Abstract
Aims
The clinical manifestation and outcomes of thrombosis with thrombocytopenia syndrome (TTS) after adenoviral COVID-19 vaccine administration are largely unknown due to the rare nature of the disease. We aimed to analyse the clinical presentation, treatment modalities, outcomes, and prognostic factors of adenoviral TTS, as well as identify predictors for mortality.
Methods and Results
PubMed, Scopus, Embase, and Web of Science databases were searched and the resulting articles were reviewed. A total of 6 case series and 13 case reports (64 patients) of TTS after ChAdOx1 nCoV-19 vaccination were included. We performed a pooled analysis and developed a novel scoring system to predict mortality. The overall mortality of TTS after ChAdOx1 nCoV-19 vaccination was 35.9% (23/64). In our analysis, age ≤60 years, platelet count <25 × 103/µL, fibrinogen <150 mg/dL, the presence of intracerebral haemorrhage (ICH), and the presence of cerebral venous thrombosis (CVT) were significantly associated with death and were selected as predictors for mortality (1 point each). We named this novel scoring system FAPIC (fibrinogen, age, platelet count, ICH, and CVT), and the C-statistic for the FAPIC score was 0.837 (95% CI 0.732–0.942). Expected mortality increased with each point increase in the FAPIC score, at 2.08, 6.66, 19.31, 44.54, 72.94, and 90.05% with FAPIC scores 0, 1, 2, 3, 4, and 5, respectively. The FAPIC scoring model was internally validated through cross-validation and bootstrapping, then externally validated on a panel of TTS patients after Ad26.COV2.S administration.
Conclusions
Fibrinogen levels, age, platelet count, and the presence of ICH and CVT were significantly associated with mortality in patients with TTS, and the FAPIC score comprising these risk factors could predict mortality. The FAPIC score could be used in the clinical setting to recognize TTS patients at high risk of adverse outcomes and provide early intensive interventions including intravenous immunoglobulins and non-heparin anticoagulants.
Keywords: Vaccine-induced thrombotic thrombocytopenia, Thrombotic thrombocytopenia syndrome, ChAdOx1 nCoV-19, COVID-19 vaccine, Cerebral venous thrombosis
Graphical Abstract
Introduction
Since its initial outbreak on 31 December 2019, coronavirus disease 2019 (COVID-19) has become a rampant pandemic with a total of 142 539 302 confirmed cases and 3 116 444 deaths as of 27 April 2021.1 At such a pivotal time, rapid, worldwide vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to achieve herd immunity has become the most pressing issue for mitigating the global threat of the virus.2,3 Currently, four vaccines have been approved either by the European Medicines Agency (EMA) or by the U.S. Food and Drug Administration (FDA), including two mRNA-based vaccines—BNT162b2 (Pfizer-BioNTech) and mRNA-173 (Moderna)—and two recombinant adenoviral vector vaccines—ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen).4,5 These vaccines have been developed and distributed at an unprecedented pace; as of 27 April 2021, a total of 570.63 million people worldwide have received at least one dose of the COVID-19 vaccine; 42.38% of the USA and 20.05% of Europe have been vaccinated at least once.6 Although these vaccines are highly efficacious in protecting against SARS-CoV-2 infection by neutralizing antibodies,7–9 there have been increasing reports of severe central vein thromboses after immunization with the ChAdOx1 nCoV-19 vaccine, some of which have been fatal.10–16
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the EMA responded by reviewing the risk of thrombosis related to SARS-CoV-2 vaccine and confirmed that the risk of venous thrombo-embolism associated with the vaccines was not higher than that in the general population, but they acknowledged that the AstraZeneca vaccine may be related to a rare but serious adverse event associated with thrombosis, such as cerebral venous thrombosis (CVT) and thrombocytopenia,17 although a causal association has not yet been confirmed.18–20 The EMA compared the clinical picture with immune-mediated heparin-induced thrombocytopenia (HIT),21 and two recently published case series have confirmed this similarity.10,12 The patients in these case series had high levels of antibodies against antigenic complexes of platelet factor 4 (PF4), which are found in HIT, though none of the patients had previously received heparin.10,12
This evidence has resulted in conflicting reports and guidelines regarding the rollout of the ChAdOx1 nCoV-19 vaccine from different parts of the world, such as Canada, Germany, the EMA, and Thailand, but many countries have cautiously opted to continue administration of the ChAdOx1 nCoV-19 vaccine.22–24 With recent outbreaks in low- and middle-income countries such as India and Brazil, it is of urgent and critical importance to rapidly and comprehensively evaluate such vaccine-related adverse effects, especially as the ChAdOx1 nCoV-19 vaccine is both the major vaccine produced intrinsically in India and the largest component of the COVAX vaccine rollout plan.25,26 Case reports and case series of rare, fatal thromboses associated with the ChAdOx1 nCoV-19 vaccine are accumulating; however, due to insufficient sample size, it is difficult to draw consistent, significant conclusions regarding the clinical presentation and treatment of these vaccine-associated thrombotic events, now called thrombosis with thrombocytopenia syndrome (TTS). Furthermore, no study to date has yet analysed risk factors for differential outcomes of TTS patients. Therefore, the present study aimed to perform a systematic review to investigate all published studies regarding TTS to analyse clinical and laboratory data, treatment modalities, and outcomes of patients and to discuss prognostic factors that may aid future therapeutic interventions.
Methods
Search strategy and selection criteria
This systematic review was performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P; Supplementary material online, Table S1).27 As reports are being updated every day, a rapid review was conducted to summarize all published cases of TTS.
We initially carried out a search of PubMed ePubs, Scopus, Embase, and Web of Science databases to include all articles available regarding patients with COVID-19 vaccine-associated thrombosis after ChAdOx1 nCoV-19 vaccination published up to 28 April 2021, without limiting our search by language or date. Our initial search yielded 673 articles. After a review of individual abstracts and full texts, we identified seven studies (three case reports and four case series) that met the inclusion criteria for this systematic review.10–16 In addition, we carried out an additional search in the same databases on 24 June 2021 and added 2 case series and 10 case reports.28–39 The search terms used are described in detail in the Supplementary material online, Table S2. The detailed selection process is depicted in Supplementary material online, Figure S1; the characteristics of individual case studies are shown in Supplementary material online, Table S3 and S4.
Cases were only included if they reported patients with a history of COVID-19 vaccination with the ChAdOx1 nCoV-19 vaccine prior to presentation, and if the patients had a haemorrhagic or thrombotic event documented by clinical and radiological findings. We excluded cases if they had received another type of COVID-19 vaccine. For the purposes of statistical analysis, we further excluded review articles, letters to the editors, abstracts, articles that did not contain sufficient information on the patients, and duplicate cases.
Three reviewers (J.I. Shin, S.H. Park, and S.B. Lee) independently examined the studies, and any disagreement among the authors was resolved by consensus. For each eligible case report and case series, we extracted data on the demographic, clinical, and laboratory findings at presentation, type of treatment, clinical course, and outcome.
Data collection
We identified 19 studies regarding TTS related to immunization with ChAdOx1 nCoV-19, and collected demographic and clinical characteristics including treatment and outcomes, comprising age, sex, onset of symptoms, ethnicity, pre-existing conditions, symptoms, laboratory results, immunological and platelet activation assays, number and location of thrombotic and/or haemorrhagic events, treatment modalities used, and mortality.
Statistical analysis
Statistical analyses were performed using the SPSS for Windows version 25.0 (SPSS Inc., IBM Corporation, Chicago, IL, USA) and R version 4.0.4 (R Core Team, Vienna, Austria). Basic demographic and clinical information was presented as the median and interquartile range (IQR) for continuous variables and the percentage for categorical variables. Continuous variables were compared with the Mann–Whitney U-test and categorical variables were compared using the Fisher’s exact test. Spearman’s correlation analysis was carried out to determine the relationships between continuous variables. Logistic regression analyses were also used to identify independent risk factors for mortality. In all statistical analyses, a two-tailed P-value of <0.05 was considered significant.
Briefly, the following steps were used to construct and validate the FAPIC score.
Step 1: construction of the FAPIC score
Demographic and clinical factors, laboratory measurements, and associated thromboses were considered as potential predictors. After univariable analyses of all parameters between survivors and non-survivors, binary variables that were significantly associated with mortality with a P-value of <0.05 were summed to create a FAPIC summary score for a logistic regression model. Only binary variables were considered in constructing the scoring model to achieve simplicity in application; cut-offs for continuous variables for dichotomization were pre-determined according to clinical relevance. Discriminative power of the FAPIC predictive model was assessed by drawing the receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC) statistic (C-statistic). Model calibration was also assessed through Hosmer–Lemeshow goodness of fit analysis.
Step 2: internal validation
Internal validation of the prediction model was undertaken by two methods: the K-step cross-validation method and bootstrapping. First, K-step cross-validation was performed by taking 10% of the whole dataset for testing, and training the model on the remaining 90%, then repeating the procedure 20 times. Second, the predictive performance of the FAPIC scoring model was re-assessed via bootstrapping, sampling the whole dataset using 100 repetitions with replacement. For each method, the predictive model accuracy was assessed by computing the C-statistic.
Step 3: external validation
The external validation step was performed independently after the development of the model using different data. We independently collected all 16 published cases of TTS after Ad26.COV2.S vaccination to extract relevant clinical characteristics and mortality data,40–44 and double-checked the data by reviewing the records in the Vaccine Adverse Event Reporting System (VAERS) of the U.S. Centers for Disease Control and Prevention (CDC). Baseline characteristics were compared between the original dataset and the validation set. Then, the performance of the FAPIC scoring model was assessed by computing the C-statistic.
Finally, a secondary analysis of steps 1–3 was performed after estimating missing values from the observed values using multiple imputation by chained equations (MICE). Twelve hundred rounds of imputation were performed, and the imputation algorithm was checked for convergence.
Results
Demographics and clinical characteristics
The 64 patients with TTS were 21–71 years of age, with a median age of 45 years and an IQR of 22.75 years. Over two-thirds (68.5%) of patients were women. None of the patients reported a pre-existing prothrombotic condition.
Overall, patients presented to the hospital from a range of 5 to 24 days after vaccination, with a median time from vaccination of 10 days. Presenting symptoms from these cases are shown in Supplementary material online, Table S5. The most common symptoms for patients were neurological; notably, 22 out of 30 (73.3%) patients for whom symptom data were available presented with headache, followed by hemiparesis (30.0%), visual disturbance (26.7%), dysphasia (16.7%), dizziness (13.3%), and seizure (13.3%). Half (50.0%) of patients reported systemic symptoms, with fever in 23.3%, reduced consciousness in 16.7%, fatigue in 10.0%, and myalgia in 6.7% of patients. Gastrointestinal manifestations were present in seven patients (23.3%), including abdominal pain (13.3%) and vomiting (10.0%). Three patients (10.0%) reported bleeding tendency, including gum bleeds (6.7%), haematoma (6.7%), and petechial rash (3.3%). Other symptoms included dyspnoea (10.0%), chest pain (6.7%), back pain (6.7%), and arthralgia (3.3%).
Laboratory findings of TTS patients are delineated in Table 1. Most patients presented with thrombocytopenia, with a median platelet count (IQR) of 35 × 103/µL (16.75 × 103–70.25 × 103/µL). Thirty-one out of 41 (70.5%) patients had abnormal protrombin time (PT) and 16 (37.2%) had abnormal partial thromboplastin time (PTT), with median PT [international normalized ratio (INR)] of 1.20 and median activated partial thromboplastin time (aPTT; s) of 29.90. More than half (52.0%) had severe hypofibrinogenemia with fibrinogen levels <150 mg/dL. All 55 patients (100.0%) who were analysed had extremely elevated D-dimer levels, with an average of 62.60 times the upper limit of normal. Furthermore, the results of the correlation analysis indicated that platelet counts, fibrinogen levels, and D-dimer levels were associated (Supplementary material online, Table S6).
Table 1.
Laboratory findings of patients with VITT after ChAdOx1 nCoV-19 vaccination according to outcome
| Laboratory findings | Total patients (n = 64a) | Survivors (n = 40) | Non-survivors (n = 23) | P-value |
|---|---|---|---|---|
| Platelet (n = 62) | ||||
| Platelet count (cells/mm3) | 35 000 (16 750, 70 250) | 40 000 (26 000, 70 000) | 19,000 (13,750, 75,750) | 0.121 |
| Platelet <25 × 103/μL | 22/62 (35.5) | 9/39 (23.1) | 13/22 (60.9) | 0.007 |
| PT (n = 41) | ||||
| PT (s) (n = 10) | 13.35 (12.95, 14.95) | 14.10 (13.15, 20.40) | 13.10 (12.80, 15.00) | 0.366 |
| PT INR (n = 28) | 1.20 (1.10, 1.40) | 1.20 (1.10, 1.30) | 1.20 (1.10, 1.66) | 0.488 |
| PT, abnormal valueb | 31/41 (70.5) | 18/27 (66.7) | 13/17 (76.5) | 0.735 |
| aPTT (n = 43) | ||||
| aPTT (s) (n = 23) | 29.90 (25.00, 39.43) | 28.70 (24.00, 37.35) | 32.70 (27.75, 43.85) | 0.258 |
| aPTT ratio (n = 14) | 1.05 (0.98, 1.33) | 1.05 (0.98, 1.33) | 1.05 (0.91, 1.55) | 0.962 |
| aPTT, abnormal valueb | 16/43 (37.2) | 10/26 (38.5) | 6/17 (35.3) | 1.000 |
| Fibrinogen (n = 50) | ||||
| Fibrinogen (mg/dL) | 140.00 (110.00, 262.50) | 210.00 (120.00, 345.00) | 120.00 (80.00, 140.00) | 0.003 |
| Fibrinogen <150 mg/dL | 26/50 (52.0) | 11/31 (35.5) | 15/19 (78.9) | 0.004 |
| D-dimer (n = 55) | ||||
| D-dimer/upper limit of normal range | 62.60 (20.80, 70.40) | 45.80 (16.30, 70.40) | 70.00 (32.22, 79.05) | 0.143 |
| D-dimer, abnormal value (>500 mg/L, FEU) | 55/55 (100) | 37/37 (100) | 17/17 (100) | 1.000 |
| Anti-PF4/heparin antibody ELISA (n = 47) | ||||
| Anti-PF4/heparin antibody ELISA OD | 2.16 (1.14, 2.92) | 1.44 (0.64, 2.63) | 2.26 (1.40, 3.13) | 0.103 |
| Anti-PF4/heparin antibody ELISA positive | 46/47 (97.9) | 26/27 (96.3) | 19/19 (100.0) | 1.000 |
| Functional HIT Assay (n = 21) | ||||
| Platelet activation assay | 19/21 (90.5) | 9/10 (90.0) | 9/10 (90.0) | 1.000 |
Values are given as n/N (%) or median (IQR).
IQR, interquartile range; ELISA, enzyme-linked immunosorbent assay; HIT, heparin-induced thrombocytopenia; OD, optical density; FEU, fibrinogen equivalent unit.
One patient had an unknown outcome.
PT (prothrombin time)/PT (s) normal range: 10.0–12.0 s/PT INR normal range: 0.9–1.1.
aPTT (activated partial thromboplastin time)/aPTT (s) normal range: 25.0–35.0 s/aPTT ratio normal range: 0.8–1.2.
Forty-seven patients in our study underwent immunological testing for HIT antibodies; 46 (97.9%) had positive HIT antibody ELISA (enzyme-linked immunosorbent assay) tests with a median optical density (OD) of 2.16. Nineteen out of 21 (90.5%) patients who tested for functional PF4-dependent platelet activation assays had positive results.
Manifestations of thrombotic and haemorrhagic events
Sixty-one (95.3%) patients were identified with at least one thrombotic event (Table 2). More than one-third (35.9%) of these patients had two or more sites of thrombosis. The most common site of thrombosis was the brain (68.8%), with CVT in 59.4% of patients with thrombosis, the middle cerebral artery thrombosis in 7.8%, and other arterial cerebral ischaemic attack in 3.1%. Thirteen patients (20.3%) had pulmonary embolism, and one patient (1.6%) had pulmonary artery thrombosis. Gastrointestinal involvement was also common (25.0%). Other sites of thrombosis included deep vein (4.7%), internal jugular vein (4.7%), and inferior vena cava (3.1%) thrombosis.
Table 2.
Thrombosis and haemorrhage of patients with VITT after ChAdOx1 nCoV-19 vaccination according to outcom
| Thrombosis/haemorrhage | Total patients (n = 64a) | Survivors (n = 40) | Non-survivors (n = 23) | P-value |
|---|---|---|---|---|
| Patients with thrombosis | ||||
| Presence of thrombosis | 61/64 (95.3%) | 38/40 (95.0%) | 22/23 (95.7%) | 1.000 |
| Two or more sites of thrombosis | 23/64 (35.9%) | 13/40 (32.5%) | 10/23 (43.5%) | 0.424 |
| Thrombosis sites | ||||
| Brain | 44/64 (68.8%) | 24/40 (60.0%) | 19/23 (82.6%) | 0.092 |
| Cerebral venous thrombosis | 38/64 (59.4%) | 19/40 (47.5%) | 18/23 (78.3%) | 0.020 |
| Acute middle cerebral artery thrombosis | 5/64 (7.8%) | 3/40 (7.5%) | 2/23 (8.7%) | 1.000 |
| Arterial cerebral ischaemic attack | 2/64 (3.1%) | 2/40 (5.0%) | 0/23 (0.0%) | 0.529 |
| Heart | 3/64 (4.7%) | 2/40 (5.0%) | 1/23 (4.3%) | 1.000 |
| Myocardial infarction | 1/64 (1.6%) | 0/40 (0.0%) | 1/23 (4.3%) | 0.365 |
| Intraventricular | 2/64 (3.1%) | 2/40 (5.0%) | 0/23 (0.0%) | 0.529 |
| Pulmonary system | 16/64 (25.0%) | 13/40 (32.5%) | 3/23 (13.0%) | 0.133 |
| Pulmonary embolism | 13/64 (20.3%) | 11/40 (27.5%) | 2/23 (8.7%) | 0.108 |
| Pulmonary artery | 1/64 (1.6%) | 1/40 (2.5%) | 0/23 (0.0%) | 1.000 |
| Not specified | 2/64 (3.1%) | 1/40 (2.5%) | 1/23 (4.3$) | 1.000 |
| Gastrointestinal system | 16/64 (25.0%) | 9/40 (22.5%) | 7/23 (30.4%) | 0.554 |
| Medium to large sized vessels | 12/64 (18.8%) | 10/40 (25.0%) | 2/23 (8.7%) | 0.183 |
| Deep vein thrombosis | 3/64 (4.7%) | 3/40 (7.5%) | 0/23 (0.0%) | 0.293 |
| Acute aortic thrombosis | 2/64 (3.1%) | 0/40 (0.0%) | 2/23 (8.7%) | 0.130 |
| Aortoiliac | 7/64 (10.9%) | 6/40 (15.0%) | 1/23 (4.3%) | 0.407 |
| Internal jugular vein thrombosis | 3/64 (4.7%) | 3/40 (7.5%) | 0/23 (0.0%) | 0.293 |
| Inferior vena cava thrombosis | 2/64 (3.1%) | 2/40 (5.0%) | 0/23 (0.0%) | 0.529 |
| Others | 7/64 (10.9%) | 5/40 (12.5%) | 2/23 (8.7%) | 1.000 |
| Patients with haemorrhage | ||||
| Presence of haemorrhage | 21/64 (32.8%) | 9/40 (22.5%) | 12/23 (52.2%) | 0.026 |
| Haemorrhage sites | ||||
| Intracerebral haemorrhage | 12/64 (18.8%) | 4/40 (10.0%) | 8/23 (34.8%) | 0.022 |
| Subarachnoid haemorrhage | 3/64 (4.7%) | 1/40 (2.5%) | 2/23 (8.7%) | 0.548 |
| Adrenal haemorrhage | 3/64 (4.7%) | 2/40 (5.0%) | 1/23 (4.3%) | 1.000 |
| Not specified | 3/64 (4.7%) | 2/40 (5.0%) | 1/23 (4.3%) | 1.000 |
One patient had an unknown outcome.
Twenty-one patients (32.8%) presented with haemorrhage. Among patients with haemorrhage, 57.1% had intracerebral haemorrhage (ICH), followed by subarachnoid haemorrhage (SAH) and adrenal haemorrhage, each at 14.3%. In three cases, the location of haemorrhage was not specified.
Treatment approaches
The treatment modalities used in patients with TTS are shown in Table 3. Among the 39 patients for whom we had information about treatment, 26 (66.7%) received heparin products; unfractionated heparin (UFH) was administered in 25.6% and low molecular weight heparin (LMWH) was used in 28.2%. Steroids were used in 31.7% of patients and intravenous immunoglobulin (IVIG) was used in 43.9% of patients. Platelet transfusions were administered in 19.5% of cases, and red blood cell (RBC) transfusions were required in one patients (2.4%). Non-heparin anticoagulants—a direct oral anticoagulant (DOAC) or a direct thrombin inhibitor—were used in 14 (34.1%) patients, 6 (14.6%) of whom used DOACs, 7 (17.1%) of whom used direct thrombin inhibitors, and 1 of whom used an unspecified non-heparin anticoagulant. Twelve patients (29.3%) required surgery.
Table 3.
Treatment modalities in patients with VITT after ChAdOx1 nCoV-19 vaccination according to outcome
| Treatment | Total patients (n = 64a) | Survivors (n = 40) | Non-survivors (n = 23) | P-value |
|---|---|---|---|---|
| Treatment received | ||||
| Heparins | 26/39 (66.7%) | 17/23 (73.9%) | 9/16 (56.3%) | 0.312 |
| Unfractionated heparin | 10/39 (25.6%) | 6/23 (26.1%) | 4/16 (25.0%) | 1.000 |
| Low molecular weight heparin | 11/39 (28.2%) | 7/23 (30.4%) | 4/16 (25.0%) | 1.000 |
| Fondaparinux | 6/39 (15.4%) | 5/23 (21.7%) | 1/16 (6.3%) | 0.370 |
| Steroids | 13/41 (31.7%) | 9/24 (37.5%) | 4/16 (25.0%) | 0.503 |
| Prednisolone | 5/41 (12.2%) | 4/24 (16.7%) | 1/16 (6.3%) | 0.631 |
| Methylprednisolone | 6/41 (14.6%) | 4/24 (16.7%) | 2/16 (12.5%) | 1.000 |
| Dexamethasone | 4/41 (9.8%) | 3/24 (12.5%) | 1/16 (6.3%) | 0.638 |
| Transfusion | ||||
| Intravenous immunoglobulin | 18/41 (43.9%) | 13/24 (54.2%) | 5/16 (31.3%) | 0.203 |
| Platelet | 8/41 (19.5%) | 2/24 (8.3%) | 6/16 (37.5%) | 0.042 |
| Red blood cell | 1/41 (2.4%) | 0/24 (0.0%) | 1/16 (6.3%) | 0.400 |
| Fibrinogen concentrate | 1/41 (2.4%) | 1/24 (4.2%) | 0/16 (0.0%) | 1.000 |
| Plasmapheresis | 1/41 (2.4%) | 1/24 (4.2%) | 0/16 (0.0%) | 1.000 |
| Surgery | 12/41 (29.3%) | 5/24 (20.8%) | 7/16 (43.8%) | 0.166 |
| Neurosurgery | 8/41 (19.5%) | 1/24 (4.2%) | 7/16 (43.8%) | 0.004 |
| Bowel resection | 3/41 (7.3%) | 3/24 (12.5%) | 0/16 (0.0%) | 0.262 |
| Thrombectomy | 2/41 (4.9%) | 1/24 (4.2%) | 1/16 (6.3%) | 1.000 |
| Tissue plasminogen activator | 1/41 (2.4%) | 1/24 (4.2%) | 0/16 (0.0%) | 1.000 |
| Non-heparin anticoagulants | 14/41 (34.1%) | 13/24 (54.2%) | 1/16 (6.3%) | 0.002 |
| Direct oral anticoagulant | 6/41 (14.6%) | 5/24 (20.8%) | 1/16 (6.3%) | 0.373 |
| Direct thrombin inhibitor | 7/41 (17.1%) | 7/24 (29.2%) | 0/16 (0.0%) | 0.029 |
| Eculizumab | 2/41 (4.9%) | 2/24 (8.3%) | 0/16 (0.0%) | 0.508 |
One patient had an unknown outcome.
Characteristics in patients according to mortality
Overall, 23 (35.9%) patients died, 40 (62.5%) were alive and recovering, and 1 (1.6%) had an unknown outcome. A number of clinical and laboratory markers were significantly associated with mortality (Table 4). Severe thrombocytopenia of <25 × 103/μL (P = 0.007), hypofibrinogenaemia of <150 mg/dL (P = 0.004), the presence of CVT (P = 0.020), and the presence of ICH (P = 0.022) were significantly associated with adverse outcome. Furthermore, we found that age over 60 was negatively associated with mortality (P = 0.010). Patients at or under 60 years of age were more likely to have adverse clinical characteristics, such as thrombosis in the brain, CVT, and fibrinogen levels <150 mg/dL than those aged >60 years (Supplementary material online, Figure S2).
Table 4.
Univariable analyses of demographic, clinical, laboratory findings, thrombosis, haemorrhage, and treatment in patients with VITT after ChAdOx1 nCoV-19 vaccination
| Variables | Survivors (n = 40) | Non-survivors (n = 23) | P-value |
|---|---|---|---|
| Demographics | |||
| Age | 46.00 (34.25, 61.00) | 37.50 (30.75, 52.50) | 0.241 |
| Age ≤60 years | 30/40 (75.0) | 23/23 (100.0) | 0.010 |
| Female sex | 26/36 (72.2) | 11/18 (61.1) | 0.536 |
| Time to presentationa | 10.00 (7.00, 14.00) | 10.00 (7.00, 10.25) | 0.309 |
| Clinical presentations | |||
| Systemic | 9/20 (45.0) | 6/10 (60.0) | 0.700 |
| Neurological | 16/20 (80.0) | 10/10 (100.0) | 0.272 |
| Bleeding | 1/20 (5.0) | 2/10 (20.0) | 0.251 |
| Gastrointestinal | 3/20 (15.0) | 4/10 (40.0) | 0.181 |
| Cardiopulmonary | 4/20 (20.0) | 0/10 (0.0) | 0.272 |
| Laboratory findings | |||
| Platelet count (cells/mm3) | 40 000 (26 000, 70 000) | 19 000 (13 750, 75 750) | 0.121 |
| Platelet <25 × 103/μL | 9/39 (23.1) | 13/22 (60.9) | 0.007 |
| Fibrinogen (mg/dL) | 210.00 (120.00, 345.00) | 120.00 (80.00, 140.00) | 0.003 |
| Fibrinogen <150 mg/dL | 11/31 (35.5) | 15/19 (78.9) | 0.004 |
| D-dimer/upper limit of normal range | 45.80 (16.30, 70.40) | 70.00 (32.22, 79.05) | 0.143 |
| HIT ELISA (OD) | 1.44 (0.64, 2.63) | 2.26 (1.40, 3.13) | 0.103 |
| Platelet activation assay | 9/10 (90.0) | 9/10 (90.0) | 1.000 |
| Thrombosis and haemorrhage | |||
| Presence of thrombosis | 38/40 (95.0) | 22/23 (95.7) | 1.000 |
| More than 2 sites of thrombosis | 9/40 (22.5) | 2/23 (8.7) | 0.301 |
| Cerebral venous thrombosis | 19/40 (47.5) | 18/23 (78.3) | 0.020 |
| Presence of haemorrhage | 9/40 (22.5) | 12/23 (52.2) | 0.026 |
| Intracerebral haemorrhage | 4/40 (10.0) | 8/23 (34.8) | 0.022 |
| Treatment | |||
| Heparins | 17/23 (73.9) | 9/16 (56.3) | 0.312 |
| Steroids | 9/24 (37.5) | 4/16 (25.0) | 0.503 |
| Intravenous immunoglobulin | 13/24 (54.2) | 5/16 (31.3) | 0.203 |
| Platelet transfusion | 2/24 (8.3) | 6/16 (37.5) | 0.042 |
| Neurosurgery | 1/24 (4.2) | 7/16 (43.8) | 0.004 |
| Non-heparin anticoagulants | 13/24 (54.2) | 1/16 (6.3) | 0.002 |
| Direct thrombin inhibitor | 7/24 (29.2) | 0/16 (0.0) | 0.029 |
| FAPIC score | 2.00 (1.00, 3.00) | 4.00 (3.00, 4.00) | < 0.001 |
Values are give as median (interquartile range), or n/N (%).
HIT, heparin-induced thrombocytopenia; OD, optical density; VITT, vaccine-induced immune thrombotic thrombocytopenia.
If time to admission after vaccination was not given, time to symptom onset after vaccination was used.
Regarding treatment, the administration of non-heparin anticoagulants was significantly associated with favourable outcome (P = 0.002). Specifically, all seven patients who received a direct thrombin inhibitor recovered and none died (p = 0.029), but the patients who received a direct thrombin inhibitor also had milder clinical profiles (Supplementary material online, Table S7). Platelet transfusion was also significantly associated with mortality (8.3% vs. 37.5%, P = 0.042), but in this case as well, patients who were administered platelets tended to have worse clinical profiles and risk factors such as lower platelet counts, and ICH. Seven out of eight (87.5%) patients who underwent neurosurgery died, while mortality was lower at 39.6% for those who did not receive surgery (P = 0.004).
Risk factors for mortality
According to logistic regression analyses, we found that platelet count <25 × 103/µL [odds ratio (OR) 4.815, 95% confidence interval (CI) 1.555–14.907, P = 0.006], fibrinogen levels <150 mg/dL (OR 6.818, 95% CI 1.811–25.672, P = 0.005), the presence of ICH (OR 4.800, 95% CI 1.253–18.384, P = 0.022), and the presence of CVT (OR 3.979, 95% CI 1.236–12.809, P = 0.021) were significantly associated with mortality (Supplementary material online, Table S8).
The FAPIC predictive scoring model for mortality
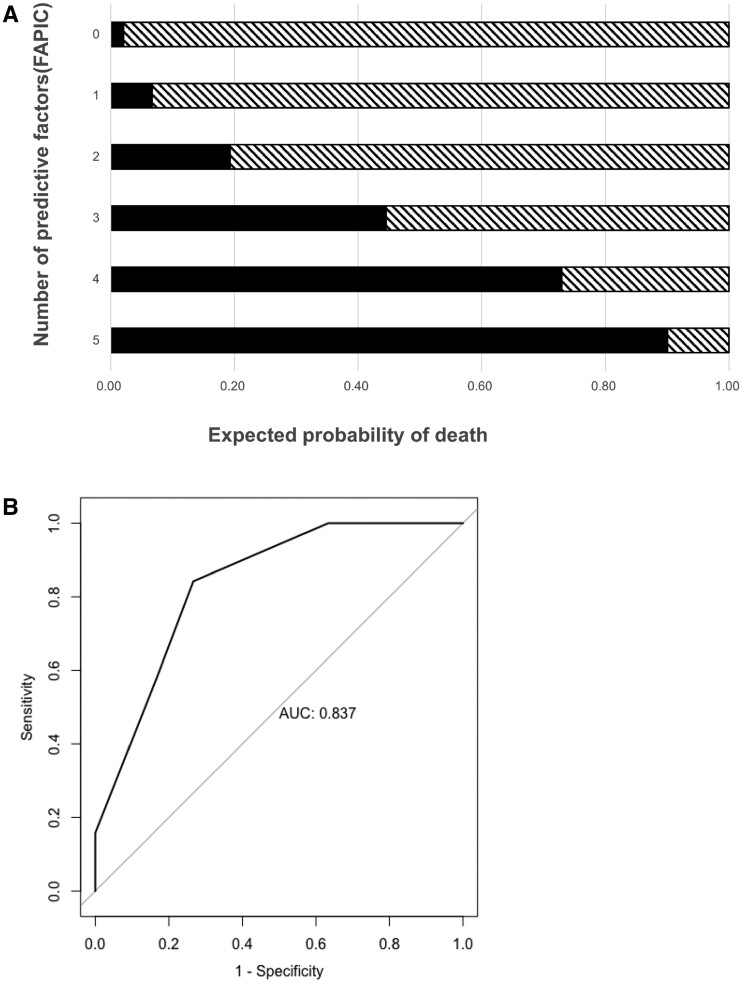
We designed a novel scoring system for mortality in TTS patients based on the predictive performance of our regression models. We included variables that were significantly associated with mortality in the univariate analyses and did not have missing values, which were age ≤60 years, platelet count <25 × 103/µL, fibrinogen <150 mg/dL, the presence of ICH, and the presence of CVT. The model was a sum of scores consisting of one point for each of these five predictors. We named this scoring system FAPIC from the components of the model: fibrinogen, age, platelet count, ICH, and CVT. The predicted mortality increased with each point increase in the FAPIC score, with expected probability of death of 2.08% with FAPIC score 0, of 6.66% with FAPIC score 1, of 19.31% with FAPIC score 2, of 44.54% with FAPIC score 3, of 72.94% with FAPIC score 4, and of 90.05% with FAPIC score 5 (Figure 1A). The calculated C-statistic for the FAPIC score was 0.837 (95% CI 0.732–0.942) (Figure 1B). The Hosmer–Lemeshow goodness of fit test yielded a test statistic of 2.857 and a P-value of 0.582, signifying a good fit between the model and the observed data.
Figure 1.
Estimated mortality in patients with TTS after ChAdOx1 nCoV-19 vaccination by FAPIC score (A) and the receiver operating characteristic (ROC) curve with the area under the curve (AUC) (B) of the FAPIC score. Variables included in the FAPIC score were: age ≤60 years, platelet count <25 × 103/µL, fibrinogen <150 mg/dL, the presence of intracerebral haemorrhage, and the presence of cerebral venous thrombosis.
Internal and external validation of the FAPIC score
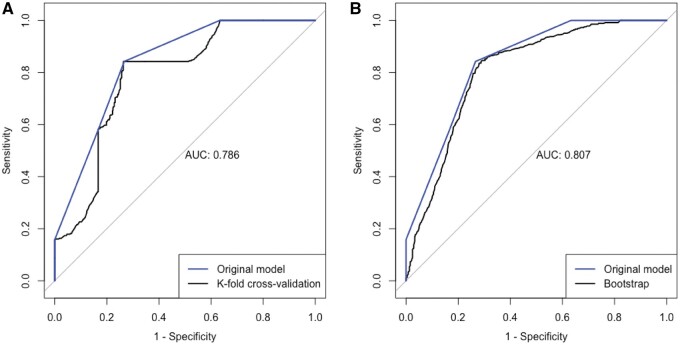
Internal validation of the FAPIC score demonstrated a good discrimination in both K-step cross-validation and bootstrapping methods. The calculated C-statistic for the FAPIC score was 0.786 (95% CI 0.757–0.814) and 0.807 (95% CI 0.787–0.827) in the K-step cross-validation and bootstrapping procedures, respectively (Figure 2).
Figure 2.
The receiver operating characteristic (ROC) curve and the area under the curve (AUC) of the FAPIC score on cross-validation (A) and bootstrapping (B).
Before externally validating the predictive performance of the FAPIC score in the Ad26.COV2.S dataset, we compared the clinical profiles of TTS after ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines, which is shown in Supplementary material online, Table S9. For the validation dataset, the risk of death increased with each point increase in the FAPIC score, with an estimated mortality of 0% with FAPIC score 0–2, of 40.0% with FAPIC score 4, and of 50.0% with FAPIC score 5. There were no patients with TTS after Ad26.COV2.S who had a FAPIC score of 5. The ROC curve is shown in Supplementary material online, Figure S3; the C-statistic was 0.771 (95% CI 0.509–1.000).
With multiple imputation, 19 observations with missing variables in the FAPIC score were added. Good discriminatory performance of the FAPIC score was replicated on the complete dataset after multiple imputation (Supplementary material online, Figure S4).
Discussion
The incidence of CVT after COVID-19 vaccination has been reported as 2.5 cases per million in 4 months, higher than 1.3 cases per million in the initially reported incidence in the general population.45 Balancing the risk of vaccine-associated adverse events and the benefits of population-wide prevention of COVID-19, many countries have opted to continue the rollout of ChAdOx1 nCoV-19 vaccinations cautiously, while some countries have halted distributions or implemented age restrictions.18,19,22–24 As massive amounts of vaccinations including the ChAdOx1 nCoV-19 vaccine are continuing to be administered at the time of writing,6 a rapid, systematic assessment of the clinical manifestations, treatment, and outcomes of TTS is crucial.
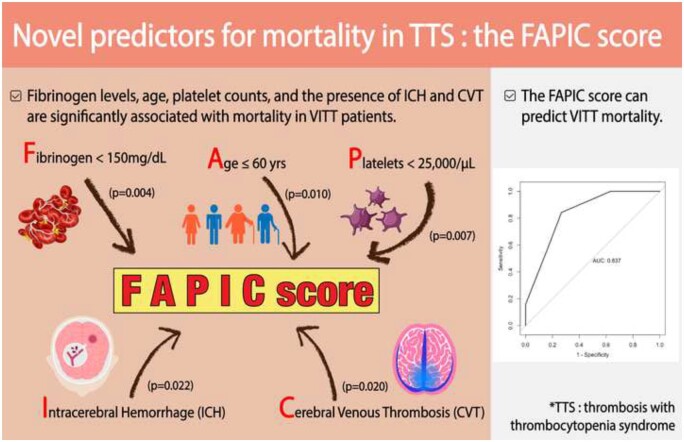
This systematic review summarizes 64 cases of TTS after ChAdOx1 nCoV-19 vaccination to analyse the clinical presentation, treatment modalities, outcomes, and prognostic factors associated with adverse outcomes (Graphical Abstract). Previously, the clinical picture of TTS has been compared with autoimmune HIT;10,12 likewise, the patients in this systematic review had a similar clinical presentation to HIT without previous exposure to heparin products. Notably, in our study, 73.3% of patients whose symptoms were reported presented with a headache at initial presentation; other neurological symptoms such as hemiparesis, visual disturbance, and hemiplegia were also common. This is concordant with the hallmark presentation of typical CVT, as subacute headache is known to be present in 90% of CVT cases.46,47 Patients could also present with a constellation of systemic, gastrointestinal, and bleeding symptoms. Furthermore, all patients had thrombocytopenia upon admission, with mean platelet count of 31 × 103/µL. Most patients (95.3%) had a thrombotic event, among which 59.4% had CVT; three patients did not present with thromboses, but with isolated haemorrhagic events. Haemorrhage was relatively common, occurring in 32.8% of patients and more than half being ICH; 8 out of 19 (80%) ICH cases were associated with CVT. This spectrum of thrombotic and haemorrhagic events shows that TTS is not only limited to CVT, but can present with varying severity and locations.
Graphical Abstract.
The FAPIC scoring model, a summary score comprising fibrinogen, age, platelet count, intracerebral haemorrhage, and cerebral venous thrombosis, can be used to predict mortality in adenoviral vaccine-associated thrombosis with thrombocytopenia syndrome. AUC, area under the curve; VITT, vaccine-induced immune thrombotic thrombocytopenia.
In most cases, patients underwent immunological testing for anti-heparin/PF4 antibodies. In our study, 97.9% of patients with TTS after ChAdOx1 nCoV-19 vaccination tested positive for anti-PF4/heparin antibodies. Most had a very high OD for HIT ELISA without having had previous exposure to heparin, similar to what is seen in autoimmune HIT.48 However, it should be noted that individual studies employed different methods for anti-heparin/PF4 antibodies, which have different diagnostic properties. The IgG ELISA tests for anti-heparin/PF4 antibodies typically have high sensitivity nearing 95–100%, but varying specificities.49–51 For example, one study reported that the Asserachrom HPIA, used by Scully et al.,11 had a 100% sensitivity and 77.8% specificity; and the LIFECODES PF4 IgG ELISA kit, used by Schultz et al.,10 demonstrated 100% sensitivity and 31.6% specificity.52 Both Asserachrom and LIFECODES anti-PF4 ELISA kits have been tested to successfully detect TTS antibodies.53
Furthermore, 19 out of 21 patients who underwent subsequent functional platelet activation assays yielded positive results as described by the original articles. Functional platelet activation assays provide more definitive, specific evidence that anti-heparin/PF4 antibodies contribute to the aberrant activation of platelets, which further support previous postulations that the mechanism of TTS may be similar to that of autoimmune HIT.10,12 However, these results must be interpreted with caution, as functional platelet assays have significant heterogeneity in their specific methodology, and the results may be subject to error or misinterpretation (Supplementary material online, Table S10–S11). Furthermore, the four studies employed different methods in evaluating platelet aggregation, namely a modified heparin-induced platelet aggregation, the multiplate method, a flow cytometry-based method, and a serotonin release assay (SRA).10–12,21,31,40,54,55 In the literature, the positive rate for functional platelet activation tests is reported to be far lower for patients with Ad26.COV.2.S-associated TTS.40 However, as all 12 patients in this study were tested with the SRA, the different properties of confirmatory tests should be considered when interpreting the results of functional platelet activation assays.
This study was the first study to analyse risk factors for mortality in TTS. Notably, the overall mortality of TTS was high at 35.9%. This may have been partially because these patients were among the initial reported cases of TTS, and many of them received heparin products—LMWH or UFH—in the early stages of presentation. One of the most significant risk factors for mortality in our study was the presence of ICH. This is consistent with the literature, as risk factors suggestive of adverse outcomes in HIT include severity of thrombocytopenia,56 and female gender has also been identified as a potential risk factor of thrombotic stroke as an outcome of HIT.57 Cerebral haemorrhage has also been identified as an adverse prognostic factor for cerebral venous sinus thrombosis.58
In addition, patients who died were more likely to have lower platelet counts, lower fibrinogen levels, ICH, and CVT. The results of the correlation analysis also indicate that platelet counts are positively associated with fibrinogen, and negatively associated with D-dimer levels, pointing to a clinical picture similar to disseminated intravascular coagulation (DIC) with thrombocytopenia, hypofibrinogenaemia, and elevated D-dimer levels, which also predisposes patients to haemorrhage. This indicates a clinical picture in which severe TTS patients progress to a DIC-like state, predisposing them to haemorrhage and thus leading to an adverse outcome. Furthermore, age above 60 was a protective factor towards survival. Patients above 60 were also less likely to have an adverse clinical profile such as CVT and low fibrinogen. This could be attributed to a less robust immune response post-vaccination due to immunosenescence,59,60 resulting in a weaker autoimmune reaction and thus a less morbid clinical course.
From these associations, we developed a novel FAPIC score to predict mortality in patients with TTS. In our dataset, we found that risk of death increased with increasing FAPIC score, with a high C-statistic of 0.837 (95% CI 0.732–0.942). When the FAPIC score was internally validated through K-step cross-validation and bootstrapping, the model was found to have good discrimination, with a C-statistic of 0.786 (95% CI 0.757–0.814) and 0.807 (95% CI 0.787–0.827), respectively. Furthermore, its predictive power was replicated on a panel of TTS patients after Ad26.COV2.S administration, showing good discrimination (C-statistic = 0.771, 95% CI 0.560–1.000).
In our study, the use of non-heparin anticoagulants—direct thrombin inhibitors, such as argatroban, or DOACs, such as rivaroxaban and apixaban—was significantly associated with a favourable outcome. In fact, 13 out of the 14 patients who were administered non-heparin anticoagulants recovered. This is in accordance with the literature on HIT which recommends limiting heparin and initiating alternative anticoagulants such as DOACs or direct thrombin inhibitors.21,61,62 The recent recommendations by the Expert Haematology Panel (EHP) and experts also suggest the use of these non-heparin-based anticoagulants in the setting of TTS.63,64 In addition, as IVIG has been utilized as a treatment adjunct in autoimmune HIT,65,66 there have been recommendations of the usage of IVIG and glucocorticoids in TTS to improve platelet counts and lower the risk of haemorrhagic transformation;64,67 in our study, although survivors had a higher likelihood of having used IVIG of 54.2% compared with 31.3%, the difference was not statistically significant. However, our results regarding treatment must be interpreted cautiously due to the small sample size and potential confounding by indication.
Previously, there has been a comparison of the clinical profiles of CVT after ChAdOx1 nCoV-19 and Ad26.COV2.S, which reported that patients who received Ad26.COV2.S tend to present with CVT later, and have a more insidious clinical course despite a higher likelihood of ICH.68 Patients with ChAdOx1 nCoV-19-associated TTS had a significantly shorter time to admission, higher rates of functional platelet assay positivity, and higher prevalence of ICH; they also tended to have higher D-dimer levels and higher prevalence of CVT with borderline significance. Other clinical characteristics were not significantly different.
A recent case series by See et al. reported all initial 12 cases of Ad26.COV2.S-associated TTS as Caucasian females aged 18–60, with additional risk factors such as obesity, hypothyroidism, and the use of combined oral contraceptives in 7 of them.40 In our panel of 64 patients, TTS affected both males and females—although females accounted for 72.2%—at varying ages of 21–71; however, three patients who died were females aged 30–55 receiving oral contraceptives. More data regarding pre-existing conditions and medication use are required to evaluate the risk of developing TTS after vaccine administration.
There are some limitations to this study. As this study was a pooled analysis of published case reports and case series, we could not directly assess the electronic medical records of the 64 patients we reviewed. Our findings should be interpreted carefully considering that the representation of clinical information in the reports summarized may have been selective and incomplete (Supplementary material online, Table S12–S13). The variables we analysed were limited to basic demographic, laboratory, and imaging findings, and the variables we included in our scoring system may reflect underlying disease progression rather than being root causes. Detailed, comprehensive review of pertinent clinical information such as comorbidities and medication history may result in more information on individuals at high risk for TTS incidence and adverse outcomes. Secondly, the sampling frame of our study was small and subject to publication bias. To mitigate this limitation, we aimed to perform an internal validation of the FAPIC score through cross-validation and bootstrapping methods, and an external validation on a distinct panel of TTS patients. More studies on national or international safety databases are also warranted to further verify the risk factors of mortality that were observed from this study. Furthermore, because of the extremely rare nature of TTS, the number of currently available cases was relatively small at 64 patients. Going forward, we expect higher statistical power and further insights from future accumulation of data. Further studies are needed to elucidate the exact pathophysiology of TTS and shed light on its clinical course; taking a step further, future investigations with more robust patient data are warranted to confirm whether the risk factors we identified play independently causal roles rather than simply being associated with mortality. Furthermore, exploration of predictors for the incidence of TTS from pre-vaccination profiles could aid clinical decision-making among available vaccines and potentially prevent the occurrence of TTS.
In conclusion, this study is the first to identify independent risk factors for mortality and propose a novel FAPIC score for predicting mortality in patients with TTS. We demonstrated that older age, severe thrombocytopenia, severe hypofibrinogenaemia, and the presence of CVT and ICH were significantly associated with adverse outcomes in TTS patients after ChAdOx1 nCoV-19 vaccination, and the sum of these factors could reliably predict mortality. Furthermore, we confirmed that the use of non-heparin anticoagulants was significantly associated with a favourable outcome, which further supports current recommendations that as soon as patients are suspected with TTS, heparin products should be halted and other forms of anticoagulation considered. The results of our study suggest that a combination of demographic, laboratory, and clinical markers may serve as predictors for mortality in TTS patients and aid identification of high-risk patients in the clinical setting.
In light of similar reports of TTS after vaccination with Ad26.COV2.S,40 and reports of thrombotic thrombocytopenia in critically ill COVID-19 patients, the precise mechanism as to how ChAdOx1 nCoV-19 vaccination gives rise to thrombotic thrombocytopenia and production of anti-PF4/heparin antibodies and whether the vaccines share a common antigenic interaction or have independent pathophysiology still remain to be elucidated. Added to the clinical severity of TTS, the sheer rarity of the disease and the paucity of available information are adding to unnecessary fear and vaccine hesitancy.69 This study has quantitatively analysed scattered evidence from clinical reports to assess risk factors and predict mortality with the largest statistical power available. We expect that our report and the FAPIC score could be utilized to evaluate TTS patients according to clinical severity, further consolidate evidence regarding better or worse outcomes, and thus ameliorate the uncertainty that still prevails regarding TTS. As evidence and experience regarding TTS are being accumulated, we expect this report to guide future management of TTS in mitigating the extremely high mortality rate in these cases, as well as inform the medical and lay community to help combat vaccine hesitancy.
Author contributions
J.H., S.H.P., S.W.L., D.K.Y., and J.I.S. designed this study. M.H.L., S.H.P., S.B.L., and J.I.S. collected the data, and J.H., S.H.P., S.W.L., D.K.Y., and J.I.S. performed the statistical analysis. J.H., S.H.P., S.W.L., D.K.Y., and J.I.S. wrote the first draft of the manuscript. All authors had full access to all the study data. All authors reviewed, wrote, and approved the final version. The corresponding authors had final responsibility for the decision to submit for publication.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: The authors disclose no financial or non-financial conflicts of interest.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Supplementary Material
References
- 1. Johns H. Coronavirus Resource Center. COVID-19 Map [Internet]. 2021. https://coronavirus.jhu.edu/map.html (28 April 2021). [Google Scholar]
- 2. Gavi C. COVID-19 Vaccine Advance Market Commitment [Internet]. 2021. https://www.gavi.org/gavi-covax-amc (28 April 2021).
- 3. Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 2021;21:e26–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Medicines Agency. COVID-19 vaccines: authorised [Internet]. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (29 April 2021).
- 5. Food and Drug Administration. COVID-19 Vaccines. FDA [Internet]. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (29 April 2021).
- 6. Our World in Data. Coronavirus (COVID-19) Vaccinations—Statistics and Research [Internet]. 2021https://ourworldindata.org/covid-vaccinations (29 April 2021).
- 7. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt A-H, Skattør TH, Tjønnfjord GE, Holme PA. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franchini M, Testa S, Pezzo M, Glingani C, Caruso B, Terenziani I, Pognani C, Bellometti SA, Castelli G. Cerebral venous thrombosis and thrombocytopenia post-COVID-19 vaccination. Thromb Res 2021;202:182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, Sztriha LK. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—a report of two UK cases. Brain Behav Immun 2021;95:514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thaler J, Ay C, Gleixner KV, Hauswirth AW, Cacioppo F, Jürgen G, Quehenberger P, Pabinger I, Knöbl P. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost 2021;19:1819–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag FG, Weißenborn K, Höglinger GU, Maasoumy B, Wedemeyer H, Ganser A. Prothrombotic immune thrombocytopenia after COVID-19 vaccine. Blood 2021;138:350–353. doi: 10.1182/blood.2021011958 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Medicines Agency. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low platelets [Internet]. 2021. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots (29 April 2021).
- 18. Medicines and Healthcare Products Regulatory Agency. UK regulator confirms that people should continue to receive the COVID-19 vaccine AstraZeneca [Internet]. 2021. https://www.gov.uk/government/news/uk-regulator-confirms-that-people-should-continue-to-receive-the-covid-19-vaccine-astrazeneca (29 April 2021).
- 19. Mahase E. AstraZeneca vaccine: blood clots are ‘extremely rare’ and benefits outweigh risks, regulators conclude. BMJ 2021;373:n931. [DOI] [PubMed] [Google Scholar]
- 20. Hunter PR. Thrombosis after covid-19 vaccination. BMJ 2021;373:n958. [DOI] [PubMed] [Google Scholar]
- 21. Greinacher A. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med 2015;373:252–261. [DOI] [PubMed] [Google Scholar]
- 22. Dyer O. Covid-19: EMA defends AstraZeneca vaccine as Germany and Canada halt rollouts [Internet]. BMJ 2021;373:n883. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low platelets [Internet]. European Medicines Agency. 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (29 April 2021).
- 24. Government of Canada HC. Health Canada provides update on the AstraZeneca and COVISHIELD COVID-19 vaccines [Internet]. 2021. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2021/75389a-eng.php (29 April 2021).
- 25. Thiagarajan K. Covid-19: India is at centre of global vaccine manufacturing, but opacity threatens public trust. BMJ 2021;372:n196. [DOI] [PubMed] [Google Scholar]
- 26. Rimmer A. Covid-19: 237m vaccine doses to be distributed worldwide over next three months. BMJ 2021;372:n631. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hocking J, Chunilal SD, Chen VM, Brighton T, Nguyen J, Tan J, Ting SB, Tran H. The first known case of vaccine-induced thrombotic thrombocytopenia in Australia. Med J Aust 2021;215:19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turi MC, Spitaleri F, Gori AM, Parruti G, Rogolino AA, Albani A, Giusti B, Agostinone L, Cesari F, Ranalli P, Pulini SD, Gioacchino G, Paganelli R, Marcucci R. A case of vaccine-induced immune thrombotic thrombocytopenia with massive artero-venous thrombosis. Blood Transfus 2021;19:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bersinger S, Lagarde K, Marlu R, Pernod G, Payen JF. using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med 2021. Jun 1; doi: 10.1097/CCM.0000000000005105 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Jones M, Boisvert A, Landry J, Petrasek PF. Limb ischemia and pulmonary artery thrombosis afer the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine: a case of vaccine-induced immune thrombotic thrombocytopenia. CMAJ 2021;193:E906–E910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, Gull D, Jäger HR, Scully MA, Werring DJ. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry 2021. May 25; doi: 10.1136/jnnp-2021-326984 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after ‘COVID-19 Vaccine AstraZeneca’ exposure. J Clin Med 2021;10:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aladdin Y, Algahtani H, Shirah B. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death following the ChAdOx1 nCoV-19 vaccine. J Stroke Cerebrovasc Dis 2021;30:105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suresh P, Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep 2021;14:e243931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guetl K, Gary T, Raggam RB, Schmid J, Wölfler A, Brodmann M. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia treated with immunoglobulin and argatroban. Lancet 2021;397:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muster V, Gary T, Raggam RB, Wölfler A, Brodmann M. Pulmonary embolism and thrombocytopenia following ChAdOx1 vaccination. Lancet 2021;397:1842. [DOI] [PubMed] [Google Scholar]
- 38. Xie C, Vincent L, Chadwick A, Peschl H. COVID-19 vaccine induced prothrombotic immune thrombocytopenia. Eur Heart J 2021. May 5; doi: 10.1093/eurheartj/ehab237 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas A-M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost 2021;19:1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, Durbin AP, Edwards K, Miller E, Harrington TA, Mba-Jonas A, Nair N, Nguyen DT, Talaat KR, Urrutia VC, Walker SC, Creech CB, Clark TA, DeStefano F, Broder KR. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021;325:2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muir K-L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021;384:1964–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abou-Ismail MY, Moser KA, Smock KJ, Lim MY. Vaccine-induced thrombotic thrombocytopenia following Ad26.COV2.S vaccine in a man presenting as acute venous thromboembolism. Am J Hematol 2021;96:E346–E349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dhoot R, Kansal A, Handran C, Haykal T, Ronald J, Kappus M, Arepally GM, Graham M, Strouse JJ. Thrombocytopenia and splanchnic thrombosis after Ad26.COV2.S vaccination successfully treated with transjugular intrahepatic portosystemic shunting and thrombectomy. Am J Hematol 2021;96:1180–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. George G, Friedman KD, Curtis BR, Lind SE. Successful treatment of thrombotic thrombocytopenia with cerebral sinus venous thrombosis following Ad26.COV2.S vaccination. Am J Hematol 2021;96:E301–E303. [DOI] [PubMed] [Google Scholar]
- 45. de Simone G, Stranges S, Gentile I. Incidence of cerebral venous thrombosis and COVID-19 vaccination: possible causale effect or just chance? Eur Heart J Cardiovasc Pharmacother 2021;7:e77–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saposnik G, Barinagarrementeria F, Brown R, Bushnell C, Cucchiara B, Cushman M, deVeber G, Ferro J, Tsai F, American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis. Stroke 2011;42:1158–1192. [DOI] [PubMed] [Google Scholar]
- 47. American Heart Association. CVST and blood clots potentially related to the J&J COVID-19 vaccine: know the symptoms [Internet]. 2021. https://newsroom.heart.org/news/cvst-and-blood-clots-potentially-related-to-the-j-j-covid-19-vaccine-know-the-symptoms (2 May 2021).
- 48. Warkentin TE, Basciano PA, Knopman J, Bernstein RA. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood 2014;123:3651–3654. [DOI] [PubMed] [Google Scholar]
- 49. Aster RH. Improving specificity in HIT testing. Blood 2010;116:1632–1633. [DOI] [PubMed] [Google Scholar]
- 50. Warkentin TE, Sheppard J-AI. Testing for heparin-induced thrombocytopenia antibodies. Transfus Med Rev 2006;20:259–272. [DOI] [PubMed] [Google Scholar]
- 51. Warkentin TE. Laboratory diagnosis of heparin-induced thrombocytopenia. Int J Lab Hematol 2019;41 Suppl 1:15–25. [DOI] [PubMed] [Google Scholar]
- 52. Mardovina T, Chromczak JG, Riley PW. Comparison of two different ELISA methods for heparin-induced thrombocytopenia (HIT) screening on an automated ELISA platform. Blood 2019;134(Suppl 1):4935. [Google Scholar]
- 53. Reilly-Stitt C, Kitchen S, Jennings I, Horner K, Jones R, Makris M, Walker ID. Anti-PF4 testing for vaccine-induced immune thrombocytopenia and thrombosis and heparin induced thrombocytopenia: results from a UK National External Quality Assessment Scheme exercise April 2021. J Thromb Haemost 2021. Jul 5; doi: 10.1111/jth.15423 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 54. Saad RA. Heparin-induced thrombocytopenia. N Engl J Med 2006;355:2598; author reply 2598–2599. [DOI] [PubMed] [Google Scholar]
- 55. Tardy B, Lecompte T, Mullier F, Vayne C, Pouplard C. Detection of platelet-activating antibodies associated with heparin-induced thrombocytopenia. J Clin Med 2020;9:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kelton JG, Hursting MJ, Heddle N, Lewis BE. Predictors of clinical outcome in patients with heparin-induced thrombocytopenia treated with direct thrombin inhibition. Blood Coagul Fibrinolysis 2008;19:471–475. [DOI] [PubMed] [Google Scholar]
- 57. LaMonte MP, Brown PM, Hursting MJ. Stroke in patients with heparin-induced thrombocytopenia and the effect of argatroban therapy. Crit Care Med 2004;32:976–980. [DOI] [PubMed] [Google Scholar]
- 58. de Bruijn SF, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry 2001;70:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lord JM. The effect of aging of the immune system on vaccination responses. Hum Vaccin Immunother 2013;9:1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol 2020;145:1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv 2018;2:3360–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J 2007;83:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pavord S, Lester W, Scully M, Hunt B, Guidance from the Expert Haematology Panel (EHP) on Covid-19 vaccine-induced immune thrombocytopenia and thrombosis (VITT). 2021. https://b-s-h.org.uk/media/19718/guidance-v20-20210528-002.pdf [DOI] [PMC free article] [PubMed]
- 64. Cines DB, Bussel JB. SARS-CoV-2 Vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021;384:2254–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Padmanabhan A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, Bryant BJ, Alperin JB, Deloughery TG, Mulvey KP, Dhakal B, Wen R, Wang D, Aster RH. IVIg for treatment of severe refractory heparin-induced thrombocytopenia. Chest 2017;152:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: a review. Expert Rev Hematol 2019;12:685–698. [DOI] [PubMed] [Google Scholar]
- 67. American Society of Hematology. Thrombosis with thrombocytopenia syndrome [Internet]. 2021. Available from: https://www.hematology.org:443/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia (2 May 2021).
- 68. Hwang J, Lee SB, Lee SW, Lee MH, Koyanagi A, Jacob L, Tizaoui K, Yon DK, Shin JI, Smith L. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J Autoimmun 2021;122:102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Elalamy I, Gerotziafas G, Alamowitch S, Laroche J-P, Van Dreden P, Ageno W, Beyer-Westendorf J, Cohen AT, Jimenez D, Brenner B, Middeldorp S, Cacoub P, Scientific Reviewer Committee. SARS-CoV-2 vaccine and thrombosis: an expert consensus on vaccine-induced immune thrombotic thrombocytopenia. Thromb Haemost 2021;121:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.