) |
FAC, fractional area change; LV, left ventricle; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion;LVEDD, left ventricle end-diastolic diameter LVESD, left ventricle end-systolic diameter; RVEDA, right ventricle end-diastolic area; RVESA, right ventricle end-systolic area; LA, left atrium
Sequential STE
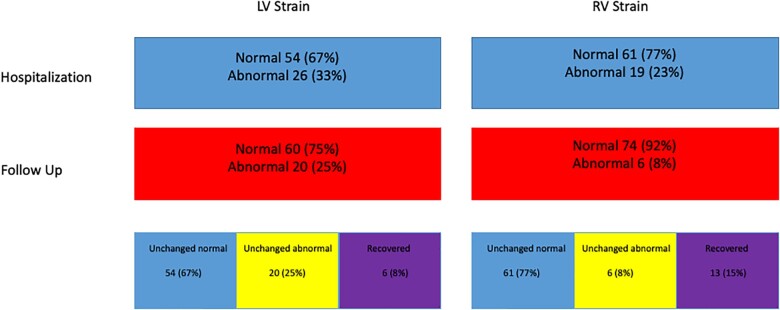
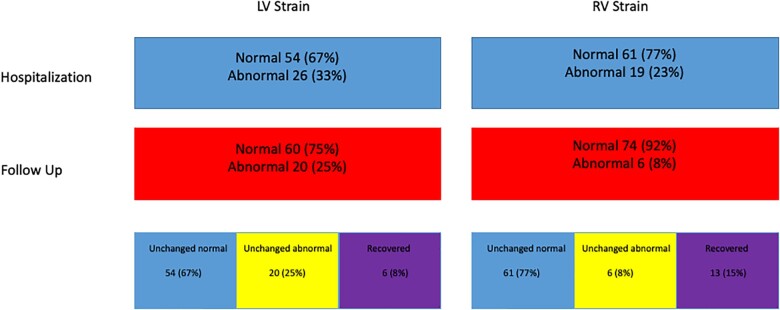
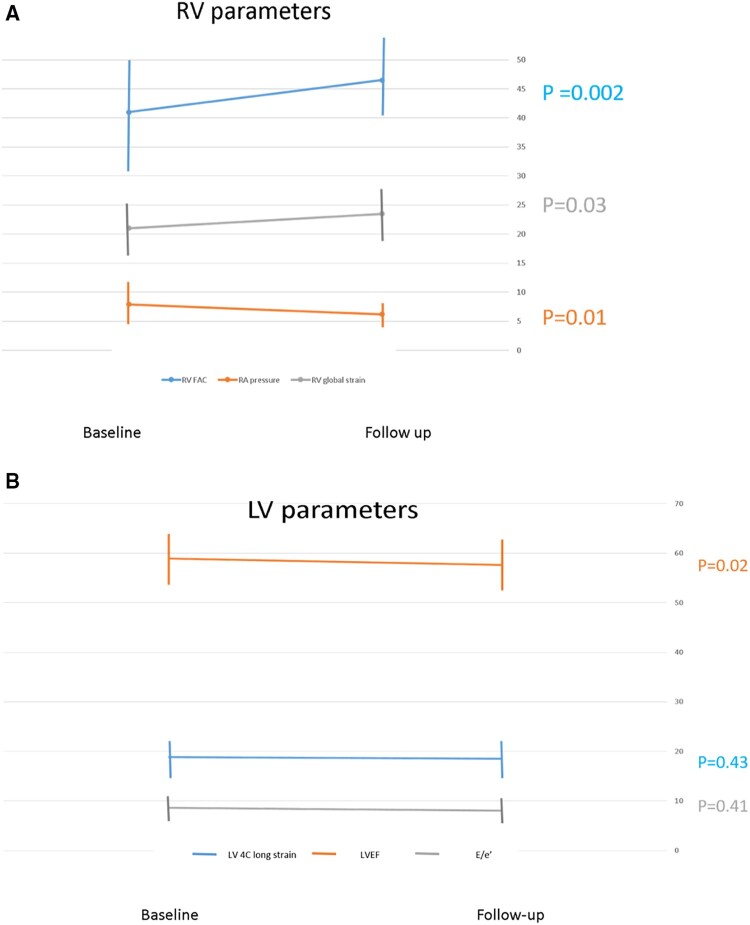
STE of RV and LV during the acute infection were compared with exams performed in the post-COVID-19 recovery clinic. RV4CLS and RV septal STE parameters improved at the recovery period (Table 3). Serial improvement in RVFWLS (24.6–26.1%) did not reach significance (P = 0.13). However, when assessing the patients with RVFWLS ≤20.0%, 19 (23%) patients who had abnormal function during hospitalization and only 6 patients (8%) had RVFWLS ≤20.0% at the follow-up (P = 0.006) evaluation (Figure 2). As shown in Figure 3A, patients showed improvement in RV radial (RVFAC), longitudinal (RV global strain), and filling pressure (RA pressure) compared with the evaluation performed during the acute phase of the disease. We assessed the correlation between lung ultrasound score and RV STE. All RV STE parameters were strongly correlated with lung ultrasound score: peak four-chamber longitudinal strain R2 = 0.21; P = 0.0001, peak free wall longitudinal strain R2 = 0.19; P = 0.0002, and peak septal longitudinal strain R2 = 0.13; P = 0.01. All LV STE parameters did not change significantly (Table 3 and Figure 2). As shown in Figure 3B, LV radial (LV EF), longitudinal (LV strain), and filling pressure (E/e′ ratio) did not change during the follow-up period. Compared with 26 (33%) patients who had LV4CLS ≤16.1 and LV4CLS ≤17.3% for male and female, respectively (the lower limit of normal strain for our vendor26) during hospitalization, a significant proportion [20 (25%)] of patients still had abnormal LV STE at the follow-up evaluation (Figure 2). Dyspnoea and hoarseness were more common in patients with persistent abnormal RV strain [dyspnoea 21/74 (28%) vs. 5/6 (83%); P = 0.005 and hoarseness 2/74 (2.5%) vs. 2/6 (33%); P = 0.0009, for normal vs. abnormal RV strain at follow-up]. Surprisingly, anosmia was more common in patients with normal LV strain [10/60 (17%) vs. 0/20 (0%); P = 0.05].
Table 3.
Evolution of RV and LV strain parameters from acute infection to recovery
| Acute disease | Recovery period | P-value | |
|---|---|---|---|
| N = 80 | N = 80 | ||
| RV speckle strain | |||
| Peak four-chamber longitudinal strain | 21.7 ± 4 | 23.2 ± 5 | 0.03 |
| Peak free wall longitudinal strain | 24.6 ± 5 | 26.1 ± 6 | 0.13 |
| Peak septal wall longitudinal strain | 19.1 ± 4 | 20.8 ± 5 | 0.01 |
| LV speckle strain | |||
| Peak four-chamber longitudinal strain | 18.3 ± 4 | 18.7 ± 4 | 0.43 |
| Peak lateral wall longitudinal strain | 18.4 ± 4 | 19.0 ± 4 | 0.36 |
| Peak septal wall longitudinal strain | 18.5 ± 4 | 18.6 ± 4 | 0.69 |
LV, left ventricle; RV, right ventricle.
Figure 2.
Flowchart showing the prevalence of patients with impaired RV and LV STE at hospitalization, follow-up, how many were unchanged, and how many recovered. LV, left ventricle; RV, right ventricle; STE, speckle-tracking echocardiography.
Figure 3.
Comparison of echocardiographic evaluations of LV and RV performed at hospitalization for acute infection vs. those performed ∼3 months post-recovery. (A) RV parameters, RV fractional area change (blue), RV global strain (grey), and RA pressure (orange). Note that all RV parameters improved at follow-up. (B) LV parameters, LV ejection fraction (orange). LV E/e′ ratio (grey) and LV four-chamber strain (blue). Note that all LV parameters did not change at follow-up. LV, left ventricle; RV, right ventricle.
We divided the patients to patients who ‘recovered’ RV STE (Supplementary data online, Table S1) or LV STE (Supplementary data online, Table S2) function vs. those with ‘unchanged’ function to check potential associated of unchanged function. We found no significant differences for LV STE possibly due to the low number of ‘recovered’ LV patients. However, patients with RV ‘recovery’ had evidence for worse RV haemodynamics, and lower LV filling pressure at baseline compared with the ‘unchanged’ RV patients.
To explore whether LV STE alterations at hospitalization are associated with co-morbidities, we compared patients with LV STE alterations at hospitalization to those who had normal LV STE (Supplementary data online, Table S3). Patients with abnormal LV STE had a trend for higher prevalence of co-morbidities as CHF, CRF, COPD, and malignancy.
Compared with the matched control group, patients 3 months post-COVID-19 infection had similar RV strain parameters but poorer LV strain (P < 0.05 for all) (Table 4).
Table 4.
Comparison of RV and LV strain parameters of recovery patients and matched control group
| COVID-19 recovery | Control group | P-value | |
|---|---|---|---|
| N = 80 | N = 20 | ||
| RV speckle strain | |||
| Peak four-chamber longitudinal strain | 23.3 ± 5 | 19.4 ± 9 | 0.12 |
| Peak free wall longitudinal strain | 25.6 ± 6 | 24.4 ± 5 | 0.21 |
| Peak septal wall longitudinal strain | 20.1 ± 4 | 18.6 ± 4 | 0.14 |
| LV speckle strain | |||
| Peak four-chamber longitudinal strain | 18.3 ± 4 | 21.7 ± 3 | 0.0002 |
| Peak lateral wall longitudinal strain | 18.6 ± 4 | 21.2 ± 3 | 0.01 |
| Peak septal wall longitudinal strain | 19.1 ± 4 | 22.6 ± 3 | <0.0001 |
LV, left ventricle; RV, right ventricle.
Inter- and intra-observer variability
Inter-observer analysis of echo variables showed good agreement between measurements: peak RV4CLS (mean difference 0.13 ± 0.45, r = 0.93, P = 0.8) and peak LV4CLS (mean difference 0.21 ± 0.22, r = 0.97, P = 0.4). Measurement variability (within-subject coefficient of variation and 95% CI) for measurements of inter-observer differences was as follows: peak RV4CLS, 1.9% and peak four-chamber longitudinal strain, 1.1%.
Intra-observer analysis of echo variables showed good agreement between measurements: peak RV4CLS (mean difference 0.29 ± 0.49, r = 0.92, P = 0.6) and peak LV4CLS (mean difference 0.20 ± 0.37, r = 0.93, P = 0.6). Measurement variability (within-subject coefficient of variation and 95% CI) for measurements of inter-observer differences was as follows: peak RV4CLS, 1.3% and peak LV4CLS, 1.9%.
Discussion
The main findings of this study are (i) in patients recovering from COVID-19 infection, RV routine echocardiographic, haemodynamic and RV STE parameters improve in the majority of patients. (ii) Although only 10% of patients recovering from COVID-19 infection had abnormal EF, ∼3 months post-acute infection, 25% of patients still had LV systolic dysfunction based on STE analysis. (iii) In patients recovering from COVID-19 infection most LV routine echocardiographic, haemodynamic, and STE parameters do not improve in the months following acute infection. In our previous prospective registries, aiming to evaluate how COVID-19 impacts the heart during the acute phase of COVID-19 infection,3,27 we demonstrated that RV and/or LV function was impaired in a large proportion of patients compared with normal values, even in patients with mild respiratory disease. The present longitudinal study is an extension of those studies, investigating for persistence of haemodynamic and/or STE echocardiographic abnormalities, ∼3 months after hospitalization for the acute infection. The results of this study emphasize the importance of both routine Doppler echocardiographic and STE analyses in evaluating myocardial function in patients recovering from COVID-19 infection.
RV routine and speckle strain echocardiography in patients recovering from COVID-19 infection
We have previously shown that the most common routine echocardiographic, and STE pattern among hospitalized patients with acute COVID-19 infection is that of RV dilatation and dysfunction.3,11 In this study, we found that most RV haemodynamic and functional parameters improved 3 months after hospitalization with acute disease. During the acute phase of COVID-19 infection RV dilatation and dysfunction are associated with shortened pulmonary acceleration time suggestive of increased pulmonary vascular resistance. There are many reasons for increased pulmonary vascular resistance in hospitalized patients with acute COVID-19 infection that may precipitate acute RV failure. These conditions include pulmonary embolism, hypoxic pulmonary vasoconstriction, decrease in lung volume, excessive positive end-expiratory pressure (in mechanically ventilated patients), super-infection with other types of pneumonia, the use of α-agonists, elevated left atrial pressure due to concomitant LV dysfunction, or combination of the above. Irrespective of its cause, the increase in RV afterload during acute COVID-19 infection results in decreased cardiac output and systemic blood pressure, which may result in decrease in coronary perfusion to the right ventricle, and additional reduction in RV contractility.3 Furthermore, the decrease in trans-septal pressure gradient between the right and left ventricles may result in septal bowing at the expense of LV, resulting in abnormal orientation of helical myofibrils and further reduction in cardiac function. In this study, we show a marked increase in pulmonary acceleration time suggestive of substantial improvement in pulmonary vascular resistance 3 months after hospitalization with acute COVID-19 infection. Furthermore, reduction in RV strain was associated with poorer lung ultrasound score, suggesting that reduced RV STE during acute infection was related to elevated pulmonary vascular resistance imposed by the worsened lung involvement. The reversal of the spiral of events described above, during recovery from acute infection, probably explains the almost uniform improvement in RV function observed already 3 months after hospitalization.
LV routine and speckle strain echocardiography in patients recovering from COVID-19 infection
Several reports suggested that patients who recover from COVID-19 infection have prevalent LV abnormalities in cardiac MRI, mostly abnormal native T1 and T2 measures and late gadolinium enhancement (LGE), suggesting myocardial inflammation and oedema. Interestingly, prevalence of cardiac involvement was independent of pre-existing conditions, severity and overall course of the acute illness.5,7 Brito et al.28 described patterns of myocardial involvement with varying degree of myocardial dysfunction in a young, otherwise healthy cohort of college athletes, assessing STE and CMR after an unknown recovery period from COVID-19 infection. However, Clark et al.29 recently published a research letter showing a much lower prevalence than initially indicated, suggesting that the true burden remains unclear and might have been overstated by earlier CMR-based publications. Similar to these MRI-based reports, recent echocardiographic studies in survivors of COVID-19 had discordant results. Moody et al.8 found that COVID-19 survivors had persisting adverse remodelling in ∼30% of their population. However, their cohort was extremely selected because they included only patients who had an echocardiography performed due to a clinical complication during their hospitalization. Furthermore, the baseline echocardiography performed during hospitalization was not performed using the same protocol, machine and personnel, as the follow-up echo. In agreement with Moody, Özer et al.30 recently published a comprehensive LV global function analysis with STE in patients recovered from COVID-19. Their findings, with a similar cohort to our study, demonstrated impaired LV-GLS values, in 30% at 1-month follow-up, and even 57% in those who developed myocardial injury during hospitalization.
On the contrary, Catena et al.9 performed a follow-up echocardiographic exam ∼40 days following hospital discharge in which they concluded that there was no evidence of persistent cardiac dysfunction on echocardiography. However, they did not perform STE analysis to detect for more subtle myocardial changes.9
This study was done on consecutive hospitalized patients, with all grades of clinical disease, unrelated to clinical complications, and with a broad age group. Moreover, this study is the first to assess changes in routine echocardiographic and STE parameters using the same vendor, equipment, protocol and personnel, both at baseline (during hospitalization) and follow-up (at the recovery clinic). We found that although low EF was rare, small LV size, abnormal LV relaxation, and low output were common 3 months post-acute COVID-19 infection. This suggests that EF should not be used as the lone routine echocardiographic parameter to follow-up these patients. Once STE was performed, a quarter of patients still had abnormal LV longitudinal function at the follow-up evaluation, based on an STE recommended cut-off.11 Moreover, on contrary to RV, in the LV there is no significant improvement in the amount of patients with abnormal LV4CLS values (26 patients during the acute phase vs. 20 patients at the follow-up clinic). Recent data in patients with other types of myocarditis and cardiomyopathies demonstrated that conventional echocardiographic parameters, such as EF do not correlate with the amount of LV myocardial oedema or fibrosis by MRI. In contrast, global longitudinal systolic myocardial strain correlated significantly with the amount of oedema by T2-weighted imaging31 and myocardial fibrosis by LGE.32 Thus, it is clear that for optimal follow-up of patients with subtle myocardial injury after COVID-19 infection, STE is superior to conventional echocardiography. Interestingly, once STE was used, some of the COVID-19 survivors showed deterioration in LV STE parameters compared with the evaluations performed during the hospitalization with acute infection. Deterioration in LV STE in some of the patients may be related to either continued adverse remodelling after the acute infection-related myocardial injury, as shown in other types of myocarditis.33 An ‘adult type’ multisystem inflammatory syndrome similar to that described in older children and adolescents34 might be another cause, however, further data and biomarker evidence are needed to support this.
Study limitations
Our study included only hospitalized COVID-19 patients, thus it does not represent patients who were asymptomatic or mildly symptomatic without the need for hospitalization. The fact that a significant proportion of patients did not show for follow-up probably led to selection bias and over-estimation of the severity of echocardiographic pathology in patients recovering from COVID-19 infection. However, 36 patients died during the acute phase of disease which may have created an opposite bias resulting in under-estimation of cardiac manifestations. Patients did not have echocardiography prior to their COVID-19 illness. Thus, it is not possible to exclude that persistent findings after COVID-19 infection may have been present and fixed prior to the initial illness. Moreover, STE analysis, has its own inherited limitations, including imperfect feasibility, mainly affected by image quality, load dependency, and re-test variability. Also, LV4CLS is not a clear substitute for LVGLS. Comparison between baseline and follow-up echo exams, especially for sub-group analyses, in our study should be interpreted with caution due to the limited number of patients. We believe that our results should serve as incentive to further explore evolution of cardiac involvement in patients recovering from COVID-19 infection in larger series.
Clinical implications
The results of this study show that subtle LV dysfunction may persist after acute COVID-19 infection despite resolution of the pulmonary disease. Nevertheless, it is important to stress that LVEF, LV haemodynamics and even LV strain are within the normal range in the majority of recovered patients, and that clinically meaningful decrease in LV strain from normal to abnormal range, during the time period between hospitalization to 3-month follow-up, is very rare. Because LV systolic abnormalities are mostly subclinical, data on the value of interventions based on such changes are still lacking. However, such subclinical changes in LV function have been associated with a worse prognosis in other types of cardiac disease,35 and even in the community,36 thus it seems prudent to follow up carefully on patients with such subclinical LV dysfunction, preferably using speckle strain imaging. Furthermore, the use of beta-blockers, ACE-inhibitors, and/or angiotensin receptor blockers may be warranted in some patients, especially if LV dysfunction persists, or worsens. Although we observed a significant degree of RV dysfunction at the time of acute infection with COVID-19, this study shows that RV function usually improves following convalescence from pulmonary disease. However, in the rare patient in which RV dysfunction persists, a thorough evaluation for persistence of elevated pulmonary vascular resistance should be considered.
Conclusions
This study demonstrates persistence of LV dysfunction in a significant proportion of patients recovering from COVID-19 infection. However, RV dysfunction improves in the majority of patients due to improved pulmonary haemodynamics. It is important to demonstrate whether the LV dysfunction is permanent or reversible with or without treatment. Therefore, a longer follow-up would be crucial to assess clinical relevance and prognosis for these patients.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Data availability
The deidentified participant data generated in this research will be shared on reasonable request to the corresponding author.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med 2020;382:2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I et al. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19) - a systematic echocardiographic study. Circulation 2020;142:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carfì A, Bernabei R, Landi F; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang D, Anguo L, Yue WS, Yin L, Tse HF, Siu CW. Refinement of ischemic stroke risk in patients with atrial fibrillation and CHA2DS2-VASc score of 1. Pacing Clin Electrophysiol 2014;37:1442–7. [DOI] [PubMed] [Google Scholar]
- 7. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021;6:116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moody WE, Liu B, Mahmoud-Elsayed HM, Senior J, Lalla SS, Khan-Kheil AM et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr 2021;34:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catena C, Colussi G, Bulfone L, Da Porto A, Tascini C, Sechi LA. Echocardiographic comparison of COVID-19 patients with or without prior biochemical evidence of cardiac injury after recovery. J Am Soc Echocardiogr 2021;34:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lichter Y, Topilsky Y, Taieb P, Banai A, Hochstadt A, Merdler I et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 2020;46:1873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothschild E, Baruch G, Szekely Y, Lichter Y, Kaplan A, Taieb P et al. The predictive role of left and right ventricular speckle-tracking echocardiography in COVID-19. JACC Cardiovasc Imaging 2020;13:2471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2014;27:911–39. [DOI] [PubMed] [Google Scholar]
- 13. Laufer-Perl M, Arnold JH, Mor L, Amrami N, Derakhshesh M, Moshkovits Y et al. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin Res Cardiol 2020;109:255–62. [DOI] [PubMed] [Google Scholar]
- 14. Houard L, Benaets MB, de Meester de Ravenstein C, Rousseau MF, Ahn SA, Amzulescu MS et al. Additional prognostic value of 2D right ventricular speckle-tracking strain for prediction of survival in heart failure and reduced ejection fraction: a comparative study with cardiac magnetic resonance. JACC Cardiovasc Imaging 2019;12:2373–85. [DOI] [PubMed] [Google Scholar]
- 15. Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score - Development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units—the experience in Sichuan Province, China. Intensive Care Med 2020;46:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. J Am Soc Echocardiogr 2015;28:183–93. [DOI] [PubMed] [Google Scholar]
- 18. Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Coll Cardiol 2020;75:3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 21. Topilsky Y, Khanna AD, Oh JK, Nishimura RA, Enriquez-Sarano M, Jeon YB et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation 2011;123:1929–39. [DOI] [PubMed] [Google Scholar]
- 22. Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 1983;68:302–9. [DOI] [PubMed] [Google Scholar]
- 23. Rigamonti F, Graf G, Merlani P, Bendjelid K. The short-term prognosis of cardiogenic shock can be determined using hemodynamic variables: a retrospective cohort study. Crit Care Med 2013;41:2484–91. [DOI] [PubMed] [Google Scholar]
- 24. Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P. Doppler echocardiography in advanced systolic heart failure: a noninvasive alternative to Swan-Ganz catheter. Circ Heart Failure 2010;3:387–94. [DOI] [PubMed] [Google Scholar]
- 25. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T et al. ; Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. [DOI] [PubMed] [Google Scholar]
- 26. Sugimoto T, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:833–40. [DOI] [PubMed] [Google Scholar]
- 27. Szekely Y, Lichter Y, Hochstadt A, Taieb P, Banai A, Sapir O et al. The predictive role of combined cardiac and lung ultrasound in coronavirus disease 2019. J Am Soc Echocardiogr 2021;34:642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging 2021;14:541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Heal 2020;8:e1003–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Özer S, Candan L, Özyıldız AG, Turan OE. Evaluation of left ventricular global functions with speckle tracking echocardiography in patients recovered from COVID-19. Int J Cardiovasc Imaging 2021;37:2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Løgstrup BB, Nielsen JM, Kim WY, Poulsen SH. Myocardial oedema in acutemyocarditis detected by echocardiographic 2Dmyocardial deformation analysis. Eur Heart J Cardiovasc Imaging 2016;17:1018–26. [DOI] [PubMed] [Google Scholar]
- 32. Pagourelias ED, Mirea O, Duchenne J, Unlu S, Van Cleemput J, Papadopoulos CE et al. Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: a comparison between 2D and 3D echo modalities. Eur Heart J Cardiovasc Imaging 2020;21:1262–72. [DOI] [PubMed] [Google Scholar]
- 33. Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390:400–14. Vol. [DOI] [PubMed] [Google Scholar]
- 34. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF et al. ; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 2015;8:1351–9. [DOI] [PubMed] [Google Scholar]
- 36. Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail 2014;16:1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified participant data generated in this research will be shared on reasonable request to the corresponding author.
Conflict of interest: none declared.