Abstract
Aims
Remdesivir is a prodrug of an adenosine triphosphate analogue and is currently the only drug formally approved for the treatment of hospitalised COVID-19 patients. Nucleoside/nucleotide analogues have been shown to induce mitochondrial damage and cardiotoxicity, and this may be exacerbated by hypoxia, which frequently occurs in severe COVID-19 patients. Although there have been few reports of adverse cardiovascular events associated with remdesivir, clinical data are limited. Here, we investigated whether remdesivir induced cardiotoxicity using an in vitro human cardiac model.
Methods and Results
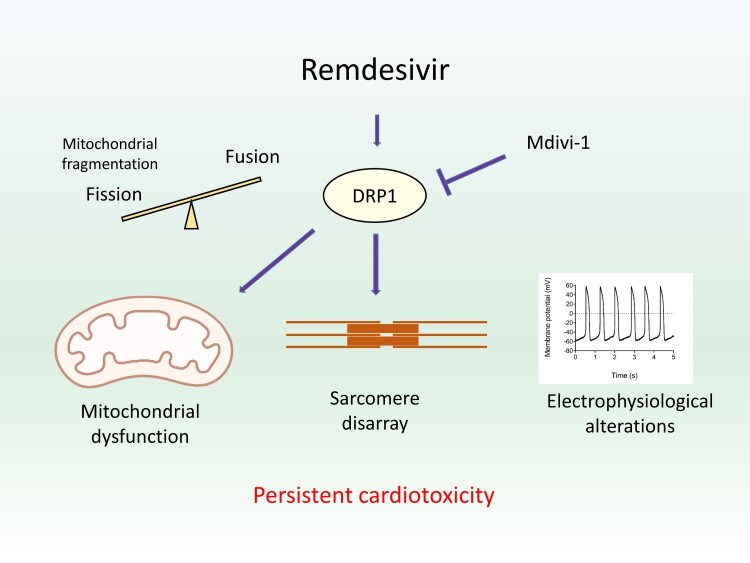
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were exposed to remdesivir under normoxic and hypoxic conditions to simulate mild and severe COVID-19 respectively. Remdesivir induced mitochondrial fragmentation, reduced redox potential and suppressed mitochondrial respiration at levels below the estimated plasma concentration under both normoxic and hypoxic conditions. Non-mitochondrial damage such as electrophysiological alterations and sarcomere disarray were also observed. Importantly, some of these changes persisted after the cessation of treatment, culminating in increased cell death. Mechanistically, we found that inhibition of DRP1, a regulator of mitochondrial fission, ameliorated the cardiotoxic effects of remdesivir, showing that remdesivir-induced cardiotoxicity was preventable and excessive mitochondrial fission might contribute to this phenotype.
Conclusions
Using an in vitro model, we demonstrated that remdesivir can induce cardiotoxicity in hiPSC-CMs at clinically relevant concentrations. These results reveal previously unknown potential side-effects of remdesivir and highlight the importance of further investigations with in vivo animal models and active clinical monitoring to prevent lasting cardiac damage to patients.
Translational perspective
Adult cardiomyocytes have limited ability to regenerate, thus treatment-induced cardiotoxicity can potentially cause irreparable harm. Remdesivir is currently the only FDA approved treatment for COVID-19 but clinical safety data are limited. Using human pluripotent stem cell-derived cardiomyocytes, we revealed that remdesivir induced persistent mitochondrial and structural abnormalities at clinically relevant concentrations. We advise confirmatory experiments in in vivo animal models, investigations of cardioprotective strategies, and closer patient monitoring such that treatment-induced cardiotoxicity does not contribute to the long term sequelae of COVID-19 patients.
Keywords: COVID-19, remdesivir, cardiotoxicity, human pluripotent stem cell derived cardiomyocytes, mitochondria
Graphical Abstract
Graphical Abstract.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.