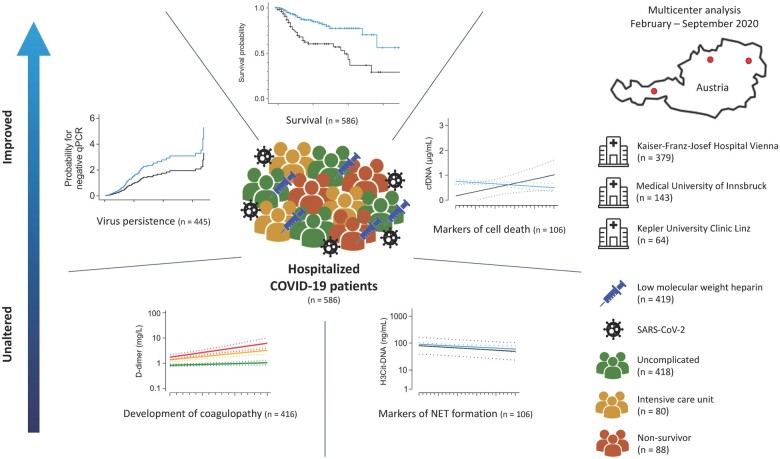
Abstract
Aims
Anticoagulation was associated with improved survival of hospitalized coronavirus disease 2019 (COVID-19) patients in large-scale studies. Yet, the development of COVID-19-associated coagulopathy (CAC) and the mechanism responsible for improved survival of anticoagulated patients with COVID-19 remain largely elusive. This investigation aimed to explore the effects of anticoagulation and low-molecular-weight heparin (LMWH) in particular on patient outcome, CAC development, thromboinflammation, cell death, and viral persistence.
Methods and results
Data of 586 hospitalized COVID-19 patients from three different regions of Austria were evaluated retrospectively. Of these, 419 (71.5%) patients received LMWH and 62 (10.5%) received non-vitamin-K oral anticoagulants (NOACs) during hospitalization. Plasma was collected at different time points in a subset of 106 patients in order to evaluate markers of thromboinflammation (H3Cit-DNA) and the cell death marker cell-free DNA (cfDNA). Use of LMWH was associated with improved survival upon multivariable Cox regression (hazard ratio = 0.561, 95% confidence interval: 0.348–0.906). Interestingly, neither LMWH nor NOAC was associated with attenuation of D-dimer increase over time, or thromboinflammation. In contrast, anticoagulation was associated with a decrease in cfDNA during hospitalization, and curtailed viral persistence was observed in patients using LMWH leading to a 4-day reduction of virus positivity upon quantitative polymerase chain reaction [13 (interquartile range: 6–24) vs. 9 (interquartile range: 5–16) days, P = 0.009].
Conclusion
Time courses of haemostatic and thromboinflammatory biomarkers were similar in patients with and without LMWH, indicating either no effects of LMWH on haemostasis or that LMWH reduced hypercoagulability to levels of patients without LMWH. Nonetheless, anticoagulation with LMWH was associated with reduced mortality, improved markers of cell death, and curtailed viral persistence, indicating potential beneficial effects of LMWH beyond haemostasis, which encourages use of LMWH in COVID-19 patients without contraindications.
Keywords: COVID-19-associated coagulopathy, Anticoagulation, Low-molecular-weight heparin, Thromboinflammation, SARS-CoV-2 viral persistence
Graphical Abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evoked an international pandemic of unforeseen global impact. While the rapid development of effective vaccines provides hope for the management of the pandemic, availability as well as the mutational burden require further research on patient care in COVID-19.1 In particular, the hypercoagulatory state induced by SARS-CoV-2 and associated thrombotic complications seen in COVID-19 represent crucial complications in hospitalized patients impacting disease course and survival.2,3 COVID-19-associated coagulopathy (CAC), characterized by a slight reduction in platelet counts accompanied by increased levels of D-dimer and a prolongation in prothrombin time (PT), has emerged as a novel type of coagulopathy distinctly different from disseminated intravascular and sepsis-induced coagulopathy.4 Hence, specific anticoagulation in COVID-19 is intensively discussed,5 and retrospective analyses revealed that prophylactic and therapeutic anticoagulations are associated with improved survival.6,7 Intriguingly, use of low-molecular-weight heparin (LMWH) in COVID-19 showed no impact of this drug on in vivo activation of coagulation or fibrinolysis.8,9 Thus, clinical evidence for the mechanism of action of anticoagulants and improved survival in COVID-19 is still missing.
Neutrophil extracellular traps (NETs), caused by decondensation of nuclear DNA from neutrophils, are associated with disease progression in COVID-19.10 NET formation is strongly linked to the presence of thromboinflammation and represents an interface between hyperinflammation and hypercoagulatory loop seen in COVID-19. Thereby, NET formation was hypothesized to be a crucial feature of CAC.
Within this observational study, we report on a multicentre cohort of hospitalized COVID-19 patients in Austria. We evaluated the predictive potential of previously reported haemostatic biomarkers for mortality and investigated markers of thromboinflammation and cell death in these patients. Further, we explored the relationship between LMWH intake and potential underlying mechanisms responsible for the observed improved outcome of COVID-19 patients receiving anticoagulation by analysing their impact on thromboinflammation, cell death, and duration of viral persistence.
2. Methods
2.1 Patients
In this retrospective multicentre study, clinical data from COVID-19 patients (n = 586) admitted to three different large-scale treatment centres in Austria (Kaiser Franz Josef Hospital, Vienna, Medical University of Innsbruck, Kepler University Hospital, Linz) between 14 February 2020 and 18 September 2020 were analysed. Patient demographics including comorbidities, use of anticoagulation, and additional medication relevant for COVID-19 treatment were recorded. Routine laboratory analysis was performed every second day. Nasopharyngeal swabs and quantitative polymerase chain reaction (qPCR) for SARS-CoV-2 were performed according to the Charité protocol11 at multiple time points (every other day excluding weekends) in order to evaluate viral persistence. Of note, no patient was using heparin-containing nasal sprays or similar compounds that might affect qPCR. In a subset of 106 patients hospitalized at Kaiser Franz Josef Hospital, Vienna, additional blood withdrawals and plasma preparation for evaluation of pathophysiological markers in circulation [i.e., citrullinated histone 3 DNA complexes (H3Cit-DNA), cell-free DNA (cfDNA)] were performed at baseline and every second day or third day in case of a weekend in between for up to four blood withdrawals (for more detail, refer to Supplementary material online, Methods and Supplementary material online, Table S1). Disease severity according to the World Health Organization (WHO) classification was assessed at admission (www.who.int; Clinical management of COVID-19 Interim guidance 27 May 2020). Accordingly, patients were classified as mild, moderate, severe, or critical based on clinical evaluation and disease dynamic within the first week after admission to hospital. Patients without COVID-19 symptoms at admission were added to the cohort of mild patients. Outcome data were available for all patients at the time of analysis. The study was conducted in accordance with the Declaration of Helsinki. The recovery of data at Kaiser Franz Josef Hospital in Vienna was part of the randomized controlled ACOVACT study (ClinicalTrials.gov: NCT04351724) approved by the local ethics committee (EK1315/2020), which aims to compare the effect of different antiviral agents and adjunctive treatments on outcome of hospitalized COVID-19 patients. Data gathered at the remaining two centres were prospectively evaluated and retrospectively analysed in this investigation. Accordingly, the study was approved by the ethics committee of the Medical University of Innsbruck (EK1167/2020) and the Kepler University Hospital Linz (EK1085/2020). Patients in the ACOVACT study gave written informed consent prior to study inclusion, while patient consent was waved for all remaining participants.
2.2 Statistical analysis
A detailed description of statistical analyses is found in the Supplementary material online. Briefly, patient demographics and laboratory parameters on admission were compared between survivors and non-survivors using Mann–Whitney U test, χ2, or Fisher’s exact test for metric or nominal and ordinal variables, respectively. Missing data were evaluated and approached using multiple imputations, as described in the Supplementary material online, Methods section.
The binary outcome death or survival was predicted using univariable and multivariable logistic regression models. Comparison of prognostic values was undertaken using receiver operating characteristics analyses. The time course of D-dimer levels during hospitalization was explored using a mixed model approach, allowing estimation of differences in D-dimer dynamic between evaluated groups. Similarly, the mixed model approach was fit for markers of NET formation and cell death. For a more detailed description of the mixed model approach, refer to the Supplementary material online, Methods section. For survival analyses, multivariable Cox proportional hazard regression models were applied. Likewise, estimation of viral persistence over time and comparison between two groups was achieved using multivariable Cox regression. It has to be mentioned that unbalanced distribution of some confounders might render statistical models unstable. Thus, the results have to be interpreted with prudence, and observed effects need to be validated in interventional trials.
All statistical analyses were performed with IBM SPSS statistics 26, and graphs were generated with GraphPad Prism 8.4. Due to the exploratory character of all analyses, no adjustment for multiple testing was performed, and results have to be interpreted accordingly. Only two-sided tests were used, and P-values ≤0.05 were considered statistically significant.
3. Results
3.1 Patient demographics
In total, 586 consecutive patients were retrospectively analysed in this study (Kaiser Franz Josef Hospital, Vienna: n = 379, Medical University of Innsbruck: n = 143, Johannes Kepler University Hospital Linz: n = 64). Patient demographics are given inTables 1 and2, and cohort comparison can be found in Supplementary material online, Table S2. Median age at admission was 64 years [interquartile range (IQR) 49–77], and 60.2% of patients were male. Incidence of complicated hospitalization and fatal outcome increased with higher age (Figure 1A). Disease severity at admission was indicative for outcome (Figure 1B). Intensive care unit (ICU) treatment was required in 111 patients (18.9%) and 66 patients (11.3%) required invasive ventilation. Overall, 88 patients (15.0%) died during their hospital stay. The main cause of death in this cohort was respiratory failure in 49 patients (55.7%), followed by multiorgan failure (18 patients, 20.5%), cardiac decompensation (8 patients, 9.1%), sepsis (7 patients, 8.0%), and thromboembolic complications (3 patients, 3.4%). Cause of death is unknown in three patients (3.4%). Missing data are presented in Supplementary material online, Table S3a and b, and frequency of missing data was compared between survivors and non-survivors showing a higher proportion of missing values for vital signs in non-survivors (Supplementary material online, Table S3a). However, missing data for routine laboratory results were distributed equally between both groups (Supplementary material online, Table S3b). In order to account for missing data in the comparison of survivors and non-survivors, multiple imputation was conducted, which is a statistical state-of-the-art method to account for missing data.12 Respective results for each variable in 20 data sets are shown in Supplementary material online, Figure S1.
Table 1.
Patient demographics
| Parameter | Missing data | Entire cohort | Survivors | Non-survivors | P-value |
|---|---|---|---|---|---|
| (N = 586) | (N = 498) | (N = 88) | |||
| N | N (%) | N (%) | N (%) | ||
| Median (IQR) | Median (IQR) | Median (IQR) | |||
| Sex | 0 | 0.157 | |||
| Female | 233 (39.8%) | 204 (41.0%) | 29 (33.0%) | ||
| Male | 353 (60.2%) | 294 (59.0%) | 59 (67.0%) | ||
| Age (years) | 0 | 64 (49–77) | 61 (47–74) | 81 (75–86) | <0.001 |
| Comorbidities | |||||
| Current smoker | 117 | 34 (5.8%) | 31 (6.2%) | 3 (3.4%) | 0.472 |
| Obesity (BMI > 25) | 69 | 260 (44.4%) | 233 (46.8%) | 27 (30.7%) | 0.383 |
| Diabetes type II | 1 | 119 (20.3%) | 92 (18.5%) | 27 (30.7%) | 0.007 |
| Hypertension | 16 | 285 (48.6%) | 223 (44.8%) | 62 (70.5%) | <0.001 |
| Cardiovascular disease (any) | 23 | 151 (25.8%) | 100 (20.1%) | 51 (58.0%) | <0.001 |
| Coronary heart disease | 25 | 77 (13.1%) | 53 (10.6%) | 24 (27.3%) | <0.001 |
| Chronic heart failure | 2 | 37 (6.3%) | 23 (4.6%) | 14 (15.9%) | <0.001 |
| Atrial fibrillation | 1 | 76 (13.0%) | 46 (9.2%) | 30 (34.1%) | <0.001 |
| Peripheral arterial disease | 1 | 25 (4.3%) | 15 (3.0%) | 10 (11.4%) | 0.002* |
| Chronic obstructive pulmonary disease | 23 | 47 (8.0%) | 34 (6.8%) | 13 (14.8%) | 0.009 |
| Asthma | 1 | 26 (4.4%) | 23 (4.6%) | 3 (3.4%) | 0.783* |
| Hypo-/hyperthyroidism | 1 | 59 (10.1%) | 49 (9.8%) | 10 (11.4%) | 0.636 |
| Chronic renal insufficiency | 1 | 65 (11.1%) | 37 (7.4%) | 28 (31.8%) | <0.001 |
| Chronic liver disease | 2 | 29 (4.9%) | 25 (5.0%) | 4 (4.5%) | 0.884 |
| Malignancy | 1 | 59 (10.1%) | 42 (8.4%) | 17 (19.3%) | 0.002 |
| Symptoms at admission | |||||
| Asymptomatic | 1 | 39 (6.7%) | 37 (7.4%) | 2 (2.3%) | 0.077 |
| Fatigue | 3 | 299 (51.0%) | 253 (50.8%) | 46 (52.3%) | 0.658 |
| Cough | 5 | 384 (65.5%) | 339 (68.1%) | 45 (51.1%) | 0.006 |
| Fever | 3 | 353 (60.2%) | 294 (59.0%) | 59 (67.0%) | 0.098 |
| Requirement of oxygen | 12 | 238 (40.6%) | 180 (36.1%) | 58 (65.9%) | <0.001 |
| Dyspnoea | 9 | 233 (39.8%) | 194 (39.0%) | 39 (44.3%) | 0.222 |
| Diarrhoea | 4 | 92 (15.7%) | 82 (16.5%) | 10 (11.4%) | 0.250 |
| Sore throat | 5 | 53 (9.0%) | 52 (10.4%) | 1 (1.1%) | 0.005 |
| Nausea or vomiting | 4 | 70 (11.9%) | 61 (12.2%) | 9 (10.2%) | 0.629 |
| Vital signs at admission | |||||
| Temperature (°C) | 145 | 36.7 (36.3–37.8) | 36.7 (36.3–37.8) | 36.7 (36.3–37.8) | 0.996 |
| Pulse rate (beats per minute) | 109 | 85 (75–97) | 85 (75–97) | 81 (71–93) | 0.165 |
| Systolic arterial pressure (mmHg) | 103 | 130 (120–140) | 130 (120–140) | 130 (120–145) | 0.501 |
| Diastolic arterial pressure (mmHg) | 105 | 80 (70–85) | 80 (70–85) | 80 (70–81) | 0.517 |
| Respiratory rate (b.p.m.) | 256 | 20 (16–24) | 20 (16–24) | 20 (18–26) | 0.010 |
| SpO2 at ambient air (%) | 113 | 95 (93–97) | 96 (94–97) | 94 (92–95) | <0.001 |
| COVID-19 classification at admissiona | 0 | <0.001 | |||
| Mild | 126 (21.5%) | 122 (24.5%) | 4 (4.5%) | ||
| Moderate | 240 (41.0%) | 212 (42.6%) | 28 (31.8%) | ||
| Severe | 165 (28.2%) | 135 (27.1%) | 30 (34.1%) | ||
| Critical | 55 (9.4%) | 29 (5.8%) | 26 (29.5%) | ||
| Anticoagulation | |||||
| LMWH | 4 | 419 (71.5%) | 375 (75.6%) | 44 (51.2%) | <0.001 |
| NOAC | 0 | 62 (10.6%) | 53 (10.6%) | 9 (10.2%) | 0.907 |
| Additional treatment | |||||
| Corticosteroids | 1 | 165 (28.2%) | 144 (29.0%) | 21 (23.9%) | 0.326 |
| Remdesivir/favipiravir | 0 | 77 (13.1%) | 58 (11.6%) | 19 (21.6%) | 0.011 |
| Lopinavir/ritonavir | 0 | 85 (14.5%) | 78 (15.7%) | 7 (8.0%) | 0.058 |
| Camostat | 0 | 37 (6.3%) | 37 (7.4%) | 0 (0.0%) | 0.008 |
| Clinical characteristics | |||||
| Total hospitalization (days) | 0 | 10 (6–17) | 10 (6–18) | 9 (6–14) | 0.116 |
| Admission to ICU | 0 | 111 (18.9%) | 80 (16.1%) | 31 (35.2%) | <0.001 |
| Invasive ventilation | 0 | 66 (11.3%) | 44 (8.8%) | 22 (25.0%) | <0.001 |
For detailed information on missing data, refer to Supplementary material online, Table S3. Bold values indicate statistically significant results.
BMI, body mass index; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range; mmHg, millimetre mercury; SpO2, peripheral capillary oxygen saturation.
COVID-19 classification was performed according to the guidelines issued by the WHO in mild (fever <38°C, no dyspnoea, no pneumonia), moderate (fever, respiratory symptoms, pneumonia), severe (respiratory distress with respiratory rate ≥30 b.p.m., SpO2 < 93% at rest), and critical (respiratory failure with requirement of mechanical ventilation, requirement of ICU).
Fisher’s exact test.
Table 2.
Laboratory parameters at admission
| Parameter | Missing data | Entire cohort | Survivors | Non-survivors | P-value |
|---|---|---|---|---|---|
| (N = 586) | (N = 498) | (N = 88) | |||
| N | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Haemoglobin (g/dL) | 173 | 13.6 | 13.7 | 12.8 | 0.002 |
| (12.3–14.8) | (12.5–14.8) | (11.2–14.2) | |||
| Red blood cell count (×1012/L) | 139 | 4.54 | 4.57 | 4.21 | 0.001 |
| (4.09–4.96) | (4.14–4.99) | (3.63–4.77) | |||
| Platelet count (×109/L) | 159 | 186 | 192 | 171 | 0.045 |
| (150–247) | (153–251) | (135–222) | |||
| Leucocyte count (×109/L) | 159 | 5.7 | 5.5 | 6.5 | 0.003 |
| (4.3–7.9) | (4.2–7.7) | (4.6–10.5) | |||
| Lymphocyte count (×109/L) | 144 | 1.15 | 1.21 | 0.87 | 0.001 |
| (0.74–9.25) | (0.78–11.55) | (0.65–3.31) | |||
| C-reactive protein (mg/L) | 162 | 50.65 | 48.2 | 84.0 | <0.001 |
| (20.18–95.93) | (16.8–89.4) | (34.0–169.5) | |||
| D-dimer (mg/L) | 366 | 0.79 | 0.70 | 1.55 | <0.001 |
| (0.52–1.56) | (0.48–1.29) | (0.89–2.04) | |||
| Prothrombin time (%) | 229 | 101 | 103 | 93 | <0.001 |
| (88–110) | (90–111) | (68–100) | |||
| International normalized ratio | 274 | 1.00 | 1.00 | 1.06 | <0.001 |
| (1.00–1.07) | (1.00–1.05) | (1.00–1.10) | |||
| Activated partial thromboplastin time (s) | 256 | 30.7 | 30.3 | 33.0 | 0.012 |
| (27.5–34.0) | (27.4–33.8) | (28.8–39.2) |
For detailed information on missing data, refer to Supplementary material online, Table S3. Bold values indicate statistically significant results.
BMI, body mass index; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range; mmHg, millimetre mercury; SpO2, peripheral capillary oxygen saturation.
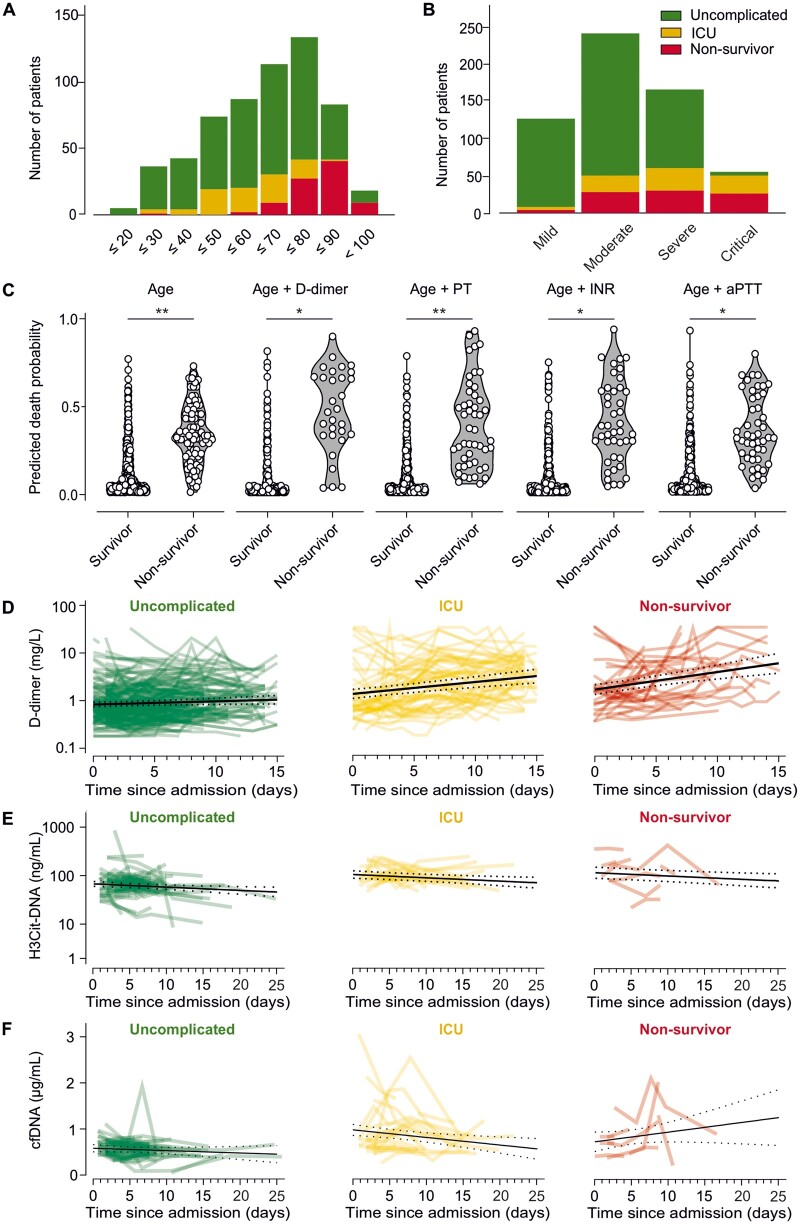
Figure 1.
Prognostic value of haematological parameters in COVID-19 patients at admission and development of CAC and thromboinflammation. (A) Total number of patients per age group and incidence of uncomplicated hospitalization, requirement of ICU, and mortality. (B) Total number of patients and incidence of uncomplicated hospitalization, requirement of ICU, and mortality according to COVID-19 classification at admission. (C) Death probability for each patient estimated by logistic regression with the predictors age, age + D-dimer, age + prothrombin time (PT), age + INR, age + aPTT. Prognostic potential was compared using receiver operating characteristic curve analyses. (D–F) Time courses of D-dimer (D), citrullinated histone 3 (H3Cit)–DNA complexes (E), and cell-free DNA (cfDNA) (F) are shown for patients with uncomplicated course of disease (green), those who were admitted to an ICU (yellow), and those who died (red). Time courses were modelled using mixed linear models, leading to respective P-values for comparison of dynamics over time between outcome cohorts. Lines with dotted error bands represent least-squared means of log-transformed D-dimer values with 95% confidence intervals (N = 586 in A–D, N = 106 in E and F, *P < 0.05, **P < 0.001).
3.2 Predictive value of haemostatic biomarkers for mortality is limited compared to older age
Age predicted fatal outcome with an odds ratio (OR) of 1.109 [95% confidence interval (95% CI): 1.082–1.137, P < 0.001; Figure 1C]. Using univariable logistic regression, a significant association with mortality was observed for D-dimer (OR = 1.058, 95% CI: 1.006–1.113, P = 0.028), PT (OR = 0.962, 95% CI: 0.948–0.977, P < 0.001), international normalized ratio (INR; OR = 2.904, 95% CI: 1.263–6.680, P = 0.012), and activated partial thrombin time (aPTT; OR = 1.034, 95% CI: 1.006–1.062, P = 0.017), showing an increased risk for mortality for patients with any deranged haemostatic biomarker. The determined predictive effects of each biomarker remained significant after addition of age to the logistic regression model (D-dimer: OR = 1.085, 95% CI: 1.015–1.158, P = 0.016; PT: OR = 0.965, 95% CI: 0.949–0.981, P < 0.001; INR: OR = 2.354, 95% CI: 1.096–5.057, P = 0.028; aPTT: OR = 1.039, 95% CI: 1.011–1.067, P = 0.006; Figure 1C). However, D-dimer and INR were not associated with mortality in the multivariable logistic regression model including age after multiple imputation (D-dimer: OR = 1.028, 95% CI: 0.972–1.087, P = 0.337; INR: OR = 1.592, 95% CI: 0.871–2.909, P = 0.130), while PT (OR = 0.979, 95% CI: 0.965–0.993, P = 0.005) and aPTT (OR = 1.028, 95% CI: 1.005–1.051, P = 0.015) remained significant in the final model. Ultimately, none of the evaluated biomarkers improved the prediction of fatal outcome based on age (age alone vs. age + D-dimer: P = 0.279, vs. age + PT: P = 0.083, vs. age + INR: P = 0.244, vs. age + aPTT: P = 0.121).
3.3 Markers of CAC and thromboinflammation are associated with disease severity in hospitalized COVID-19 patients and elevated in patients with poor outcome
In this cohort, D-dimer levels, H3Cit–DNA complexes, and cfDNA measured upon admission were associated with higher COVID-19 disease severity at admission (P = 0.046, P < 0.001, P = 0.041, respectively; Supplementary material online, Figure S2A–C). Further, patients with requirement of ICU treatment and non-survivors displayed increased D-dimer (uncomplicated vs. ICU: P = 0.014, uncomplicated vs. non-survivors: P < 0.001, ICU vs. non-survivors: P = 0.058; Supplementary material online, Figure S2D) and H3Cit–DNA complexes (uncomplicated vs. ICU: P = 0.001, uncomplicated vs. non-survivors: P = 0.013, ICU vs. non-survivors: P = 0.467; Supplementary material online, Figure S2E) at admission. A similar pattern was observed for cfDNA (uncomplicated vs. ICU: P = 0.018, uncomplicated vs. non-survivors: P = 0.151, ICU vs. non-survivors: P = 0.897; Supplementary material online, Figure S2F). There was no correlation of D-dimer levels and H3Cit–DNA complexes (r = −0.069, P = 0.531; Supplementary material online, Figure S2G), while cfDNA and D-dimer showed a weak, yet statistically significant correlation (r = 0.280, P = 0.010; Supplementary material online, Figure S2H).
3.4 CAC develops in COVID-19 patients with complicated hospitalization irrespective of disease severity upon admission
D-dimer dynamics during hospitalization were assessed and modelled using a mixed linear model approach. There was no difference in D-dimer increase between patients with mild or moderate COVID-19 and patients with severe or critical disease, as underlined by similar slopes for D-dimer increase (P = 0.440, Supplementary material online, Figure S2I–J). However, patients with severe and critical disease displayed increased D-dimer levels throughout the entire observation period (difference between D-dimer intercepts P < 0.001, Supplementary material online, Figure S2K).
Interestingly, the slopes of D-dimer showed a significant inclination in ICU patients and non-survivors whereas patients with uncomplicated disease did not display a relevant D-dimer increase during hospitalization (P < 0.001, Figure 1D, Supplementary material online, Figure S3A and B). However, there was no evidence for different slopes between patients who required ICU treatment and patients with fatal outcome (P = 0.230, Supplementary material online, Figure S3C).
H3Cit–DNA complexes in circulation decreased during hospitalization (P = 0.005; Figure 1E). There was no difference in H3Cit-DNA dynamics over time between patients with different outcomes (P = 0.123), while patients with ICU treatment and non-survivors displayed higher levels of H3Cit-DNA throughout the entire observational period when compared to patients with uncomplicated hospitalization (P < 0.001). In contrast, cfDNA dynamics varied according to outcome (P = 0.041, Figure 1F). In particular, patients with uncomplicated hospitalization and patients requiring ICU treatment showed a decrease over time, while non-survivors displayed a steady increase in cfDNA throughout hospitalization.
3.5 LMWH use is significantly associated with improved survival in hospitalized COVID-19 patients after adjusting for confounders
In order to evaluate which parameters influence hospital mortality, a multivariable Cox regression analysis was conducted (Table 3). Of note, the reported multivariable Cox regression model was carried out on the data set obtained after multiple imputations to account for missing data. Age, hypertension, cardiovascular diseases, chronic renal insufficiency, history of malignancy, requirement of oxygen at admission, oxygen saturation at admission, COVID-19 disease severity at admission, and use of LMWH were significantly associated with survival in univariable Cox regression. The multivariable model included all parameters being significant upon univariable Cox regression. Ultimately, older age, history of malignancy, and increasing COVID-19 disease severity increased the risk for mortality. In addition, LMWH remained significantly associated with reduced risk for mortality, as depicted by a hazard ratio (HR) of 0.561 (95% CI: 0.348–0.906). Additionally, a multivariable Cox regression model for adjustment to potential confounders and disparities in baseline demographics between survivors and non-survivors was fit. Here, we also included parameters that were not significant upon univariable Cox regression (Supplementary material online, Table S4). Again, use of LMWH was found to be independently associated with a decreased risk for mortality (HR = 0.539, 95% CI: 0.336–0.866). Of note, information on use of anticoagulants was missing in four patients. In total, 72.0% of patients were treated with LMWH from admission onwards, and 10.7% of patients were using non-vitamin-K oral anticoagulants (NOACs) (Supplementary material online, Table S5). Differences in baseline characteristics, disease severity, treatment, and outcome between anticoagulation subgroups were evaluated (Supplementary material online, Table S5). As use of NOAC was underrepresented in this cohort and not statistically associated with survival, the following analyses focused on LMWH. Importantly, only nine patients receiving LMWH were treated with therapeutic doses (i.e., ≥1.5 mg/kg daily), while the remaining 410 patients using LMWH received prophylactic doses.
Table 3.
Cox regression analysis in multiple-imputation data set
| Parameter | Univariable Cox regression |
Multivariable Cox regression |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Sex | 1.125 | 0.720–1.757 | 0.650 | |||
| Age (years) | 1.075 | 1.055–1.096 | <0.001 | 1.068 | 1.043–1.093 | <0.001 |
| Comorbidities | ||||||
| Current smoker | 0.735 | 0.246–2.197 | 0.581 | |||
| Obesity (BMI > 25) | 0.779 | 0.498–1.218 | 0.273 | |||
| Diabetes type II | 1.317 | 0.835–2.077 | 0.237 | |||
| Hypertension | 1.838 | 1.156–2.920 | 0.010 | 1.136 | 0.692–1.865 | 0.613 |
| Cardiovascular disease (any) | 3.493 | 2.248–5.427 | <0.001 | 1.246 | 0.711–2.183 | 0.442 |
| Chronic obstructive pulmonary disease | 1.682 | 0.935–3.024 | 0.082 | |||
| Asthma | 0.829 | 0.261–2.627 | 0.750 | |||
| Hypo-/hyperthyroidism | 1.196 | 0.617–2.319 | 0.597 | |||
| Chronic renal insufficiency | 3.466 | 2.205–5.448 | <0.001 | 1.579 | 0.937–2.662 | 0.086 |
| Chronic liver disease | 0.689 | 0.252–1.883 | 0.468 | |||
| Malignancy | 1.978 | 1.163–3.364 | 0.012 | 2.054 | 1.184–3.561 | 0.010 |
| Symptoms at admission | ||||||
| Asymptomatic | 0.401 | 0.105–1.527 | 0.180 | |||
| Fatigue | 1.054 | 0.690–1.610 | 0.806 | |||
| Cough | 0.634 | 0.414–0.972 | 0.036 | 0.850 | 0.537–1.346 | 0.489 |
| Fever | 1.324 | 0.838–2.091 | 0.229 | |||
| Requirement of oxygen | 2.077 | 1.306–3.304 | 0.002 | 1.639 | 1.001–2.682 | 0.050 |
| Dyspnoea | 1.205 | 0.788–1.842 | 0.389 | |||
| Diarrhoea | 0.583 | 0.301–1.130 | 0.110 | |||
| Sore throat | 0.185 | 0.027–1.259 | 0.085 | |||
| Nausea or vomiting | 0.897 | 0.449–1.791 | 0.758 | |||
| Vital Signs at admission | ||||||
| Temperature (°C) | 0.882 | 0.698–1.114 | 0.291 | |||
| Pulse rate (beats per minute) | 0.988 | 0.973–1.003 | 0.128 | |||
| Systolic arterial pressure (mmHg) | 0.997 | 0.986–1.008 | 0.637 | |||
| Diastolic arterial pressure (mmHg) | 0.989 | 0.968–1.010 | 0.290 | |||
| Respiratory rate (b.p.m.) | 1.006 | 0.981–1.033 | 0.633 | |||
| SpO2 at ambient air (%) | 0.933 | 0.881–0.988 | 0.017 | 1.038 | 0.957–1.125 | 0.368 |
| COVID-19 classification at admissiona | 1.654 | 1.303–2.099 | <0.001 | 1.639 | 1.234–2.176 | 0.001 |
| Clinical characteristics | ||||||
| Admission to ICU | 1.161 | 0.738–1.827 | 0.517 | |||
| Invasive ventilation | 1.196 | 0.722–1.982 | 0.487 | |||
| Anticoagulation | ||||||
| LMWH | 0.413 | 0.270–0.634 | <0.001 | 0.561 | 0.348–0.906 | 0.018 |
| NOAC | 0.750 | 0.376–1.495 | 0.413 | |||
| Additional treatment | ||||||
| Corticosteroids | 0.611 | 0.366–1.019 | 0.059 | |||
| Remdesivir/Favipiravir | 1.082 | 0.648–1.805 | 0.764 | |||
| Lopinavir/Ritonavir | 0.475 | 0.219–1.031 | 0.060 | |||
| Camostat | 0.044 | 0.001–1.829 | 0.101 | |||
BMI, body mass index; CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; LMWH, low-molecular-weight heparin; mmHg, millimetre mercury; NOAC, non-vitamin K oral anticoagulants; SpO2, peripheral capillary oxygen saturation.
COVID-19 classification was performed according to the guidelines issued by the WHO in mild (fever <38°C, no dyspnoea, no pneumonia), moderate (fever, respiratory symptoms, pneumonia), severe (respiratory distress with respiratory rate ≥30 b.p.m., SpO2 < 93% at rest), and critical (respiratory failure with requirement of mechanical ventilation, requirement of ICU).
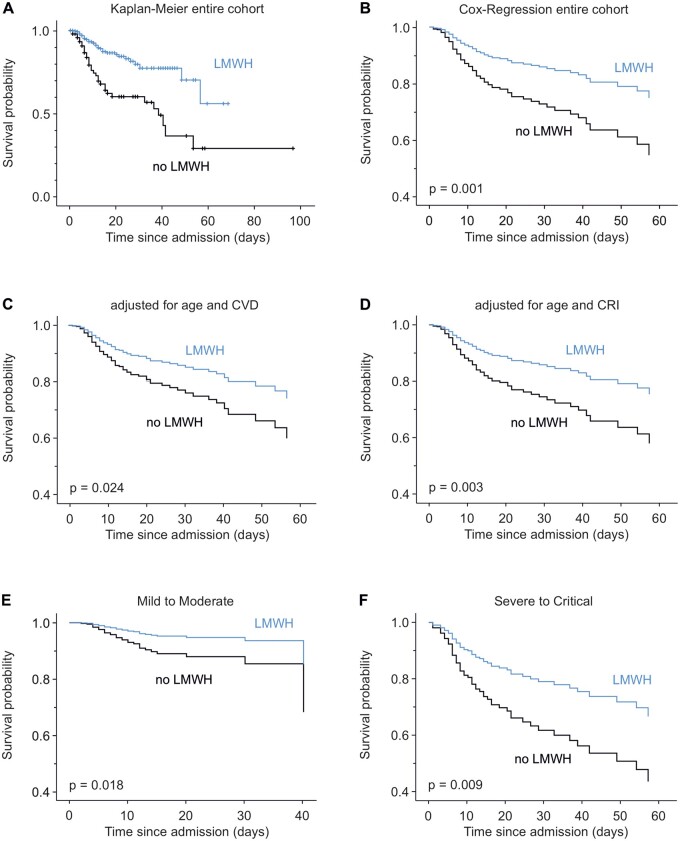
After observing an association of LMWH with survival in a data set including multiple imputations, we aimed for description of these data on the original data set. Kaplan–Meier analysis according to status of LMWH intake is shown in Figure 2A. Corresponding Kaplan–Meier curves for each evaluated centre are shown in Supplementary material online, Figure S4A–C. In order to further evaluate the effect of LMWH on hospital mortality, additional Cox regression analyses including confounders and sub-groups were conducted. LMWH users showed improved survival upon age-adjusted Cox regression analysis (HR = 0.478, 95% CI: 0.312–0.733, P = 0.001, Figure 2B). Of note, we observed an increase in the proportion of LMWH treatment throughout the observed study period (Supplementary material online, Figure S5), which is due to the inclusion of anticoagulation in guidelines for treatment of COVID-19 in July 2020. In parallel, we observed a reduction of risk for mortality in this cohort, which was evaluated using Cox regression analysis for a time-dependent variable beginning with the inclusion of the first patient (HR = 0.991, 95% CI: 0.986–0.996, P = 0.001). Importantly, the association of LMWH with improved hospital survival was not affected by the time point of study inclusion, as the interaction term of LMWH × time of inclusion was not significantly associated with survival (HR = 1.013, 95% CI: 0.999–1.028, P = 0.075), while both the main effect of LMWH use and time of inclusion remained significant in the Cox regression analysis. The association of LMWH with survival further remained significant after including presence of cardiovascular diseases (HR = 0.586, 95% CI: 0.368–0.933, P = 0.024; Figure 2C) or chronic renal insufficiency (HR = 0.518, 95% CI: 0.336–0.800, P = 0.003; Figure 2D) as confounders for LMWH use. Additionally, as patients on anticoagulants frequently used further drugs (Supplementary material online, Table S4), multivariable Cox regression including treatment with corticosteroids, remdesivir/favipiravir, lopinavir/ritonavir, or camostat was computed and revealed a robust association of LMWH with hospital survival (Supplementary material online, Figure S6). Further, LMWH use was associated with improved survival in patients with mild-to-moderate disease (HR = 0.419, 95% CI: 0.204–0.862, P = 0.018; Figure 2E), as well as in patients with severe and critical COVID-19 at admission (HR = 0.488, 95% CI: 0.284–0.839, P = 0.009; Figure 2F). Respective data for use of NOAC can be found in Supplementary material online, Figure S7.
Figure 2.
Anticoagulation with LMWH is associated with improved survival in COVID-19. (A) Kaplan–Meier curve showing survival for patients treated with LMWH (blue) and patients not using LMWH (black). Intersections represent censored patients. (B–H) Age-adjusted Cox regression plots show the effect of LMWH on survival for the entire cohort (B), after additional adjustment for CVD (C) or chronic renal insufficiency (CRI, D), as well as in patients with mild-to-moderate (E) or severe-to-critical (F) COVID-19 disease classification at admission (N = 582 in A–D, N = 365 in E, N = 217 in F).
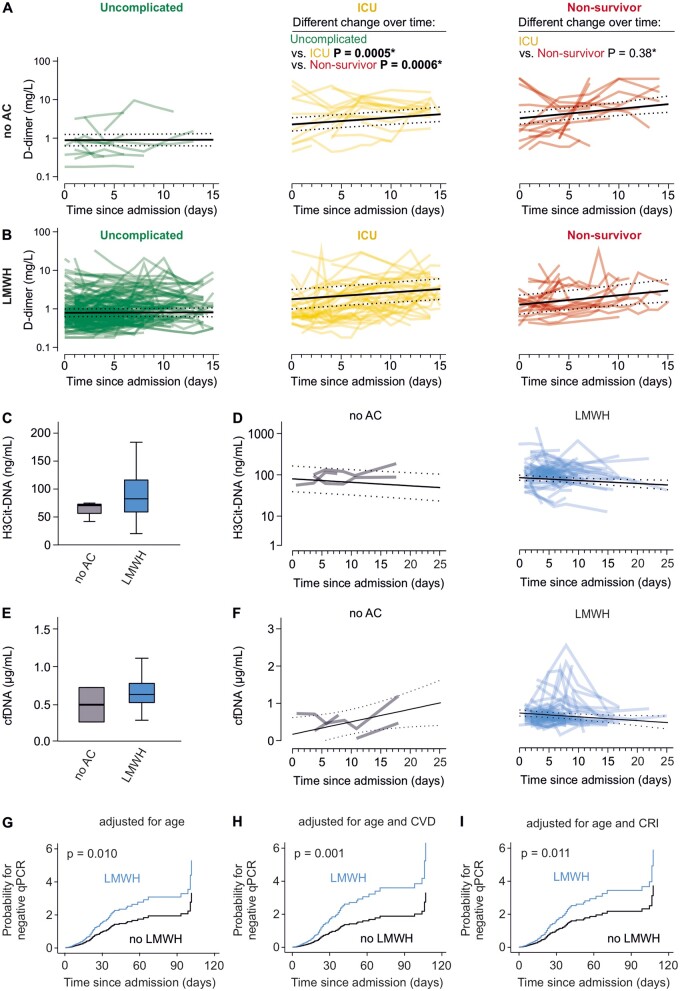
3.6 Anticoagulation is not associated with development of CAC, or reduction of markers of thromboinflammation
No difference in D-dimer dynamic was observed between patients with and without use of LMWH (Figure 3A and B). In particular, the effect of clinical course on D-dimer slopes was similar between patients treated with LMWH and patients who were not anticoagulated (P = 0.890). Likewise, use of NOAC was not associated with D-dimer increase in ICU patients and non-survivors (Supplementary material online, Figure S8). Due to the limited count of non-survivors taking NOAC, no conclusion can be made regarding these patients.
Figure 3.
Anticoagulation has no effect on development of CAC and thromboinflammation while impacting viral persistence. (A and B) D-dimer time courses for patients with uncomplicated course of disease (green), those who were admitted to an ICU (yellow), and those who died (red) are plotted, and computed regression lines resulting from the applied mixed linear model are given including 95% CI (dotted lines) for patients without use of anticoagulation (no AC, A) and for patients treated with low-molecular heparin (LMWH, B). (C) Distribution of citrullinated histone 3 (H3Cit)–DNA complexes in circulation is illustrated for patients not using AC and patients treated with LMWH using box plots. Mann–Whitney U tests were used for comparison. (D) Time course of H3Cit–DNA complexes is modelled using a mixed linear model and shown for patients not using AC (grey) and patients treated with LMWH (blue). Computed regression lines for each group are given including 95% CI (dotted lines). (E) Distribution of cell-free DNA (cfDNA) in circulation is illustrated for patients not using AC and patients treated with LMWH using box plots. Mann–Whitney U tests were used for comparison. (F) Time course of cfDNA is modelled using a mixed linear model and shown for patients not using AC (grey) and patients treated with LMWH (blue). Computed regression lines for each group are given including 95% confidence interval (dotted lines). (G–I) Age-adjusted differences in viral persistence as estimated via Cox regression are shown in hazard plots comparing the probability of negative SARS-CoV-2 qPCR results over time for patients with and without use of LMWH in the entire cohort (G) and after additional adjustment for cardiovascular diseases (CVD, E) or chronic renal insufficiency (CRI, F) (N = 101 in A, N = 419 in B, N = 106 in C–F, N = 445 in G–I).
H3Cit–DNA complexes and cfDNA levels did not differ between patients with and without LMWH use at baseline (P = 0.360 and P = 0.173, respectively; Figure 3C–F). There was no difference in H3Cit-DNA dynamics over time between LMWH users and patients without LMWH use (P = 0.104; Figure 3D). Results obtained in patients using NOAC showed a similar pattern (Supplementary material online, Figure S9A). H3Cit-DNA concentration decreased over time (P = 0.009) irrespective of anticoagulation. Interestingly, cfDNA dynamics differed according to LMWH use (P = 0.039; Figure 3F), which was comparable between patients using NOAC (Supplementary material online, Figure S9B). In detail, cfDNA concentrations increased in patients without use of LMWH or NOAC, while a decrease over time of hospitalization was observed in patients using LMWH or NOAC.
3.7 Use of LMWH is associated with curtailed persistence of SARS-CoV-2 infection
Differences in viral persistence were assessed and compared according to use of anticoagulants. In total, information on viral persistence was available in 447 patients, with 60 patients (13.5%) not receiving anticoagulants, 337 (75.7%) receiving LMWH, and 48 patients (10.8%) receiving NOAC. Patients with LMWH use displayed a significantly increased probability for earlier negativity of SARS-CoV-2 qPCR upon age-adjusted Cox regression (HR = 1.593, 95% CI: 1.119–2.267, P = 0.010; Figure 3G), leading to a reduction in median time of viral persistence (median viral persistence no LMWH = 13 [IQR: 6-24] days vs. median viral persistence LMWH = 9 [IQR: 5–16] days, P = 0.009). Of note, the association of LMWH with curtailed viral persistence remained significant after additional adjustment for corticosteroids, antiviral agents, such as remdesivir/favipiravir or lopinavir/ritonavir, and camostat (Supplementary material online, Figure S10). Conversely, NOAC intake was not associated with curtailed SARS-CoV-2 persistence upon age-adjusted Cox regression (HR = 0.679, 95% CI: 0.410–1.123, P = 0.131; Supplementary material online, Figure S11A) and tended to be associated with prolonged viral persistence. Time of viral persistence did not differ between patients with and without NOAC intake [median viral persistence no NOAC = 10 (IQR: 5–17) days vs. median viral persistence NOAC = 12 (5–22) days, P = 0.331]. The beneficial effect of LMWH use was still observed after correction for cardiovascular diseases (HR = 1.914, 95% CI: 1.295–2.828, P = 0.001; Figure 3H) and after correction for chronic renal insufficiency (HR = 1.579, 95% CI: 1.108–2.251, P = 0.011; Figure 3I). Similarly, correction for these parameters did not alter the results obtained for NOAC which still did not associate with shortened viral persistence (Supplementary material online, Figure S11B and C). Importantly, the effect of LMWH on viral persistence was comparable in patients hospitalized at each evaluated centre (Supplementary material online, Figure S12A–F).
4. Discussion
In this observational multicentre investigation of 586 patients hospitalized for COVID-19 in Austria, we were able to show that the use of LMWH was associated with curtailed viral persistence in COVID-19 leading to a reduction of virus shedding of 4 days. LMWH use war further associated with increased survival and diminished circulating markers of cell death, while no differences in biomarkers of CAC development and thromboinflammation were observed between LMWH users and non-users.
Coagulation and the development of a hypercoagulable state play a central role in COVID-19 pathophysiology.3,13–17 In fact, CAC as depicted by D-dimer increase during hospitalization majorly evolved in patients requiring ICU and in non-survivors. While the mechanisms leading to CAC are a matter of ongoing investigations, increased D-dimer levels were postulated as a surrogate marker for presence of CAC, development of thrombotic complications, and ultimately as a predictor of mortality in COVID-194,18 As previously reported, analysis of D-dimer and other haemostatic biomarkers revealed that these markers are of limited prognostic value for prediction of mortality in COVID-19.19 This indicates that more specific biomarkers are necessary to reliably predict patient outcome.
Thromboinflammation represents a central link between systemic hypercoagulability, respiratory failure, and mortality in COVID-19 patients, and NETs are frequently observed in CAC20,21 Investigation of post-mortem biopsies in COVID-19 patients showed occluding thrombi positive for citrullinated histone 3 as a specific marker for NETs not only in pulmonary tissue but also in kidney and cardiac tissue.20 In the present study, increased circulating markers of NET formation were observed in patients with higher COVID-19 disease severity at admission, which was paralleled by increased D-dimer levels, indicating that thromboinflammation might be a potential determinant of disease severity, as previously suggested.21 However, patients in the ICU and in the non-survivor sub-groups showed increased levels of H3Cit-DNA in circulation throughout the entire hospitalization. To our knowledge, this study is the first to assess markers of NET formation in a longitudinal approach, while previous studies focused on only one time point during disease onset. Intriguingly, we observed a general decrease in H3Cit-DNA regardless of outcome. This finding stands in clear contrast to the observed increase in D-dimer in patients requiring ICU treatment and non-survivors, suggesting a minor role of NET formation in CAC development or in later phases of COVID-19. In fact, there was no direct correlation of H3Cit-DNA and D-dimer in our study. Accordingly, while a role of NETs in COVID-19 pathophysiology could be validated, the contribution of thromboinflammation to CAC has to be questioned.
Importantly, we found that anticoagulation was associated with improved survival upon multivariable Cox regression analysis including age, cardiovascular diseases, chronic renal insufficiency, and concomitant treatment with other drugs affecting the course of COVID-19 as potential confounders, as well as in all evaluated sub-groups. This finding is in line with previous observational studies in COVID-19 patients, showing comparable effects of prophylactic and therapeutic anticoagulation on survival of COVID-19 patients.6,7,17 The use of anticoagulation and LMWH in particular entered the guidelines for treatment of COVID-19 in July 2020. Accordingly, we observed an increase in probability of LMWH use throughout the study period (Supplementary material online, Figure S5). Importantly, we could not evaluate an influence of the inclusion time point on the association of LMWH and improved survival, even though the biggest proportion of patients in the no-LMWH sub-group was included in the beginning of the study period. Intriguingly, in our cohort, anticoagulation with either LMWH or NOAC was not associated with altered dynamics of D-dimer indicating a minor effect of LMWH on CAC development. Alternatively, D-dimer levels in patients who received LMWH could be suppressed to levels observed in patients who did not receive LMWH, which was previously suggested by Blasi et al.9 Nonetheless, the observations made in this longitudinal approach do not allow to link improved CAC or altered haemostasis in LMWH treated COVID-19 patients to the observed improved survival. Accordingly, we aimed for investigation of potential off target effects of anticoagulation. We observed a decrease in the cell death marker cfDNA in patients using LMWH and NOAC during hospitalization. This is of specific interest, as an increase in cfDNA was only observed in non-survivors. Noticeably, a correlation of cfDNA and D-dimer could already be observed at baseline, potentially linking cell death to haemostatic derangements. Yet, these findings have to be interpreted with caution due to the observational character and the low number of patients not receiving anticoagulation in this evaluated sub-group. Nonetheless, our data are suggestive for a potential protective role of anticoagulants in COVID-19 beyond haemostasis. In this context, direct factor Xa inhibitors and heparin were shown to reduce oxidative stress and to yield anti-inflammatory properties, thereby potentially altering the inflammatory environment in vivo and affecting cell death in COVID-19.22,23 Preservation of vascular integrity via inhibition of endothelial cell heparinase or impairment of hepcidin formation and concomitant reduction of hyperferritinaemia might be other potential mechanisms by which LMWH exerts its beneficial effects.24,25
Importantly, we observed curtailed SARS-CoV-2 viral persistence upon qPCR in patients treated with LMWH, when compared to patients without anticoagulation or those using NOAC. These data provide exploratory clinical evidence compatible with a direct effect of LMWH on virus pathology, which was previously suggested in in vitro studies, where heparin was found to interfere with SARS-CoV-2 binding on ACE2 expressing cells, thereby limiting its infectivity.26 The effect on viral persistence was specific for LMWH in our analyses and increased odds for curtailed SARS-CoV-2 infection culminated in a median 4-day reduction of viral shedding. While direct interaction of LMWH with SARS-CoV-2 binding is one potential mechanism explaining the observed viral dynamics,27 the underlying study design does not allow to evaluate the exact pathomechanism responsible for these observations.
Notably, our study has certain limitations. In particular, the retrospective character of the study only allows to hypothesize a potential interaction of LMWH with SARS-CoV-2 which diminishes viral persistence. This limitation is further important for the interpretation of survival analyses, as the retrospective study design is associated with a notable amount of missing data. Accordingly, interventional studies are necessary to establish causality. In the light of the ongoing clinical trials, we aim to raise awareness for these potentially beneficial off-target effects of anticoagulants and encourage further research within this area. Moreover, we did not analyse effects of different doses of LMWH in our cohort, as only nine patients (2.2% of LMWH-treated patients) received therapeutic doses of LMWH and the statistical power for the respective analyses was not sufficient. Of note, previous reports were not able to assess a difference between prophylactic and therapeutic uses of LMWH in more than 4,000 patients.6 Further, recent data from interventional studies comparing therapeutic doses of LMWH to standard of care thromboprophylaxis showed a beneficial effect of high-dose treatments on hospital survival in non-critically ill patients, while critically ill patients did not benefit from these schemes.28,29 However, we cannot rule out that a difference in LMWH dosage might affect the data obtained for SARS-CoV-2 viral persistence in the present study. Ultimately, we want to point out that patients using NOAC were underrepresented in our cohorts, which renders the findings for this sub-cohort explorative and hypothesis generating. While we tried to take various possible confounders, for example comorbidities and age, into account, we cannot exclude that our data only reflect the situation in Austria and the virus mutations present.
Taken together, the present investigation confirms an association of anticoagulants with improved survival of COVID-19 patients in a large Central European Multicentre Cohort and suggests a beneficial effect of LMWH use on SARS-CoV-2 viral persistence. While the exact pathomechanisms underlying these observations cannot be investigated due to the retrospective observational study design, the present study encourages the evaluation of viral persistence in randomized controlled trials assessing the effect of LMWH in COVID-19 patients in order to establish a causal relation of the presented findings. Limiting viral persistence, thereby shortening hospitalization and contagiousness is a relevant aspect during this pandemic.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
D.P. collected and analysed the data, prepared the figures, and wrote the manuscript; S.H. performed statistical analyses, prepared the figures, and wrote the manuscript; W.C.S., J.S., A.P., A.S., K.K., D.A., T.S., F.F., H.H., E.P., P.K., B.R., M.T.T., P.G., C.F., C.S., T.S., and M.K. collected and analysed the data; I.P., C.B., P.S., G.W., R.B.W., H.J.F.S., B.J., and A.Z. provided resources and interpreted the data; A.A. conceived the study, analysed and interpreted the data, and wrote the manuscript.
Conflict of interest: none declared.
Funding
This work is part of the ACOVACT study of the Medical University of Vienna and is financially supported by the Austrian Federal Ministry of Education, Science and Research, the Medical-Scientific Fund of the Mayor of Vienna (COVID024), and the Austrian Science Fund (P32064; SFB-54).
Data availability
The data presented in this article are represented in the main text and in the article’s Supplementary material online. Additional information can be shared after reasonable request to the corresponding author.
Supplementary Material
Time for primary review: 19 days
Translational perspective
The data gathered in this retrospective multicentre observational study could confirm an association of improved survival and anticoagulation and could link LMWH use to improved biomarkers of cell death and curtailed persistence of SARS-CoV-2. This encourages the use of this drug in patients without known contraindications in case this effect can be consolidated in randomized controlled trials. Currently performed prospective trials using LMWH in COVID-19 should focus on viral persistence in order to explore an additional field of application for LMWH during this pandemic.
References
- 1.The Lancet Haematology. COVID-19 and thrombosis: a continuing story. Lancet Haematol 2021;8:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S.. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020;173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, Gao Y, Cai L, Wang Z, Yin P, Wang Y, Tang L, Deng J, Mei H, Hu Y.. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 2020;7:e671–e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miesbach W, Makris M.. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost 2020;26:1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno P, Pujadas E, Arvind V, Bose S, Charney AW, Chen MD, Cordon-Cardo C, Dunn AS, Farkouh ME, Glicksberg B, Kia A, Kohli-Seth R, Levin MA, Timsina P, Zhao S, Fayad ZA, Fuster V.. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J Am Coll Cardiol 2020;76:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN.. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lisman T, Thachil J.. Differentiating biochemical from clinical heparin resistance in COVID-19. J Thromb Thrombolysis 2020;50:1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibañez C, Perdomo J, Reverter JC, Lisman T.. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemostasis 2020;18:2646–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicolai L, Leunig A, Brambs S, Kaiser R, Joppich M, Hoffknecht M, Gold C, Engel A, Polewka V, Muenchhoff M, Hellmuth JC, Ruhle A, Ledderose S, Weinberger T, Schulz H, Scherer C, Rudelius M, Zoller M, Keppler OT, Zwißler B, von Bergwelt-Baildon M, Kääb S, Zimmer R, Bülow RD, von Stillfried S, Boor P, Massberg S, Pekayvaz K, Stark K.. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J Thromb Haemostasis 2021;19:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C.. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR.. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, Zhang C, Li H, Xia X, Kong S, Liao J, Jia H, Pang X, Song Y, Tian Y, Wang B, Wu C, Yuan H, Zhang Y, Li Y, Sun W, Zhang Y, Zhu S, Wang S, Xie Y, Ge S, Zhang L, Hu Y, Xie M.. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation 2020;142:114–128. [DOI] [PubMed] [Google Scholar]
- 14. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H.. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macías M, Toledo-Samaniego N, García-García A, García-Fernández-Bravo I, Ji Z, de-Miguel-Diez J, Álvarez-Sala-Walther LA, Del-Toro-Cervera J, Galeano-Valle F.. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 2020;192:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP.. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z.. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemostasis 2020;18:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pereyra D, Heber S, Jilma B, Zoufaly A, Assinger A.. Routine haematological parameters in COVID-19 prognosis. Lancet Haematol 2020;7:e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Höchter D, Keppler O, Teupser D, Zwißler B, von Bergwelt-Baildon M, Kääb S, Massberg S, Pekayvaz K, Stark K.. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 2020;142:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng H, Havervall S, Rosell A, Aguilera K, Parv K, von Meijenfeldt FA, Lisman T, Mackman N, Thalin C, Phillipson M.. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler Thromb Vasc Biol 2021;41:988–994. Atvbaha120315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al-Harbi NO, Imam F, Alharbi MM, Khan MR, Qamar W, Afzal M, Algahtani M, Alobaid S, Alfardan AS, Alshammari A, Albekairi TH, Alharbi KS.. Role of rivaroxaban in sunitinib-induced renal injuries via inhibition of oxidative stress-induced apoptosis and inflammation through the tissue nacrosis factor-α induced nuclear factor-κappa B signaling pathway in rats. J Thromb Thrombolysis 2020;50:361–370. [DOI] [PubMed] [Google Scholar]
- 23. Shi M, Wang L, Zhou J, Wang JS, Tong N, Bi L, Song J, Hu Y, Chen J.X.. Direct factor Xa inhibition attenuates acute lung injury progression via modulation of the PAR-2/NF-κB signaling pathway. Am J Transl Res 2018;10:2335–2349. [PMC free article] [PubMed] [Google Scholar]
- 24. Buijsers B, Yanginlar C, de Nooijer A, Grondman I, Maciej-Hulme ML, Jonkman I, Janssen NAF, Rother N, de Graaf M, Pickkers P, Kox M, Joosten LAB, Nijenhuis T, Netea MG, Hilbrands L, van de Veerdonk FL, Duivenvoorden R, de Mast Q, van der Vlag J.. Increased plasma heparanase activity in COVID-19 patients. Front Immunol 2020;11:575047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asperti M, Denardo A, Gryzik M, Arosio P, Poli M.. The role of heparin, heparanase and heparan sulfates in hepcidin regulation. Vitam Horm 2019;110:157–188. [DOI] [PubMed] [Google Scholar]
- 26. Kwon PS, Oh H, Kwon SJ, Jin W, Zhang F, Fraser K, Hong JJ, Linhardt RJ, Dordick JS.. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov 2020;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buijsers B, Yanginlar C, Maciej-Hulme ML, de Mast Q, van der Vlag J.. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMed 2020;59:102969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, Carrier M, Rosenson RS, Reynolds HR, Turgeon AF, Escobedo J, Huang DT, Bradbury CA, Houston BL, Kornblith LZ, Kumar A, Kahn SR, Cushman M, McQuilten Z, Slutsky AS, Kim KS, Gordon AC, Kirwan BA, Brooks MM, Higgins AM, Lewis RJ, Lorenzi E, Berry SM, Berry LR, Aday AW, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Costantini TW, de Brouwer S, Derde LPG, Detry MA, Duggal A, Džavík V, Effron MB, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Galanaud JP, Galen BT, Gandotra S, García-Madrona S, Girard TD, Godoy LC, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Hamburg NM, Haniffa R, Hanna G, Hanna N, Hegde SM, Hendrickson CM, Hite RD, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Hudock K, Hunt BJ, Husain M, Hyzy RC, Iyer VN, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski AL, King AJ, Knudson MM, Kornblith AE, Krishnan V, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Lima FG, Linstrum K, Litton E, Lopez-Sendon J, Lopez-Sendon Moreno JL, Lother SA, Malhotra S, Marcos M, Saud Marinez A, Marshall JC, Marten N, Matthay MA, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Moore SC, Morillo Guerrero R, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nunez-Garcia B, Pandey A, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pérez González YS, Pompilio M, Prekker ME, Quigley JG, Rost NS, Rowan K, Santos FO, Santos M, Olombrada Santos M, Satterwhite L, Saunders CT, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Shankar-Hari M, Sheehan JP, Singhal AB, Solvason D, Stanworth SJ, Tritschler T, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Wells BJ, Widmer RJ, Wilson JG, Yuriditsky E, Zampieri FG, Angus DC, McArthur CJ, Webb SA, Farkouh ME, Hochman JS, Zarychanski R; REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Eng J Med 2021;385:790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, Carrier M, Reynolds HR, Kumar A, Turgeon AF, Kornblith LZ, Kahn SR, Marshall JC, Kim KS, Houston BL, Derde LPG, Cushman M, Tritschler T, Angus DC, Godoy LC, McQuilten Z, Kirwan BA, Farkouh ME, Brooks MM, Lewis RJ, Berry LR, Lorenzi E, Gordon AC, Ahuja T, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Contreras A, Costantini TW, de Brouwer S, Detry MA, Duggal A, Džavík V, Effron MB, Eng HF, Escobedo J, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Froess JD, Fu Z, Galanaud JP, Galen BT, Gandotra S, Girard TD, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Haniffa R, Hegde SM, Hendrickson CM, Higgins AM, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Huang DT, Hudock K, Hunt BJ, Husain M, Hyzy RC, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski A, King AJ, Knudson MM, Kornblith AE, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Gallego Lima F, Linstrum K, Litton E, Lopez-Sendon J, Lother SA, Marten NS, Marinez A, Martinez MM, Garcia E, Mavromichalis S, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nicolau JC, Nunez-Garcia B, Park JJ, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pompilio M, Quigley JG, Rosenson RS, Rost NS, Rowan K, Santos FO, Santos M, Santos MO, Satterwhite L, Saunders CT, Schreiber J, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Singhal AB, Slutsky AS, Solvason D, Stanworth SJ, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Widmer RJ, Wilson JG, Yuriditsky E, Zhong Y, Berry SM, McArthur CJ, Neal MD, Hochman JS, Webb SA, Zarychanski R.. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Eng J Med 2021;385:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this article are represented in the main text and in the article’s Supplementary material online. Additional information can be shared after reasonable request to the corresponding author.