Abstract
Background and Aims:
Transversus abdominis plane (TAP) block is commonly used to treat post-operative pain after lower abdominal surgeries. The aim of this randomised controlled study was to assess the efficacy of addition of dexmedetomidine or dexamethasone to ropivacaine in TAP block and compare the two for post-operative pain relief in caesarean section.
Methods:
A hundred parturients (18–45 years) undergoing caesarean section under spinal anaesthesia received ultrasound-guided (USG) bilateral TAP block with 50ml of 3mg/kg ropivacaine along with 0.1mg/kg dexamethasone (Group A) or 1μg/kg dexmedetomidine (Group B) in this prospective, randomised, double-blind study. Time to initial self-reporting of post-operative pain, time to first rescue analgesic demand, visual analogue scale (VAS) for pain haemodynamic parameters and adverse effects if any were noted, anda P value < 0.005 was considered as statistically significant.
Results:
Time to initial self-reporting of post-operative pain (411.35 vs. 338.20 min, P < 0.005) and time to first rescue analgesic (474.30 vs. 407.30 min, P < 0.005) were significantly longer in group B as compared to group A. VAS score at the time of initial self-reporting of pain was significantly lower in group B. No significant haemodynamic changes or side-effects were noted.
Conclusion:
Addition of dexmedetomidine to ropivacaine as compared with dexamethasone in bilateral TAP block following caesarean section prolongs the time to initial post-operative pain and time to first rescue analgesic consumption.
Keywords: Dexamethasone, dexmedetomidine, post-operative pain, transversus abdominis plane block
INTRODUCTION
Post-caesarean pain control is crucial particularly during the first 24 hours to facilitate early ambulation and establishment of breast feeding. Pain management after caesarean section is usually multimodal, incorporating regional nerve blocks as part of opioid sparing analgesic technique.[1]
Ultrasound-guided (USG) transversus abdominis plane (TAP) block inhibits the neural afferents from T7-L1 lying between the internal oblique and transverse abdominis muscle with the help of local anaesthetics.[2] The accuracy of local anaesthetic deposition is enhanced with the help of ultrasound, thereby blocking the sensory nerves more effectively and enhancing the efficacy of analgesia.
Various adjuvants to local anaesthetics like opioids, ketamine, dexamethasone and alpha-2 agonists like dexmedetomidine have been utilised successfully in peripheral nerve blocks and field blocks to enhance the duration of post-operative analgesia.[1,3,4,5]
A thorough literature search did not reveal comparison of dexamethasone and dexmedetomidine as an adjuvant to ropivacaine in TAP block. The primary objective of our study was to comparatively evaluate the analgesic efficacy of dexamethasone and dexmedetomidine as an adjuvant to ropivacaine in USG TAP block for post-operative pain relief in caesarean section. Any change in the haemodynamic parameters and time to administer first rescue analgesic was considered as our secondary objective.
METHODS
After approval from our Institutional Ethics Committee, a prospective, randomised, double- blind controlled trial was conducted among American Society of Anesthesiologists (ASA) physical status II-III parturients, aged between 18 and 45 years scheduled for elective caesarean section under subarachnoid block (SAB). This trial was registered in Clinical Trials Registry-India (CTRI no-CTRI/2020/08/026995) and was conducted during August 2020–February 2021 in accordance with the Declaration of Helsinki. A written informed consent was taken from all the parturients. Parturients who refused to give consent for spinal anaesthesia or for TAP block, history of recent opioid use, sensitivity to study drugs, history of significant cardiovascular, renal and hepatic disease, any contraindication to regional anaesthesia and local anaesthetic hypersensitivity were excluded from the study.
All parturients were randomly allocated using computer-generated random numbers contained in sequentially sealed envelopes into two groups of fifty each to receive USG TAP block bilaterally with 50 ml of 3mg/kg ropivacaine along with 0.1mg/kg dexamethasone in Group A or 1μg/kg dexmedetomidine in Group B.
The anaesthesiologist not involved in the study prepared the drug and handed it over to the anaesthesiologist who administered TAP block. All the parturients were made familiar with visual analogue scale (VAS) with 0 representing no pain and 10 representing worst imaginable pain prior to the administration of subarachnoid block.
All parturients received spinal anaesthesia with 0.5% hyperbaric bupivacaine 10mg along with fentanyl 25 μg. An USG bilateral TAP block was performed under complete aseptic technique in all the patients at the end of surgery after skin closure by using Sonosite Edge II model ultrasonography machine. A linear array high-frequency probe (13-6 MHz) was placed transversely between the costal margin and iliac crest in midaxillary line. A 100-mm, 21-gauge stimuplex A-insulated needle was advanced from medial to lateral under real time ultrasound visualisation usingin-line approach and advanced until it reached the plane between the internal oblique and transversus abdominis muscles. Upon reaching the plane, 2 ml of saline was injected to confirm correct needle position, which was followed by an injection of total volume of 25 ml of drug solution on each side. The same process was repeated on the other side. The time of institution of TAP block was taken as time zero and the time from giving of TAP block to the time for first analgesic request in the post-operative period was recorded. All patients received intravenous (iv) paracetamol 1 g at the end of surgery.
Post-operative pain was evaluated using VAS every 30 min for first 2 hours and then at hourly interval till VAS ≥3. Time to initial self-reporting of post-operative pain and time of first rescue analgesic administration at VAS ≥3 was also recorded. Slow iv injection of diclofenac sodium 75 mg was administered as first rescue analgesic and continued on eight hourly basis thereafter.
Haemodynamic parameters like non-invasive blood pressure, heart rate, saturation and respiratory rate were also noted along with VAS every 30 min for first 2 hours and then at hourly intervals till VAS ≥3. A fall in blood pressure and heart rate by more than 10% was treated with iv ephedrine 6mg and iv atropine 0.6mg respectively. The study ended once the patient received her first rescue analgesic dose. Any patient sleeping comfortably in the bed at any time of observation was not disturbed.
Data was described in terms of range, mean ± standard deviation (±SD), median, frequencies (number of cases) and relative frequencies (percentages) as appropriate. Comparison of quantitative variables between the study groups was done using Student's t-test and Mann–Whitney U test for independent samples for parametric and non-parametric data respectively. For comparing categorical data, Chi-square test was performed and Fisher's exact test was used when the expected frequency was less than 5. A probability value (P value) less than 0.05 was considered statistically significant. All statistical calculations were done using Statistical Package for Social Sciences (SPSS) software version 21 (SPSS Inc., Chicago, IL, USA). Using time to first recue analgesia as our primary outcome, the sample size was calculated with the power of 80% and 5% level of significance with expected mean difference of 1.33hours and SD of 2.29 and it came out to be 50 patients in each group.
RESULTS
A total of 100 patients were recruited in this randomised controlled trial [Figure 1]. The mean time to initial self-reporting of post-operative pain was significantly prolonged in dexmedetomidine group (411.00 ± 143.35 min vs. 338.20 ± 196.13 min, P value = 0.001) [Figure 2] and mean VAS at that time was also significantly less in dexmedetomidine group (1.22 ± 0.42) when compared with dexamethasone group (1.52 ± 0.50, P value = 0.002) [Figure 3]. The mean time to first analgesic requirement when VAS ≥3 was significantly longer in parturients receiving dexmedetomidine (474.30 ± 153.99 min) as compared with those receiving dexamethasone (407.30 ± 202.31 min, P < 0.005) [Figure 2].
Figure 1.
CONSORT Flow diagram. n: number of parturients
Figure 2.
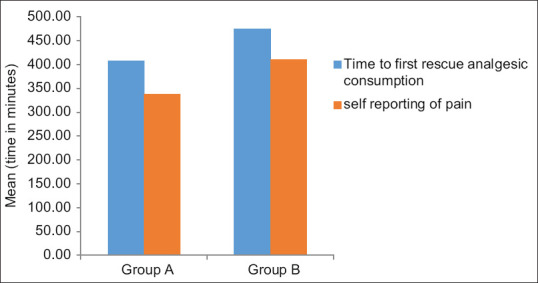
Time of initial self-reporting of pain (in min) and first rescue analgesic consumption (in min)
Figure 3.
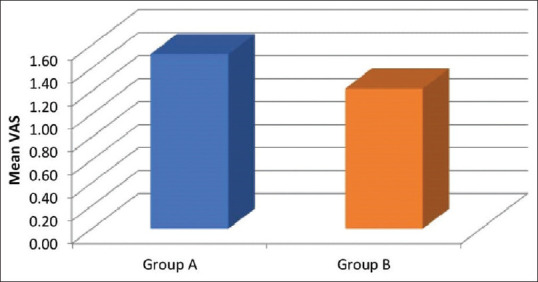
Mean Visual Analogue Scale (VAS) at the time of initial self-reporting of pain
The two groups were comparable with regards to demographic profile and duration of surgery [Table 1]. There was no significant difference in post-operative haemodynamic parameters between the two groups [Figure 4]. In our study, two parturients in dexmedetomidine group and one in dexamethasone group did not complain of pain until 24 hours after surgery.
Table 1.
Distribution of subjects according to demographic variables and clinical features
| Group A | Group B | t/Z/Chisquare value | P | |
|---|---|---|---|---|
| Age in years (SD) | 29.18 (3.51) | 28.82 (3.61) | 0.506 | 0.614 |
| BMI (SD) | 27.74 (3.80) | 27.8 (2.76) | -0.096 | 0.924 |
| Duration of surgery in minutes (SD) | 72.3 (14.75) | 69.2 (11.04) | 1.190 | 0.237 |
| Selfreporting of pain in minutes (SD) | 338.20 (196.13) | 411.00 (143.35) | -3.303 | 0.001* |
| VAS initial | 1.52 (0.50) | 1.22 (0.42) | 3.236 | 0.002* |
| Time to first rescue analgesic consumption in minutes (SD) | 407.30 (202.31) | 474.30 (153.99) | -2.942 | 0.003* |
| Adverse effects: | ||||
| Bradycardia, hypotension (%) | 0 (0) | 5 (10) | 5.263 | 0.056 |
*P≤0.05 is considered significant. BMI: body mass index, SD: standard deviation, VAS: visual analogue scale
Figure 4.
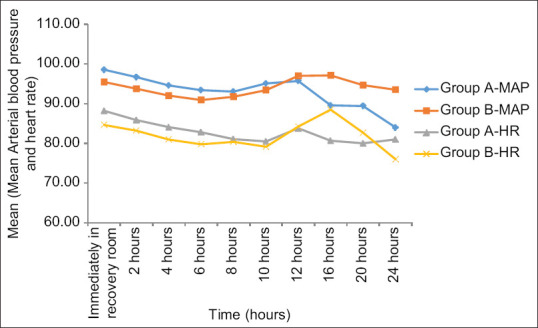
Trends in haemodynamic parameters
Hypotension and bradycardia were reported in 10% of patients in dexmedetomidine group.
DISCUSSION
Post-operative caesarean section pain is mainly because of somatic and visceral components. Surgical trauma of tissues due to surgical incision leads to somatic pain whereas visceral pain is mainly due to ‘inflammation.[6] TAP block inhibits the neural afferents from T7-L1 leading to somatic pain antagonism.[2,7] TAP block is a multifaceted block, which works through local field effects leading to distal effects causing far spread of local anaesthetic.[8] However, the combined use of local anaesthetics and opioid in intrathecal space, along with iv paracetamol administration at the end of surgery minimises the visceral component of pain.[9]
The main results of our study reveal superiority of dexmedetomidine as an adjuvant to ropivacaine in TAP block in terms of longer time to initial self-reporting of post-operative pain and prolonged mean time to initial rescue analgesic administration. The mean VAS score of initial self-reporting of pain was significantly less in dexmedetomidine group.
Similar findings have been reported in a study comparing dexamethasone and dexmedetomidine as adjuvant to bupivacaine in USG-guided TAP block. The investigators concluded that addition of dexmedetomidine reduces post-operative pain, prolongs duration of analgesia and decreases demand for additional analgesic requirement as compared with dexamethasone with bupivacaine in TAP block for post-operative pain relief in caesarean section.[10]
Various studies have been carried out to demonstrate the analgesic efficacy and safety of dexamethasone and dexmedetomidine as an adjunct in neuraxial and peripheral blocks. Dexamethasone produces analgesia through its anti-inflammatory or immunosuppressive action.[11,12] It potentiates the action of local anaesthetic through modulation of function of the potassium channels.[12] Neuroprotection and anti-hyperalgesia effects with clinically relevant dosing of perineural dexamethasone along with local anaesthetics have also been studied.[13,14]
A study was conducted using different doses (4mg or 8mg) of dexamethasone along with 0.25% isobaric bupivacaine in TAP block for post-operative analgesia in bariatric surgery. It was observed that addition of 4mg dexamethasone was equipotent to 8mg of dexamethasone for TAP block.[15] This encouraged us to choose a dose of 0.1mg/kg dexamethasone in our study, as the mean weight of our study parturients was 70 kg.
Dexmedetomidine was chosen as another adjuvant to ropivacaine in our study, as it is a highly selective central alpha-2 adrenergic agonist.[16] It has profound sedative, anxiolytic and analgesic properties. It reduces the inflammation and prolongs the duration of nerve block through vasoconstriction and inhibiting hyperpolarisation-activated cationic current. Dexmedetomidine acts by blocking the conduction of nerve signals through C and A delta fibres and may stimulate the release of encephalin-like substances at peripheral sites.[17] In this way, dexmedetomidine potentiates the local anaesthestic effects and prolongs their analgesic duration.
We observed that time to initial self-reporting of post-operative pain was significantly less in dexmedetomidine group as compared with dexamethasone group. Also, the mean time to first rescue analgesic administration when VAS ≥3 was prolonged in parturients receiving dexmedetomidine as an adjunct to ropivacaine as compared with those parturients receiving dexamethasone.
Diclofenac sodium (iv) 75mg was used as a rescue analgesic, once the patient complained of pain at VAS ≥3. The mean time to first rescue analgesic was 67 minutes later in the patients belonging to dexmedetomidine group as compared with dexamethasone group, which was statistically significant. Our findings are similar to the results of Bansal P et al.[1] who compared the efficacy of TAP block after addition of dexmedetomidine (1μg/kg) to 3mg/kg of ropivacaine for post-operative analgesia in caesarean section. They concluded that the patients receiving dexmedetomidine as an adjuvant to ropivacaine were 90 minutes more pain free than the patients receiving ropivacaine alone and mean time to first rescue analgesic was 84 minutes later in dexmedetomidine group as compared with ropivacaine alone group. This difference could be because of the usage of adjuvants in both the groups, whereas only dexmedetomidine was used in one of the groups in their study.
Bradycardia and hypotension were recorded in 10% of parturients in dexmedetomidine group, whereas heart rate, systolic and diastolic blood pressure were better maintained in dexamethasone group. This can be explained by the action of dexmedetomidine on centrally acting alpha 2A adrenergic receptors, causing decreased release of noradrenaline from the sympathetic nervous system.[18]
Despite our attempt to compare these promising adjuvants with each other in TAP block for post-operative pain relief in post-caesarean parturients, our study does have certain limitations all of which can be improvised in future studies. The methodology used in our study incorporated a brief follow-up period; hence we did not evaluate 24-hour analgesic consumption. In addition, we measured the pain score of parturients at rest and could not monitor the analgesic requirement during movement, which is essential for these lactating parturients.
CONCLUSION
The addition of dexmedetomidine to ropivacaine in TAP block significantly reduces initial post-operative pain and prolongs the time to first rescue analgesic with minimal adverse effects, as compared with dexamethasone in parturients undergoing lower segment caesarean section.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bansal P, Sood D. Effect of dexmedetomidine as an adjuvant to ropivacaine in ultrasound guided transversus abdominis plane block for post- operative pain relief in cesarean section. J Obstet Anaesth Crit Care. 2018;8:79–82. [Google Scholar]
- 2.Mukhtar BYK. Transversus abdominis plane (TAP) block. Br J Anaesth. 2009;102(6):763–7. doi: 10.1093/bja/aep067. [DOI] [PubMed] [Google Scholar]
- 3.Krishna Prasad GV, Khanna S, Jaishree SV. Review of adjuvants to local anesthetics in peripheral nerve blocks: Current and future trends. Saudi J Anaesth. 2020;14:77–84. doi: 10.4103/sja.SJA_423_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diab DG, Roshdy H. Efficacy of transversus abdominis plane block with ketamine for inguinal hernioplasty: A controlled study. Ain-Shams J Anaesthesiol. 2014;7:346–9. [Google Scholar]
- 5.Kartalov A, Jankulovski N, Kuzmanovska B, Zdravkovska M, Shosholcheva M, Spirovska T, et al. Effect of adding dexamethasone as a ropivacaine adjuvant in ultrasound-guided transversus abdominis plane block for inguinal hernia repair. Pril (MakedonAkadNaukUmet Odd Med Nauki) 2015;36:35–41. doi: 10.1515/prilozi-2015-0076. [DOI] [PubMed] [Google Scholar]
- 6.Storti E, Vaccari S, Bruno R, Ricchi A, Buffagni J, Neri I. Guidelines for management of analgesics after caesarean section: Cognitive survey. Int J Womens Health Wellness. 2018;4:075. [Google Scholar]
- 7.Jadon A, Bagai R. Effective pain relief after caesarean section; Are we on the right path or still on thecrossroad. J Obstet Anaesth Crit Care. 2019;9:3–6. [Google Scholar]
- 8.Salama ER. Post-operative bilateral continuous ultrasound-guided transversus abdominis plane block versus continuous local anaesthetic wound infusion in patients undergoing abdominoplasty. Indian J Anaesth. 2018;62:449–54. doi: 10.4103/ija.IJA_221_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirabayashi Y, Saitoh K, Fukuda H, Shimizu R. Visceral pain during caesarean section: Effect of varying dose of spinal amethocaine. Br J Anaesth. 1995;75:266–8. doi: 10.1093/bja/75.3.266. [DOI] [PubMed] [Google Scholar]
- 10.Thakur J, Gupta B, Gupta A, Verma RK, Verma A, Shah P. A prospective randomized study to compare dexmedetomidine and dexamethasone as an adjunct to bupivacaine in transversus abdominis plane block for post-operative analgesia in caesarean delivery. Int J Reprod Contracept Obstet Gynecol. 2019;8:4903–8. [Google Scholar]
- 11.McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs. 1994;47:28–45. doi: 10.2165/00003495-199400475-00006. [DOI] [PubMed] [Google Scholar]
- 12.Ahlgren SC, Wang JF, Levine JD. C-fiber mechanical stimulus-response functions are different in inflammatory versus neuropathic hyperalgesia in the rat. Neuroscience. 1997;76:285–90. doi: 10.1016/s0306-4522(96)00290-4. [DOI] [PubMed] [Google Scholar]
- 13.Williams BA, Butt MT, Zeller JR, Coffee S, Pippi MA. Multimodal perineural analgesia with combined bupivacaine-clonidine-buprenorphine-dexamethasone: Safe in vivo and chemically compatible in solution. Pain Med. 2015;16:186–98. doi: 10.1111/pme.12592. [DOI] [PubMed] [Google Scholar]
- 14.An K, Elkassabany NM, Liu J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS One. 2015;10:0123459. doi: 10.1371/journal.pone.0123459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Sharnouby NM, El Gendy HA. Ultrasound-guided single injection transversus abdominis plane block of isobaric bupivacaine with or without dexamethasone for bariatric patients undergoing laparoscopic vertical banded gastroplasty: A comparative study of different doses. Ain Shams J Anaesthesiol. 2015;8:194–9. [Google Scholar]
- 16.Sivakumar RK, Pannerselvam S, Cheian, Rudingwa P, Menin J. Perineural vs.intravenous dexmedetomidine as an adjuvant to bupivacaine in ultrasound guided fascia iliaca compartment block for femur surgeries: A randomized control trial. Indian J Anaesth. 2018;62:851–7. doi: 10.4103/ija.IJA_397_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P, Liu S, Zhu J, Rao Z, Liu C. Dexamethasone and dexmedetomidine as adjuvants to local anesthetic mixture in intercostal nerve block for thoracoscopic pneumonectomy: A prospective randomized study. Reg Anesth Pain Med. 2019 doi: 10.1136/rapm-2018-100221. rapm-2018. [DOI] [PubMed] [Google Scholar]
- 18.Xue Y, Yuan H. Effects of dexmedetomidine as an adjuvant in transversus abdominis plane block during gynaecological laparoscopy. Exp Ther Med. 2018;16:1131–6. doi: 10.3892/etm.2018.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]