Abstract
Background
The use of dexamethasone in patients infected with Strongyloides stercoralis can cause severe complications. It is necessary to investigate the relationship between coronavirus disease 2019 (COVID-19) and strongyloidiasis infection.
Methods
A retrospective, longitudinal, descriptive study was undertaken to review all patients admitted with a diagnosis of COVID-19 infection at the Complejo Asistencial Universitario de Salamanca, Spain, during 1 March–31 December 2020.
Results
A total of 2567 patients received a diagnosis of COVID-19. Eighty-six patients from endemic areas were included. Seven patients had strongyloidiasis. Five patients were female. The mean age (±SD) was 39 (±10.8) y. Six patients were Latin-American and only one patient was from Africa. Six patients had previous symptoms compatible with strongyloidiasis infections. Only three patients received dexamethasone (6 mg once daily) for 10 d. In all cases, the clinical courses of the patients were satisfactory. No patient died or was admitted to the ICU.
Conclusions
Screening programmes using serological techniques should be implemented in COVID-19 patients to prevent strongyloidiasis. Our study suggested that drugs used against COVID-19 in patients with strongyloidiasis did not affect the evolution of the disease. However, more studies are necessary to elucidate the role of dexamethasone in COVID-19 patients infected with Strongyloides.
Keywords: COVID-19, immigrants, Spain, strongyloidiasis
Introduction
Coronavirus disease 2019 (COVID-19), which can cause serious respiratory illnesses such as pneumonia and pulmonary failure, was first reported in Wuhan, the capital of Hubei, China. The aetiological agent has been confirmed as a novel coronavirus, and the disease is now known as coronavirus disease 19 (COVID-19). The novel coronavirus (SARS-CoV-2) most likely originated from zoonotic coronaviruses, such as SARS-CoV-1, which emerged in 2002 Within a few months of the first reported case, SARS-CoV-2 had spread across China and worldwide, eventually reaching pandemic level.1
Most patients with COVID-19 have mild or moderate disease; however, 5–10% of patients present with severe and even life-threatening disease. Mortality rates are approximately 2%. Case reports have demonstrated bacterial and fungal coinfections among COVID-19–positive individuals.2
In hospitalised patients with COVID-19, several treatments and drugs are being evaluated, and dexamethasone and tocilizumab are currently the most widely used drugs.
Strongyloidiasis, with an estimated global prevalence of 30–100 million individuals,3 is endemic in Southeast Asia, Africa, Latin America and the Western Pacific regions and occurs sporadically in temperate areas such as North America, Southern Europe, Japan and Australia. In Europe, rates of infection are highest among individuals who have resided in endemic areas (including immigrants, refugees and travellers). In endemic areas, the overall regional prevalence may exceed 25%. Strongyloidiasis is often asymptomatic in immunocompetent adults but may also present with mild gastrointestinal or respiratory symptoms or with Larva currens, a rapidly moving pruritic linear skin eruption. In general, dexamethasone treatment is safe when used in low doses and for short periods of time. However, in patients coinfected with undetected Strongyloides stercoralis, the use of dexamethasone can cause severe but rare complications such as hyperinfection, as well as a wide dissemination and migration of larvae that is often accompanied by severe enterocolitis, which can potentially lead to a fatal Gram-negative sepsis. To date, there are only two published case reports of strongyloidiasis in COVID-19 patients immunosuppressed from dexamethasone and tocilizumab.4,5 Further research is necessary to investigate the relationship between COVID-19 and parasitic coinfections, particularly given that patients with COVID-19 could receive immunosuppressive treatment, a potential risk factor for severe strongyloidiasis infection. Therefore, in the current study, we aimed to evaluate the frequency of strongyloidiasis in immigrants with COVID-19 and the clinical characteristics of these patients.
Patients and Methods
Study design and participant selection
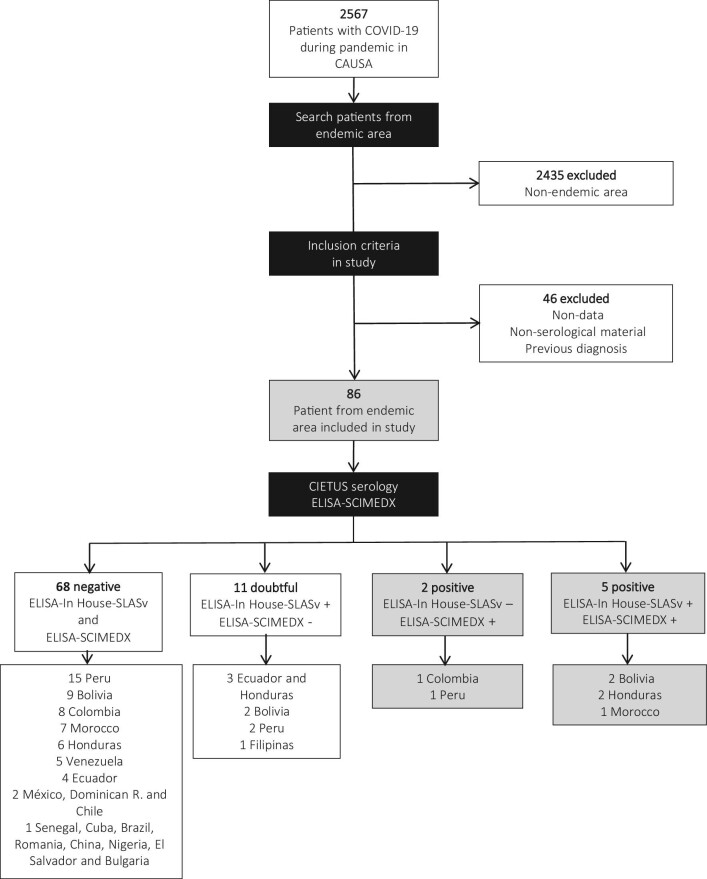
A retrospective, longitudinal, descriptive study was undertaken to review all patients admitted with a diagnosis of COVID-19 infection at the Complejo Asistencial Universitario de Salamanca (CAUSA) from 1 March to 31 December 2020. CAUSA is a tertiary-care hospital that covers an area of 12 350 km2, with 329 245 inhabitants in 2020 (National Institute of Statistics; www.ine.es), and is located in central-western Spain. The inclusion criteria were as follows: (1) patients with a diagnosis of COVID-19 infection; (2) patients aged >18 y; and (3) patients from endemic areas. The exclusion criteria were as follows: (1) patients with missing data; (2) patients without serological test results; and (3) patients with a previous diagnosis of strongyloidiasis. Figure 1 shows the flowchart of participant inclusion in the study. The clinical and epidemiological data were collected after a review of the medical records. All data analysed were anonymised.
Figure 1.
Flowchart of participant inclusion in the study.
Serological studies
IgG antibodies against S. stercoralis were measured in serum using an ELISA technique (SCIMEDX, Dover, NJ, USA). Samples in the microwell plates were processed in a fully automated ELISA system, Dynex DS-2 (Dynex Technologies, Chantilly, VA, USA) and read at 450/620–650 nm, according to the manufacturer's instructions. Simultaneously, in-house ELISA using the somatic larval antigen of Strongyloides venezuelensis (SLASv) was performed for all patients. Briefly, 96-well polystyrene plates (Costar, Washington DC, USA) were coated with 5 µg of SLASv then blocked with 2% bovine serum albumin.6
Sera were then added at 1:100 dilution, followed by the addition of peroxidase-labelled anti-human IgG antibodies at 1:2000 dilution (Merck Life Science, Darmstadt, Germany). The reaction was developed with H2O2 and orthophenylenediamine (Sigma), and the absorbance at 492 nm was measured in an ELISA Ear400FT reader (Lab Instruments S.r.l., Putignano, Italy). All samples were tested in duplicate, and sera were considered positive if the optical density (OD) was more than 50% when considering the calculation: OD negative control–OD analysed sera/OD negative control–OD positive control x 100.
Statistical analysis
The data were statistically analysed using SPSS Statistics 25.0 software (Statistical Package for the Social Sciences). Proportions were calculated for the qualitative variables, central tendency positions were means and medians, followed by the measures of variability, standard deviation and IQR, respectively.
Results
From 1 March to 31 December 2020, a total of 2567 patients received a diagnosis of COVID-19 at CAUSA: 2435 patients were excluded due to their geographical origin, while 46 were excluded due to missing data, not having serological samples available for testing or because of a previous diagnosis of strongyloidiasis. Finally, 86 patients, from endemic areas with a diagnosis of COVID-19, were included in the initial study.
A double serological study was carried out on 86 immigrants (Figure 1). The main clinical and epidemiological data of the seven patients with a serological diagnosis of strongyloidiasis and a concurrent COVID-19 diagnosis are shown in Table 1. All patients were SARS-CoV-2 PCR-positive. Five patients were female and two were male. The mean age (±SD) was 39 (±10.8) y, with ages ranging from 22 to 69 y. Six patients were Latin American, and only one patient was from Africa (Morocco). Two patients had underlying immunosuppressive conditions: patient number 5 had immunosuppressive treatment (infliximab, adalimumab, steroids and azathioprine) for Crohn's disease, while patient number 7 was pregnant. Six patients had previous strongyloidiasis infections, which included four patients who had digestive symptoms and three patients who had asthma. No patient presented cutaneous manifestations. Six of the seven patients had symptoms compatible with SARS-CoV-2 pneumonia, but only one patient (#7 patient) was positive on PCR for SARS-CoV-2 but was asymptomatic. The diagnosis for that one patient was made through a prepartum screening programme. With regards to the treatment of SARS-CoV-2 infection in the patients, only three patients received oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 d. No patient received tocilizumab. In all cases, the clinical courses of the patients were satisfactory, and the patients had a mean hospital stay of 10.1±3.8 d. No patient died or was admitted to the ICU during the study.
Table 1.
Main epidemiological and clinical data of patients included in the current study
| Immigrants | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 | Patient #7 |
|---|---|---|---|---|---|---|---|
| Demographical data | |||||||
| Age, y | 37 | 47 | 33 | 38 | 22 | 69 | 27 |
| Gender | Female | Female | Female | Male | Male | Female | Female |
| Country of origin | Bolivia | Bolivia | Honduras | Honduras | Morocco | Colombia | Peru |
| Immunosuppression | No | No | No | No | Yes | No | No |
| Previous clinical data | |||||||
| Non-strongyloidiasis disease | Obesity | Chagas disease | None | None | Crohn disease | Trigeminal neuralgia | Vitiligo |
| Diabetes Dyslipidaemia | |||||||
| Eosinophilia | No | No | Yes | No | No | No | No |
| Asthma | No | Yes | Yes | Yes | No | No | No |
| Digestive symptoms | Yes | Yes | Yes | No | Yes | No | Yes |
| Covid clinical data | Yes | Yes | Yes | Yes | Yes | Yes | No/Delivery |
| Fever | Yes | No | Yes | Yes | Yes | Yes | No |
| Dyspnoea | Yes | No | Yes | Yes | No | No | No |
| Cough | Yes | No | Yes | Yes | No | Yes | No |
| Ageusia/anosmia | No | Yes | No | Yes | No | No | No |
| Myalgia | Yes | Yes | Yes | No | No | Yes | No |
| Asthenia | No | Yes | No | No | Yes | Yes | No |
| Diarrhoea | No | Yes | No | No | Yes | No | No |
| Serological diagnosis | |||||||
| ELISA-SCIMEDX | + | + | + | + | + | + | + |
| ELISA-In House-SLASv | + | + | + | + | + | − | − |
| Specific treatment for COVID-19 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Steroids | Yes/recovery | Yes/recovery | Yes/recovery | No | No | No | No |
| Remdesevir | No | Yes | No | No | No | No | No |
| Heparin | Yes | Yes | Yes | No | No | No | No |
| Others | No | Yes | Yes | Azithromycin/hydroxychloroquine/lopinavir/ritonavir | Azithromycin/hydroxychloroquine/lopinavir/ritonavir | Azithromycin/hydroxychloroquine/lopinavir/ritonavir | No |
| Evolution of COVID-19 | Fully recovered without sequellae | fully recovered without sequellae | Fully recovered without sequellae | Fully recovered without sequellae | Fully recovered without sequellae | Fully recovered without sequellae | Fully recovered without sequellae |
| Stay, d | 7 | 12 | 7 | 4 | 20 | 12 | 9 |
| Other diagnosis during COVID-19 stay | No | No | No | Bacterial superinfection | Active Crohn disease | No | Normal delivery |
Discussion
Strongyloidiasis is caused by the soil-transmitted nematode, S. stercoralis, which is endemic in tropical and subtropical regions. The COVID-19 pandemic is a challenge due to the difficulty in the management of coinfected patients from these areas. The study approached an immigrant population from strongyloidiasis endemic areas, and all patients were infected with SARS-CoV-2. Our data indicate the importance of the relationship between COVID-19 infection and strongyloidiasis.
Screening programmes should be developed during the SARS-CoV-2 pandemic in geographical areas with a high incidence of strongyloidiasis or with a significant impact of immigration. Differences between countries with high and medium-low resources were observed, in particular in patients who have COVID-19 and receive dexamethasone therapy. Dexamethasone treatment is safe when using low doses and when use is for short periods of time. However, its use in patients coinfected with undetected S. stercoralis can have severe, rare complications, such as S. stercoralis hyperinfection syndrome (SHS). Several methods to detect the presence of S. stercoralis are used. Serology by ELISA has the highest sensitivity and specificity. However, ELISA is not always available, and serology may remain positive even after the resolution of the infection in immigrant patients. To prevent this, some authors propose a risk assessment and screening algorithm for Strongyloides spp. in COVID-19 patients with underlying risk exposures.7 This epidemiological stratification for the risk of infection from Strongyloides spp. is given according to the country of birth, current residence or long-term travel.8 Moreover, presumptive treatment with ivermectin, with an 85% efficacy as a single dose, could be used in migrants and travellers with COVID-19, and for patients at a high risk of exposure not treated previously for Strongyloides spp. if serological tests are not available.9
Despite the high incidence described in our study (8%), low clinical impact was observed. Therefore, infection is often paucisymptomatic or asymptomatic during COVID-19 infections. Interestingly, in endemic areas for strongyloidiasis, such as Brazil and India, symptomatic strongyloidiasis in COVID-19 patients has not been described. This phenomenon could be explained by several reasons: (1) early suspicion of strongyloidiasis; (2) implementation of screening programmes and empirical treatment of strongyloidiasis in patients affected by COVID-19. Therefore, prophylactic doses of ivermectin for S. stercoralis before starting corticosteroids are recommended in low- and middle-income countries10; (3) ivermectin as an empirical and/or prophylactic treatment has been used against COVID-19 during the pandemic11,12; (4) COVID-19 infection could influence the development of strongyloidiasis. Furthermore, postmortem studies have not routinely been performed in COVID-19 patients. Thus, the hyperinfection syndrome secondary to Strongyloides has already been overlooked in fatal cases of COVID-19 infection; and (5) for optimal clinical management, suspicion of hyperinfection and disseminated strongyloidiasis is essential because the use of immunosuppressive therapies for treatment of COVID-19, such as tocilizumab and dexamethasones, is common. The use of corticosteroids is one of the strongest risk factors associated with Strongyloides dissemination or SHS, which is probably from the inhibition of eosinophil and lymphocyte activation.13 In addition, corticosteroids increase the fertility of adult female worms and cause a high number of eggs to be produced and subsequent rhabditiform larvae.14 The correlation between corticosteroid treatment duration and the development of strongyloidiasis is not clear.13 However, some authors have suggested that even low-dose corticosteroids could induce SHS and dissemination with a very high fatality rate.8
Patients are treated with an anti-IL-6 receptor antibody such as tocilizumab, which has been used to mitigate the COVID-19–related cytokine release syndrome.15 Currently, tocilizumab has not been linked to the development of disseminated strongyloidiasis. In our study, no patient received tocilizumab, and only three received dexamethasone (as a RECOVERY trial dose). In the current literature, there are only two described cases of strongyloidiasis, which developed after receiving tocilizumab with concurrent high-dose corticosteroid treatment.4 Both patients presented good clinical evolution in the previous study, which was similar to the patients in our study.
Finally, we should consider false positive results for COVID-19 in patients affected with SHS in endemic areas of strongyloidiasis given the clinical similarities between COVID-19 and SHS, and an accurate diagnosis would allow for the correct therapeutic management in patients. This is a phenomenon that we did not find in the study but that is theoretically possible.
In summary, asymptomatic Strongyloides infection is common, particularly in migrants from tropical and subtropical regions. More studies are needed to analyse the real impact of COVID-19 drugs such as tocilizumab and steroids, as these drugs could potentially influence the development of severe strongyloidiasis. Screening programmes using serological techniques should be implemented in COVID-19 patients to prevent strongyloidiasis.
Contributor Information
Helena Lorenzo, Servicio de Microbiología y Parasitología, Complejo Asistencial Universitario de Salamanca (CAUSA), Salamanca 37007, Spain.
Cristina Carbonell, Servicio de Medicina Interna, Unidad de Infecciosas, CAUSA, Centro de Investigación de Enfermedades Tropicales de la Universidad de Salamanca (CIETUS), Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca 37007, Spain.
María Belén Vicente Santiago, Infectious and Tropical Diseases Group (e-INTRO), CIETUS, IBSAL, Facultad de Farmacia, Universidad de Salamanca, Salamanca 37007, Spain.
Amparo López-Bernus, Servicio de Medicina Interna, Unidad de Infecciosas, CAUSA, CIETUS, IBSAL, Salamanca 37007, Spain.
Josué Pendones Ulerio, Servicio de Microbiología y Parasitología, CAUSA, IBSAL, Salamanca 37007, Spain.
Juan Luis Muñoz Bellido, Servicio de Microbiología y Parasitología, CAUSA, CIETUS, IBSAL, Departamento de Ciencias Biomédicas y del Diagnóstico, Universidad de Salamanca, CSIC, Salamanca 37007, Spain.
Antonio Muro, e-INTRO, CIETUS, IBSAL, Facultad de Farmacia, Universidad de Salamanca, Salamanca 37007, Spain.
Moncef Belhassen-García, Servicio de Medicina Interna, Unidad de Infecciosas, CAUSA, e-INTRO, CIETUS, IBSAL, Salamanca 37007, Spain.
Authors' contributions
HL and CC contributed equally as first authors of this manuscript. Study design: HLJ, JLMB, AMA and MBG. Study implementation: CC, BV, ALB, JP and JLMB. Analysis and interpretation of data: AMA and MBG. Major contribution to writing: AMA and MBG. Read and approved the final version of the manuscript: all the authors.
Acknowledgements
To all who have faced SARS-CoV-2 infection in CAUSA.
Funding
This research was funded by the Consejería de Educación de la Junta de Castilla y León [COV20EDU/00657] and European Union cofinancing by Fondo Europeo de Desarrollo Regional (FEDER), ‘Una manera de hacer Europa’.
Competing interests
All authors declare no potential conflicts of interest and no sources of support.
Ethical approval
The study protocol was approved by the Clinical Research Ethics Committee of Investigation with Drugs of HUS, Salamanca, Spain (CEIm PI 2021 06 794). The procedures described here were carried out in accordance with the ethical standards described in the revised declaration of Helsinki in 2013. All clinical and epidemiological data were anonymised.
Data availability
None.
References
- 1. Ahn D-G, Shin H-J, Kim M-Het al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechn. 2020;30(3):313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang G, Hu C, Luo Let al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buonfrate D, Bisanzio D, Giorli Get al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9(6):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lier AJ, Tuan JJ, Davis MWet al. Case report: fisseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg. 2020;103(4):1590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marchese V, Crosato V, Gulletta Met al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2021;49(3):539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia MB, Pardo-Lledias J, Villar LPDet al. Relevance of eosinophilia and hyper-IgE in immigrant children. Medicine. 2014;93:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Wilton A, Nabarro LE, Godbole GSet al. Risk of Strongyloides hyperinfection syndrome when prescribing dexamethasone in severe COVID-19. Travel Med Infect Di. 2021;40:101981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stauffer WM, Alpern JD, Walker PF.. COVID-19 and dexamethasone. JAMA. 2020;324:623–4. [DOI] [PubMed] [Google Scholar]
- 9. Buonfrate D, Salas-Coronas J, Muñoz Jet al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (strong treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect Dis. 2019;19:1181–90. [DOI] [PubMed] [Google Scholar]
- 10. Olivera MJ. Dexamethasone and COVID-19: strategies in low- and middle-income countries to tackle steroid-related Strongyloides hyperinfection. Am J Trop Med Hyg. 2021;104:1611–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hellwig MD, Maia A.. A COVID-19 prophylaxis? Lower incidence associated with prophylactic administration of ivermectin. Int J Antimicrob Ag. 2021;57:106248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed S, Karim MM, Ross AGet al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2020;103:214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keiser PB, Nutman TB.. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soares EG, Machado ER, Souza DIet al. Dexamethasone effects in the Strongyloides venezuelensis infection in a murine model. Am J Tropical Med Hyg. 2011;84:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanders JM, Monogue ML, Jodlowski TZet al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19). JAMA. 2020;323:1824–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.