Abstract
Context
COVID-19 morbidity and mortality are increased in type 1 diabetes (T1D), but few data focus on age-based outcomes.
Objective
This work aimed to quantify the risk for COVID-19–related hospitalization and adverse outcomes by age in people with T1D.
Methods
For this observational, multisite, cross-sectional study of patients with T1D and laboratory-confirmed COVID-19 from 56 clinical sites in the United States, data were collected from April 2020 to March 2021. The distribution of patient factors and outcomes across age groups (0-18, 19-40, and > 40 years) was examined. Descriptive statistics were used to describe the study population, and multivariate logistic regression models were used to analyze the relationship between age, adverse outcomes, and hospitalization. The main outcome measure was hospitalization for COVID-19.
Results
A total of 767 patients were analyzed. Fifty-four percent (n = 415) were aged 0 to 18 years, 32% (n = 247) were aged 19 to 40 years, and 14% (n = 105) were older than 40 years. A total of 170 patients were hospitalized, and 5 patients died. Compared to the 0- to 18-years age group, those older than 40 years had an adjusted odds ratio of 4.2 (95% CI, 2.28-7.83) for hospitalization after adjustment for sex, glycated hemoglobin A1c, race, insurance type, and comorbidities.
Conclusion
Age older than 40 years is a risk factor for patients with T1D and COVID-19, with children and younger adults experiencing milder disease and better prognosis. This indicates a need for age-tailored treatments, immunization, and clinical management of individuals affected by T1D.
Keywords: type 1 diabetes, COVID-19, age, hospitalization
Clinical manifestations of COVID-19 caused by the new coronavirus SARS-CoV-2 infection in children are distinct from adults (1-3). Children with COVID-19 rarely develop severe respiratory symptoms and often remain asymptomatic (2). In contrast, adults experience respiratory symptoms of varying severity, with older adults and those with comorbidities such as hypertension and diabetes having higher risks of developing COVID-19–associated acute respiratory distress syndrome with high mortality (2, 4).
Individuals with diabetes are at higher risk for COVID-19–related complications (4-6). Early reports did not differentiate diabetes type (4). More recent reports have established a similar risk for people with type 1 (T1D) and type 2 diabetes and for COVID-19–related complications (7-10). Although more data regarding COVID-19 in individuals with T1D are becoming available, these reports focus on adults, including older individuals with additional comorbidities (6-12). Thus, there is still a scarcity of studies comparing the risk of COVID-19–related outcomes in younger vs older patients with established T1D.
The T1D Exchange Quality Improvement Collaborative was established to improve care delivery for people with T1D (13), and in April 2020 it initiated a multisite COVID-19 surveillance study to gather data about outcomes in people with T1D who were infected with this novel virus. The initial report on 33 COVID-19–positive patients had a mean age 24.8 years (range, 7-79 years), with the most prevalent comorbidity being obesity (39.4%), followed by hypertension or cardiovascular disease (12.1%). Diabetic ketoacidosis (DKA) was the most prevalent adverse outcome (45.5%) (14). A later report from this registry provided a description of COVID-19 in a larger adult population (age ≥ 19 years, n = 113). There were 58 patients who had required hospitalization and 5 deaths. Older patients were more likely to be hospitalized, as were patients who identified as non-Hispanic Black, used public insurance, had hypertension, and were less likely to use continuous glucose monitoring (CGM) or insulin pumps. Higher glycated hemoglobin A1c (HbA1c) was positively associated with hospitalization, which persisted after adjustment for age, sex, race, and obesity (15). Another analysis from this registry reported diabetes technology use was associated with fewer adverse COVID-19 outcomes and confirmed the known existence of racial disparities in the use of diabetes technology (16). Most recently, data from 266 patients with established T1D and COVID-19 who were younger than 19 years revealed that higher HbA1c was the only predictor for hospitalization and DKA was the most common adverse outcome in this age group (17).
The primary objectives of this study are to describe a larger pediatric and adult population of patients with T1D and COVID-19 and to determine if there is an age-based hospitalization risk stratification. Understanding how T1D affects COVID-19 severity in different age groups is critical to designing tailored interventions regarding public health advice, treatment, immunization, and clinical management of individuals with T1D.
Materials and Methods
A cross-sectional study was sponsored and coordinated by the T1D Exchange Quality Improvement Collaborative. The study was reviewed by a central review board (Western Institutional Review Board) and approved as exempt. All participating centers also obtained local institutional review board approvals as appropriate.
As of March 31, 2021, a total of 56 endocrinology sites across 23 US states participated in the multicenter surveillance study. Diabetes providers from 44 pediatric sites and 12 adult sites completed a retrospective chart review of patients with confirmed COVID-19 from April 2020 to March 2021. Deidentified data were obtained via a 33-item questionnaire using Qualtrics software, version XM (www.qualtrics.com). One team member from each participating site was identified for completing the questionnaires to avoid duplicate case submission.
Questionnaires were reviewed by the coordinating center for possible errors in data entry or incomplete information, and random validation of data was performed for quality assurance. Patients with previously established T1D who had confirmed COVID-19 were included. Data from the T1D COVID-19 registry has previously been reported (14-19).
The primary outcome for this study was hospitalization. This was defined as a binary outcome, with patients grouped under hospitalized when they have been an inpatient or admitted to an intensive care unit, and nonhospitalized when they were seen in outpatient clinics, the emergency department, or received care at home.
Descriptive statistics were used to summarize the data. Patient comorbidities and outcomes were analyzed as categorical variables. Insurance types were classified as public, private, and uninsured. Mean (SD) or median (interquartile range) were reported for continuous variables. Categorical data were shown as the percentage of patients. P values were calculated using the Fisher exact or chi-square tests to examine the association between the categorical variables. Logistic regression analysis was generated, and odds ratio (ORs) obtained to verify the association between age group and hospitalization status. Models were adjusted for, sex, HbA1c, race and ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, and other), insurance type (public, private, and uninsured), and comorbidities (obesity, hypertension/cardiovascular disease, asthma, and chronic kidney disease).
Logistic regression analysis was generated and ORs obtained to verify the association between hospitalization status as well as adverse outcomes (death, DKA, severe hypoglycemia) and HbA1c, sex, minority race and ethnicity (Non-Hispanic Black, Hispanic, and other), and presence of comorbidities.
Patient characteristics were described and stratified by age groups. Age in years was analyzed as a categorical variable (0-18, 19-40, and > 40). The age cutoff of 0 to 18 years was chosen to be in line with the most common COVID-19 state reporting practices because 47 of 56 reporting sites use a similar age cutoff for delineating pediatric from adult patients, with 19 sites using 0 to 17 and 28 sites using 0 to 19 years (20). The age cutoff of older than 40 years was chosen because it agrees with evidence suggesting hospitalization rates rise considerably after age 40 years (21). An additional age cutoff was determined using tertiles (0-15, 15-20, and > 21 years). Newly diagnosed patients defined as having a simultaneous presentation with a new diagnosis of TID and COVID-19 were excluded from this analysis. In addition to mean HbA1c, median HbA1c values were compared between groups using the nonparametric Kruskal-Wallis test, given the nonnormal distribution of HbA1c. All statistical tests were 2-sided, with a type 1 error set at 5%. Analyses were performed using statistical software R, version 3.6.2 (R: A Language an Environment for Statistical Computing, R Core Team, R Foundation for Statistical computing, 2020, https://www.R-project.org).
Results
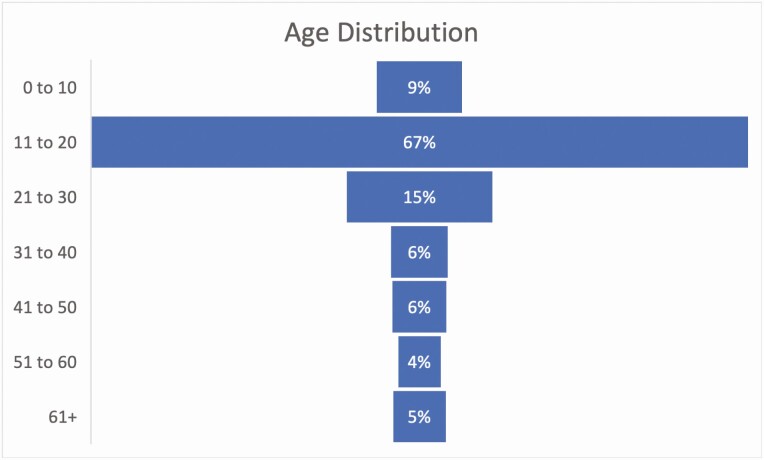
A total of 767 patients with established T1D and confirmed COVID-19 from 56 endocrinology sites across 23 US states (Fig. 1) were included in this analysis, with a mean age of 22.4 years (range, 2-87 years); 52% were female. This population represents all patients with T1D and COVID-19 included in the registry. The distribution by age in percentage is shown in Fig. 2. Patients were clustered in age groups with 415 (54%) patients in the 0 to 18 age group, 247 (32%) in the 19 to 40 age group, and 105 (14%) in the group older than 40. Demographics, diabetes history, comorbidities, and outcomes are summarized in Table 1. Characteristics of patients by age group based on tertiles (0-15, 15-20, and > 21 years) are summarized in Supplementary Table 1 (22). Compared with the other age groups, a larger number of older patients (age > 40 years) were publicly insured (49% for the ≥ 41 group and 45% for the age 0- to 18-year group vs 31% for the age 19- to 40-year group) (P < .001).
Figure 1.
Geographical distribution of pediatric and adult clinical sites across the United States (n = 23).
Figure 2.
Distribution by age as percentage (n = 767).
Table 1.
Characteristics of patients by age group with COVID-19 and type 1 diabetes. data collected April 7, 2020 to March 31, 2021, total N = 767
| 0-18 y Age group (n = 415) | 19-40 y Age group (n = 247) | 41+ y Age group (n = 105) | |
|---|---|---|---|
| Sex | |||
| Female | 219 (53) | 130 (53) | 48 (46) |
| Race/Ethnicity a | |||
| NH White | 244 (59) | 164 (66) | 71 (68) |
| NH Black | 55 (13) | 32 (13) | 13 (20) |
| Hispanic | 97 (23) | 32 (13) | 13 (6) |
| Other | 19 (5) | 19 (8) | 8 (8) |
| Insurance type a | |||
| Public | 186 (45) | 76 (31) | 51 (49) |
| Private | 218 (53) | 161 (65) | 51 (49) |
| Uninsured | 11 (3) | 10 (4) | 3 (3) |
| HbA 1c median (IQR)a | 8.6 (2.9) | 8 (2.5) | 8.2 (1.8) |
| HbA 1c mean (SD)a | 9.2 (2.3) | 8.6 (2.4) | 8.4 (1.7) |
| Duration of T1D a , y | |||
| < 1 | 43 (10) | 1 (0) | 2 (2) |
| 1-5 | 211 (51) | 42 (17) | 4 (4) |
| 6-10 | 111 (27) | 64 (26) | 7 (7) |
| 11-20 | 50 (12) | 107 (43) | 20 (19) |
| > 20 | 0 (0) | 33 (13) | 72 (69) |
| CGM use a | |||
| Yes | 286 (69) | 145 (59) | 50 (48) |
| Insulin pump use | |||
| Yes | 199 (48) | 119 (48) | 41 (39) |
| Most prevalent comorbidities a | |||
| Obesity | 23 (6) | 17 (7) | 20 (19) |
| Hypertension/CVD | 0 (0) | 21 (9) | 70 (67) |
| Asthma | 23 (6) | 12 (5) | 7 (7) |
| CKD | 0 (0) | 15 (6) | 31 (30) |
| Highest level of care a | |||
| In patient | 47 (11) | 20 (8) | 34 (32) |
| ICU | 35 (8) | 20 (8) | 14 (13) |
| Nonhospitalized | 333 (80) | 207 (84) | 57 (54) |
| Adverse outcome a | |||
| Death | 0 (0) | 2 (1) | 3 (3) |
| DKA | 62 (15) | 24 (10) | 15 (14) |
| Severe hypoglycemia | 6 (1) | 1 (0) | 5 (5) |
| Other | 21 (5) | 15 (6) | 7 (7) |
| None | 326 (79) | 207 (84) | 76 (72) |
Data are presented as n (%) unless stated otherwise. Other race/ethnicity included Asian, Pacific islander, and people with more than one race. Other adverse outcomes included hospitalization for other non–COVID-19 or diabetes reasons, for example, prescheduled urologic procedures, salmonella enteritis, suicidal ideation, etc.
Abbreviations: CGM, continuous glucose monitoring; CKD, chronic kidney disease; CVD, cardiovascular disease; DKA, diabetic ketoacidosis; HbA1c, glycated hemoglobin A1c; ICU, intensive care unit; IQR, interquartile range; NH, non-Hispanic; T1D, type 1 diabetes.
a Statistically significant intergroup differences with P value less than .001.
Median HbA1c was highest for the 0- to 18-year age group with 8.6% (interquartile range, 7.5-10.5; 70 mmol/mol [58-91 mmol/mol]) vs 8% [7.2-9.8], (65 mmol/mol [55-84 mmol/mol]) for the 19- to 40-year age group, and 8.2% (7.3-9) (66 mmol/mol [56-75 mmol/mol]) for the older than 40-year age group (P < .001). Older patients were more likely to be hospitalized for COVID-19 (47% for the > 40 years group vs 20% for the 0-18 group and 16% for the 19-40 group, P < .001) and to experience an adverse outcome including death, DKA, or severe hypoglycemia (28% for the > 40 group vs 21% for the 0-10 group and 17% for the 19-40 group, P < .001). Patients older than 40 years had a significantly higher prevalence of comorbidities such as obesity, hypertension or cardiovascular disease, and chronic kidney disease when compared to the younger groups (P < .001) (see Table 1).
Of the 170 hospitalized patients, 69 were admitted to an intensive care unit, and 5 died during hospitalization. Characteristics of the 5, all men, who died have been previously described (15). No deaths occurred in the youngest group (age 0-18). Compared with the 0 to 18 age group, patients older than 40 years were less likely to be using CGM (P < .001) (see Table 1).
Table 2 shows results from a logistic regression analysis examining the association between different age groups (0-18, 19-40, and > 40 years) and hospitalization among patients with confirmed COVID-19 and T1D. The adjusted model was controlled for sex, HbA1c, race and ethnicity (minority vs nonminority), and insurance type (public vs private). The odds of being hospitalized increased with age. Compared with the 0-18 age group, the adjusted OR for hospitalization was 6.93 (95% CI, 4.00-12.18) for the older than 40 age group. Even after adjusting for comorbidities, we found that patients older than 40 years had an OR for hospitalization of 4.20 (95% CI, 2.28-7.83). Table 3 shows the results from the same logistic regression analysis but between age groups obtained by tertiles (0-15, 15-20, and > 21 years).
Table 2.
Logistic regression for hospitalization among patients with confirmed COVID-19 and type 1 diabetes
| n = 743 | Model A | Model B | Model C |
|---|---|---|---|
| Reference (0-18 y age group) | – | – | – |
| 19-40 y age group | 0.81 (0.52-1.23) | 1.24 (0.75-2.03) | 1.01 (0.60-1.69) |
| 40+ y age group | 3.48 (2.19-5.54) a | 6.93 (4.00-12.18) a | 4.20 (2.28-7.83) a |
a P less than .001. bP less than .01. cP less than .05.
Table 3.
Logistic regression for hospitalization among patients with confirmed COVID-19 and type 1 diabetes
| n = 743 | Model A | Model B | Model C |
|---|---|---|---|
| Reference (0-15 y age group) | |||
| 15-20 y age group | 0.79 (0.50-1.25) | 0.95 (0.56-1.60) | 0.84 (0.49-1.43) |
| 21+ y age group | 1.68 (1.10-2.56) b | 3.11 (1.91-5.15) a | 1.91 (1.11-3.30) b |
Model A: unadjusted. Model B: adjusted for sex (male vs female), A1c (as a continuous variable), race (minority vs nonminority), and insurance type (public vs private). Model C: adjusted for sex (male vs female), A1c (as a continuous variable), race (minority vs nonminority), insurance type (public vs private), and comorbidity (yes or no).
a P less than .001.
b P less than .01.
c P less than .05.
Compared with the 0 to 15 age group, the adjusted OR for hospitalization was 3.11 (95% CI, 1.91-5.15) for the older than 21 age group. After adjusting for comorbidities, we found that patients older than 21 years had an OR for hospitalization of 1.91 (95% CI, 1.11-3.30).
Table 4 shows results from logistic regression analysis for adverse outcomes (death, DKA, and severe hypoglycemia) among patients with confirmed COVID-19 and T1D between the 0 to 18, 19 to 40, and older than 40 years age groups. After adjustment, patients older than 40 years had an OR for an adverse outcome of 2.03 (95% CI, 1.07-3.77). However, when adjusted for comorbidities, this finding was not statistically significant (adjusted OR 1.62 [95% CI, 0.79-3.27]). The logistic regression analysis for adverse outcomes between age groups obtained by tertiles (0-15, 15-20, and > 21 years) was not statistically significant (Table 5).
Table 4.
Logistic regression for adverse outcomes (death, diabetic ketoacidosis, severe hypoglycemia) among patients with confirmed COVID-19 and type 1 diabetes
| n = 743 | Model A | Model B | Model C |
|---|---|---|---|
| Reference (0-18 y age group) | – | – | – |
| 19-40 y age group | 0.60 (0.36-0.99) | 0.82 (0.46-1.44) | 0.76 (0.42-1.34) |
| 40+ y age group | 1.32 (0.74-2.27) c | 2.03 (1.07-3.77) c | 1.62 (0.79-3.27) |
a P less than .001. bP less than .01.
c P less than .05.
Table 5.
Logistic regression for adverse outcomes (death, diabetic ketoacidosis, severe hypoglycemia) among patients with confirmed COVID-19 and type 1 diabetes
| n = 743 | Model A | Model B | Model C |
|---|---|---|---|
| Reference (0-15 y age group) | |||
| 15-20 y age group | 0.67 (0.40-1.11) | 0.73 (0.41-1.29) | 0.70 (0.39-1.24) |
| 20+ y age group | 0.89 (0.54-1.45) | 1.38 (0.80-2.40) | 1.12 (0.61-2.07) |
Model A: unadjusted. Model B: adjusted for sex (male vs female), A1c (as a continuous variable), race (minority vs nonminority), and insurance type (public vs private). Model C: adjusted for sex (male vs female), A1c (as a continuous variable), race (minority vs nonminority), insurance type (public vs private), and comorbidity (yes or no).
Table 6 shows results from logistic regression analysis examining the association between hospitalization among patients with T1D and confirmed COVID-19 and HbA1c, sex, minority race and ethnicity (non-Hispanic Black, Hispanic, and other), and comorbidities. The odds of being hospitalized increased with higher HbA1c values (OR 1.46, 95% CI, 1.35-1.58; P < .001). Minorities had more than 3 times greater odds of requiring hospitalization (OR 3.46, 95% CI, 2.42-4.99; P < .001). Hospitalization was also more likely with the presence of comorbidities (OR 3.09, 95% CI, 2.15-4.47; P < .001).
Table 6.
Logistic regression for hospitalization among patients with confirmed COVID-19 and type 1 diabetes
| n = 743 | Unadjusted |
|---|---|
| HbA1c (continuous) | 1.46 (1.35-1.58) a |
| Sex (female) | 0.79 (0.56-1.128) |
| Race (minority)b | 3.46 (2.42-4.99) a |
| Comorbidities (yes) | 3.09 (2.15-4.47) a |
Abbreviation: HbA1c, glycated hemoglobin A1c.
a P less than .001.
b Minority includes non-Hispanic Black, Hispanic, and other.
Table 7 shows results from logistic regression analysis examining the association between adverse outcomes (death, DKA, or severe hypoglycemia) and HbA1c, sex, race, and comorbidities. The odds of developing adverse outcomes increased with higher HbA1c values (OR 1.5, 95% CI, 1.37-1.66; P < .001), minority race or ethnicity (OR 3.31, 95% CI, 2.17-5.09; P < .001), and presence of comorbidities (OR 1.61, 95% CI, 1.07-2.44; P < .05).
Table 7.
Logistic regression for adverse outcomes (death, diabetic ketoacidosis, severe hypoglycemia) among patients with confirmed COVID-19 and type 1 diabetes
| n = 743 | Unadjusted |
|---|---|
| HbA1c (continuous) | 1.50 (1.37-1.64) a |
| Sex (female) | 0.79 (0.52-1.19) |
| Race (minority)c | 3.31 (2.17-5.09) a |
| Comorbidities (yes) | 1.61 (1.07-2.44) b |
Abbreviation: HbA1c, glycated hemoglobin A1c.
a P less than .001.
b P less than .05.
c Minority includes non-Hispanic Black, Hispanic, and other.
Discussion
Patients with T1D and confirmed COVID-19 who were older than 40 years were 7 times more likely to be hospitalized compared with patients aged 0 to 18 years after adjustment for sex, HbA1c, race (minority vs nonminority), and insurance type (public vs private). Importantly, even after adjusting for comorbidities such as obesity, asthma, hypertension/cardiovascular disease, and chronic kidney disease, the odds of hospitalization were still 4 times higher for patients older than 40 years and 2 times higher for patients older than 21 years if comparing age groups obtained by tertiles (0-15, 15-20, and > 21 years).
Several reports have shown that people with T1D have increased odds for hospitalization and poor outcomes due to COVID-19 when compared with people without diabetes (6-10). However, there is still a scarcity of data regarding how COVID-19 affects patients with T1D from different age groups. This study is a systematic evaluation of age-based hospitalization risk stratification in people with T1D and COVID-19 using a diverse cohort with wide representation regarding sex, location, and race/ethnicity across the United States.
The risk of fatal or critical care unit–treated COVID-19 increases with age, with just 30 (2.8%) of 1082 people in one study with fatal or critical care unit–treated COVID-19 being younger than 50 years (all aged > 20 years), whereas 972 (89.9%) were aged 60 years or older (12). Our data align with previous evidence suggesting that hospitalization is infrequent in children and young adults, rising after age 40 years (21). It has been also reported that people with T1D and COVID-19 have a higher probability of hospitalization than patients without diabetes, with age being the most important factor in the multivariable ordinal regression model for illness severity (10).
In addition to having longer duration of T1D and more comorbidities, characteristics of patients in the oldest group (> age 40 years) included a large percentage using public insurance and less use of CGM than the younger age groups. Although not statistically significant, there was also a tendency toward less insulin pump use in the group older than 40 years. Decreased rates of diabetes technology use as well as a higher rate of public insurance are likely correlated to lower access to diabetes care and has been shown to be associated with increased hospitalization in adults and children with T1D and COVID-19 (14-17). Patients with T1D and COVID-19 were more likely to be hospitalized and to develop adverse outcomes if they had a higher HbA1c, identified as minority race and ethnicity, or had comorbidities in alignment with earlier results from this registry on 133 patients aged 19 years and older (15). The present study builds on the earlier findings with a more powered cohort size and broader population representation across the lifespan (2-87 years). Higher HbA1c level has also been reported by others to increase the odds of hospitalization in this population (14, 17). Similar to previous reports from the T1D Exchange Clinical Registry, our cohort HbA1c was significantly higher among the youngest patients (age 0-18 years), although this group had a lower number of hospitalizations. Our data and others’ suggest that risk of hospitalization in COVID-19 among youth with T1D is more comparable to youth without diabetes than older adults with diabetes (1, 2, 23). Among people with T1D and consistent with general population data, age is clearly protective against severe outcomes even in the face of relatively higher HbA1c in the younger age group compared to the older than 40 age group.
Adults with COVID-19 may present with respiratory symptoms, which can lead to acute respiratory distress syndrome in the most severe form, whereas children are largely spared respiratory disease but can develop a life-threatening multisystem inflammatory syndrome (24). The immune systems of children and adults are different with respect to their composition and overall responsiveness (25). Weisberg et al (3) recently reported that the anti-SARS-CoV-2 antibody response generated in children is predominantly antispike immunoglobulin G antibodies independent of clinical syndrome. On the other hand, adults generate broader antibody responses to infection and exhibit increased magnitude of the antispike antibody response with more severe disease.
Experts caution against extrapolating poor outcomes from COVID-19 among adults with diabetes to children (26, 27). Adults living with T1D have a far greater burden of diabetes-related vascular complications than children, largely from the much longer average duration of T1D in adult cohorts. Aside from the risk of DKA, there have been very few deaths and long-term adverse outcomes reported in children with T1D and COVID-19 (28, 29). In an earlier report of pediatric patients from this registry, higher HbA1c was the only predictor for inpatient hospital admission. DKA was the most common poor outcome, with severe outcomes being relatively rare. Only one of 266 patients experienced COVID-19–associated multisystem inflammatory syndrome, and 10 of 61 hospitalizations were unrelated to T1D or COVID-19 (17). It has been reported that although pediatric hospitalization rates are used as a marker of COVID-19 disease severity, it is likely overestimated by the detection of mild or asymptomatic infection via universal screening of patients (30).
The primary strength of this study is the broad representation of patients from 56 diabetes clinics across the United States. One limitation is that we report only patients who were known to have COVID-19 and do not compare them to the rest of the population of patients with T1D or to people with COVID-19 who do not have T1D. In addition, as a multicenter registry, the data are prone to selection bias. This could increase the proportion of hospitalized patients, decrease the proportion of milder and asymptomatic cases, and lead to missing or incomplete data on diabetes control, COVID-19 management, and timing of molecular testing positivity. Most important, this study likely overestimates the proportion of young patients with T1D and COVID-19 who were hospitalized and underestimates those with minimal symptoms who were either not tested for COVID-19 or whose illness did not come to the attention of the diabetes team. Additionally, more pediatric than adult clinics contributed data to this registry.
These data and others indicate that risk for hospitalization for people with T1D is age dependent, with the majority of children and young adults being spared from hospitalization and adverse outcomes. Public health recommendations need to be followed by all to reduce risk of contracting COVID-19, and if a person with T1D contracts COVID-19, then appropriate sick-day management including contact with the diabetes care team is recommended.
Acknowledgments
The authors thank the Helmsley Charitable Trust, which funds the T1D Exchange QI Collaborative. The authors also thank all participating clinics for their contribution.
Funding: This work was supported by the Helmsley Charitable Trust, which funds the T1D Exchange QI Collaborative. The T1D Exchange received financial support for COVID-19 response from Abbott Diabetes, JDRF, Dexcom, Medtronic, Insulet Corporation, Eli Lilly, and Tandem Diabetes Care.
Author Contributions: C.D.B. wrote the manuscript; C.D.B., O.E., S.R., and D.M.M. developed the concept for the manuscript; and O.E. and S.R. analyzed the data and had full access to the data. S.R. is the guarantor for the data and analysis. O.E., D.M.M., and C.J.L. critically revised the early draft of the manuscript; all authors researched the references, revised, and approved the final version of the manuscript.
Glossary
Abbreviations
- CGM
continuous glucose monitoring
- DKA
diabetic ketoacidosis
- HbA1c
glycated hemoglobin A1c
- OR
odds ratio
- T1D
type 1 diabetes
Additional Information
Disclosures: OE has consulted for Medtronic and is a member of Medtronic Diabetes Health Equity Advisory Board. OE has received research support from Medtronic, Dexcom, Eli Lilly paid through his institution. CJL has received research support from Insulet, Abbott Diabetes, Tandem Diabetes and Dexcom paid to her institution, and has received consulting fees from Dexcom and Eli Lilly. DMM has had research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust and his institution has had research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. DMM has consulted for Abbott, Aditxt, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Insulet, and Dompe. All the other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239-1242. [DOI] [PubMed] [Google Scholar]
- 3. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22(1):25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068-1077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wargny M, Gourdy P, Ludwig L, et al. ; CORONADO investigators . Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGurnaghan SJ, Weir A, Bishop J, et al. ; Public Health Scotland COVID-19 Health Protection Study Group; Scottish Diabetes Research Network Epidemiology Group . Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance Study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance registry. Diabetes Care. 2021;44(8):e160-e162. [DOI] [PubMed] [Google Scholar]
- 17. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. [DOI] [PubMed] [Google Scholar]
- 19. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Academy of Pediatrics. Children and COVID-19: a state data report. Published April 29, 2021. Accessed May 9, 2021. https://www.aap.org/
- 21. Centers for Disease Control and Prevention. COVID-19 hospitalization and death by age, 2020. Accessed 17 April 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html
- 22. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Data for “Age and hospitalization risk in people with type 1 diabetes and COVID-19: data from the T1D Exchange Surveillance Study.” Supplementary Table S1.docx. figshare. Dataset. Deposited August 1, 2021. 10.6084/m9.figshare.15088167 [DOI] [Google Scholar]
- 23. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DiMeglio LA, Albanese-O’Neill A, Muñoz CE, Maahs DM. COVID-19 and children with diabetes—updates, unknowns, and next steps: first, do no extrapolation. Diabetes Care. 2020;43(11):2631-2634. [DOI] [PubMed] [Google Scholar]
- 27. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care 2021;44:526-532. Diabetes Care 2021;44(5):e102. [DOI] [PubMed] [Google Scholar]
- 28. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A; Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes . Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43(11):2870-2872. [DOI] [PubMed] [Google Scholar]
- 29. Unsworth R, Wallace S, Oliver NS, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43(11):e170-e171. [DOI] [PubMed] [Google Scholar]
- 30. Kushner LE, Schroeder AR, Kim J, Mathew R. “For COVID” or “With COVID”: classification of SARS-CoV-2 hospitalizations in children. Hosp Pediatr. 2021;11(8):e151-e156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.